The response of the neuronal adaptive system to background illumination and readaptation to dark in the immature retina
Abstract
Purpose
Developmental characteristics of the neuronal adaptive system of the retina, focusing on background light (BGL) adaptation and readaptation functions, were studied by measuring the oscillatory response (SOP) of the electroretinogram (ERG).
Methods
Digitally filtered and conventional ERGs were simultaneously recorded. Rats aged 15 and 17 days were studied during exposure to BGLs of two mesopic intensities and during readaptation to dark.
Results
Results were compared to adult rats. In ‘low mesopic’ BGL SOP instantly dropped significantly to about half of its dark-adapted (DA) value contrary to mature rats, in which the SOP significantly increased. In ‘high mesopic’ BGL SOP decreased to about 20% and 30% of DA values in immature and adult rats, respectively. The process of recovery of SOP in darkness lacked the transient enhancement immediately as BGL was turned off, characteristic of adult rats. There were no major age differences in adaptive behaviour of a-wave. In young rats, recovery of b-wave was relatively slower.
Conclusions
Properties of BGL adaptation and readaptation functions of the neuronal adaptive system in baby retina differed compared to the adult one by being less forceful and more restrained. Handling of mesopic illumination and recovery in the dark was immature. Development of these functions of the neuronal adaptive system progresses postnatally and lags behind that of the photoreceptor response and seems to be delayed also compared to that of the bipolar response.
Introduction
The oscillatory potentials (OPs) of the electroretinogram (ERG) are low amplitude high frequency components with different characteristics compared to the slow graded responses of retinal cells represented in the a- and b-waves (Heynen et al. 1985; see also Wachtmeister 1998 for overview, Wang et al. 2001). The OPs are superimposed on the b-wave and the first oscillation seems to be a postreceptor part of the a-wave being related to the positive trough of the double-peaked a-wave (el Azazi & Wachtmeister 1993; Rangaswamy et al. 2003; Dong et al. 2004, Lundström et al. 2007). The OPs may be the only postsynaptic components that can be recorded in the conventional ERG (Wachtmeister & Dowling 1978; Wachtmeister 1980, 1981a,b; Heynen et al. 1985). Furthermore, it is generally agreed that the OP activity is generated in the inner plexiform layer (IPL), where dendrites of bipolars, amacrines and ganglion cells connect (Wachtmeister & Dowling 1978; Heynen et al. 1985; Rangaswamy et al. 2003; Dong et al. 2004).
The OPs reflect both scotopic and photopic activities in the retina related not only to cone function but also to rod function (Algvere & Wachtmeister 1972; Wachtmeister 1973a,b, 1974a,b; King-Smith et al. 1986; Peachey et al. 1987; Janaky et al. 1996; Lei et al. 2006; Lundström et al. 2007). The oscillatory response is known to be maximized at an adaptational level at which the cone threshold just has been reached when the rods still are sensitive, that is, at a mesopic state of adaptation to light (Wachtmeister 1973b). To obtain an oscillatory response of maximal energy in mesopic conditions, the dark-adapted retina must be exposed to a series of bright flashes, including a conditioning flash which reduces the rod contribution, achieved by variation of interstimulus interval (ISI) or flash intensity (Peachey et al. 1987; see also Wachtmeister 1998 for overview).
Most likely, the oscillatory response represents activity in neuronal inhibitory feed-back circuits initiated by amacrine cells receiving input from both rods and cones (Wachtmeister & Dowling 1978; Heynen et al. 1985; Friedburg et al. 2004; Lei et al. 2006; Lundström et al. 2007; Perry & George 2007). As the OPs respond rapidly to changes of illumination signalling back to distal retina, the OP system offers a good tool to study the neuronal adaptive system.
In adult rats, the oscillatory response of the retina has been described to have specific characteristics showing increased activity upon exposure to low mesopic illumination (Wang et al. 2001; el Azazi et al. 2004). Further, a pronounced enhancement of the OP response in mature rats has been observed to occur after set-off of background light (BGL) of mesopic intensities (el Azazi M, Wang L, Eklund A, Wachtmeister L, unpublished data).
Previous studies of the postnatal development of the oscillatory response in the rat retina have shown that the OPs matured later than the a- and b-waves (el Azazi & Wachtmeister 1990). In the immature retina, the oscillatory activity was most pronounced during relatively more scotopic conditions. Scotopically induced OPs reached adult levels at 17 days of age, whereas relatively more photopically induced OPs reached full maturity later, at 25–30 days of age (el Azazi & Wachtmeister 1991a,b). At postnatal day 19, the immature scotopic oscillatory response shifted to a more mature photopic one.
Other postnatal studies of the OPs in the rat ERG have focused on the differences of the OP responses in dark-adapted Sprague–Dawley rats with retinopathies induced by prematurity or vulnerability to light compared to age-matched controls (Joly et al. 2006; Liu et al. 2006). However, the developmental qualities of the neuronal adaptive system during BGL exposure and its recovery in the dark have not yet been systemically addressed.
Therefore, in the present study, the retinal neuronal adaptive system in the context of OPs was studied focusing on its behaviour during and after exposure to steady, non-bleaching, mesopic background illumination. To describe the postnatal development of this complex system, we investigated and compared the results from immature rats with those of the adult ones.
Materials and Methods
The animal preparation, the recording technique and the method of measurements have been described previously in detail (Wang et al. 2001; el Azazi et al. 2004). For OP, recording the bandwidth was set at 30–250 Hz for the 15- and 17-days-old rats, and at 40–250 Hz for the adult animals.
Electroretinograms of dark-adapted albino rats were recorded during exposure to mesopic BGL of two different intensities and during subsequent readaptation to darkness. The rats had been dark-adapted (DA) for at least 12 h beforehand, prepared in dim red light and kept another 5 min in the dark. Simultaneously, filtered and conventional ERGs in response to single flashes using an ISI of 1 min were recorded from the right eye as follows:
- In DA, five recordings. Three recordings, that is, those in response to the third, fourth and fifth single flashes in the dark were averaged and referred to as the DA control value.
- Immediately as the BGL was turned on and additional seven recordings during BGL illumination. The last three recordings in response to the single flashes given at the sixth, seventh and eighth minute of BGL illumination were averaged and referred to as the background (BG) on 6-min response.
- Immediately as the BGL was turned off and at every minute during readaptation to the dark after the different BGL exposures. The last three recordings in response to the single flashes given at the 15th, 16th and 17th minute in the dark were averaged and referred to as the BG off 15-min response.
The recordings were performed using Dawson, Trick, Litzkow (DTL) electrodes. The intensities of the two mesopic BGLs were 1.43 × 10−2 cd/m2 (Log IB = −2) and 1.43 × 100 cd/m2 (Log IB = 0) and defined as previously described, ‘low mesopic’ and ‘high mesopic’, respectively (Wang et al. 2001; el Azazi et al. 2004). The ‘low mesopic’ BGL bleached negligible amounts of photopigments. The ‘high mesopic’ BGL bleached <1% of rhodopsin. The intensity of the stimulus flash was 1.43 × 102 cd/m2 and its duration 75 msecond. The stimulating and the BGL sources were two tungsten filament lamps (6 V, 25 W) regulated by electromagnetic shutters (Uniblitz 325; Vincent Lab Inc., Rochester, NY, USA). A total of 25 animals were studied, 10 baby rats at the age of 15 days, another 10 pups aged 17 days and five adults at the age of 25–29 days. The mean body weight of the baby rats was 27.5 g (±1.02) at 15 days and 30.2 g (±1.13) at 17 days. Five baby rats of each age and five adult animals were exposed to ‘low mesopic’ BGL illumination and the same set up was used for recordings in ‘high mesopic’ BGL.
The summed amplitude of the individual OPs was calculated as the oscillatory response (SOP). The amplitude of each OP wavelet was measured from its peak to a line connecting adjacent troughs. The amplitude of the a-wave was measured from the base line to its negative trough and that of the b-wave from its positive peak to the trough of the a-wave.
If not otherwise stated, the ERG responses obtained during adaptation to the two BGL illuminations and during readaptation to dark after the BGL was turned off are in the following text and Figures compared to those of the DA state, that is, before exposure to BGL illumination.
The means and standard error of the means of the amplitudes of the responses of the filtered and conventional ERGs obtained in DA, during and after exposure to the two different BGL intensities were calculated for the three age groups. The 95% confidence interval for the mean difference of the amplitudes in DA in comparison to the responses recorded at BG on, at BG on 1 min, at BG on 6 min, at BG off and at every minute during the course of readaptation to dark were determined. The same analysis was applied on data obtained for the two different BGLs. The differences were evaluated using repeated measures anova. Significance level was set at 0.05.
The present study was approved of by the Ethical Committee for Animal Research at Umeå University, Sweden. Animal care and use followed The Association for Research in Vision and Ophthalmology (ARVO) standard.
Results
‘Low mesopic’ background illumination (Log IB = −2)
Morphology
In DA, five oscillatory wavelets were clearly recordable in all animals regardless of age (Fig. 1A indicated by numbers in the top row tracings in the left, middle and right columns). During exposure to low mesopic BGL, the recordings from the adult rat retina (tracings in the right column) followed the expected pattern as previously described (Wang et al. 2001; el Azazi et al. 2004). The first two OPs (O1, O2), indicated by numbers in the top row tracing in the right column, are minute in DA. During BGL illumination, these OPs are obscured by the fast rising third OP (O3) the latter indicated by number in the second row tracing in the right column. Thus, the three later OPs (O3, O4 and O5) become dominant in the light-adapted ERG from the adults. After the BGL had been turned off for 1 min, O1 reappeared in all of the mature rats whereas O2 was detectable in two of the adult rats.
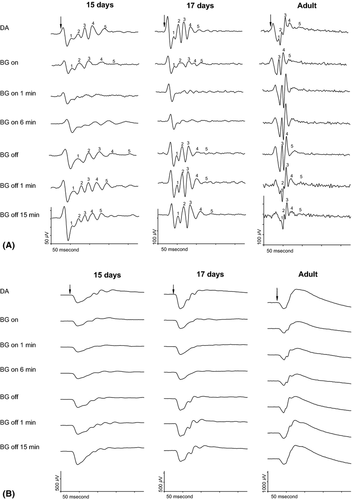
During BG illumination, the ERGs of the 15- and 17-days-old rats (tracings in left and middle columns) showed the five wavelets to be diminished in amplitudes (indicated by numbers in the second row tracings in the left and middle columns) compared to those in DA state. After the BGL was turned off, the OPs gradually regained in amplitude in darkness in all the immature animals.
Oscillatory response
At exposure to this BGL, the summed amplitudes of the OPs (SOP) started to enhance significantly in the adult rats as earlier described (Fig. 3A, Table 1). The enhancement continued progressively to a conspicuous increase up to about 50% at the end of the light exposure. However, in the baby rats, the SOP decreased significantly to about half its value in DA.
| ERG | Age | DA | BG on | 1 min | 6 min | BG off | 1 min | 2 min | 3 min | 4 min | 5 min | 6 min | 15 min |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SOP | 15 days | 210.1 ± 43.7 | 135.1a ± 25.5 | 106.7a ± 22.9 | 109.8a ± 23.2 | 136.7a ± 26.9 | 201.1 ± 41.4 | 216.5 ± 46.5 | 213.6 ± 48.7 | 229.6 ± 55.2 | 213.3 ± 53.2 | 236.3 ± 48.6 | 228.5 ± 41.0 |
| 17 days | 340.6 ± 21.5 | 180.7a ± 42.2 | 141.9a ± 32.0 | 143.7a ± 33.0 | 244.0a ± 57.7 | 359.6 ± 43.7 | 352.2 ± 36.4 | 354.4 ± 38.7 | 345.7 ± 35.5 | 365.1 ± 37.7 | 360.9 ± 32.7 | 328.1 ± 33.7 | |
| Adult | 399.9 ± 15.2 | 385.1 ± 17.9 | 527.6a ± 17.7 | 596.0a ± 17.8 | 781.8a ± 56.5 | 657.4a ± 52.2 | 578.1a ± 40.1 | 520.9a ± 46.7 | 489.7a ± 35.9 | 524.9a ± 44.2 | 484.4 ± 45.0 | 410.8 ± 41.6 | |
| a-wave | 15 days | 382.4 ± 81.7 | 258.9a ± 56.5 | 247.0a ± 53.5 | 272.3a ± 56.0 | 362.3 ± 65.4 | 396.0 ± 70.7 | 391.6 ± 70.4 | 398.5 ± 69.5 | 403.7 ± 68.1 | 405.7 ± 66.9 | 409.0 ± 69.8 | 427.5 ± 65.3 |
| 17 days | 506.4 ± 100.0 | 326.1a ± 65.2 | 331.1a ± 63.8 | 332.6a ± 76.3 | 462.2 ± 99.7 | 498.8 ± 101.3 | 490.5 ± 108.9 | 492.6 ± 108.1 | 492.4 ± 106.7 | 489.5 ± 105.2 | 497.5 ± 109.5 | 470.6 ± 96.7 | |
| Adult | 751.5 ± 42.4 | 513.9a ± 60.7 | 533.0a ± 58.5 | 534.3a ± 58.2 | 720.2 ± 77.9 | 686.5 ± 81.7 | 706.6 ± 75.9 | 694.5 ± 79.2 | 701.8 ± 76.1 | 692.7 ± 82.2 | 695.5 ± 81.8 | 679.4 ± 77.7 | |
| b-wave | 15 days | 574.8 ± 100.9 | 403.1a ± 68.8 | 352.7a ± 62.8 | 386.5a ± 67.6 | 470.3a ± 77.5 | 563.1 ± 86.7 | 582.8 ± 84.2 | 595.2 ± 82.5 | 592.8 ± 80.9 | 600.7 ± 86.1 | 611.0 ± 83.4 | 624.2 ± 67.9 |
| 17 days | 761.0 ± 82.2 | 526.0a ± 77.3 | 423.3a ± 52.9 | 439.4a ± 61.5 | 583.7a ± 101.8 | 701.1 ± 89.2 | 731.5 ± 98.1 | 732.8 ± 98.3 | 744.4 ± 96.7 | 758.8 ± 97.9 | 763.7 ± 102.0 | 773.0 ± 99.8 | |
| Adult | 1841.9 ± 95.0 | 1113.0a ± 99.1 | 1088.3a ± 98.5 | 1176.4a ± 107.8 | 1779.5 ± 162.3 | 1900.8 ± 175.6 | 1936.1 ± 176.9 | 1979.6 ± 181.4 | 1992.9 ± 184.4 | 2009.4 ± 184.9 | 2008.9 ± 184.4 | 2015.4 ± 174.1 |
- a Indicates a significant change of the amplitudes of the SOP, the a- and b-waves of the ERG compared to values in DA (p < 0.05).
Immediately at BG off, a significant, additional increase up to about twice its DA size occurred in the adult rat. Thereafter, the SOP decreased from its enhanced level and slowly levelled off to reach its DA value after 6 min in the dark.
However, in the baby rats, the SOP immediately augmented from its reduced value to return after 1 min in darkness to its previous DA value. There was no obvious difference between the courses of recovery in the 15-days-old compared to the 17-days-old pups.
a-wave
In all the animals regardless of age, the a-wave behaved similarly (Fig. 1B, Table 1). During the BGL illumination, its amplitude was reduced to about 70% of its DA value and returned to the DA level promptly as the BGL was turned off.
b-wave
As the BGL was turned on, the b-wave instantly and significantly decreased in amplitude to about 60–70% of its DA value in all animals independently of maturity (Fig. 1B, Table 1). In the 15- and 17-days-old baby rats, a further decrease occurred during the first minute of BGL exposure.
As the BGL was turned off, the b-wave amplitude immediately increased to a size not statistically different to the DA value in the mature rat. In the baby rat, the b-wave amplitude returned to its DA value after 1 min in darkness.
‘High mesopic’ background illumination (Log IB = 0)
Morphology
As previously described in the adult rat (Fig. 2A tracings in the right column), the first two OPs (O1, O2), indicated by numbers in the top tracing in the right column, are minute in DA (Wang et al. 2001; el Azazi et al. 2004). As expected during exposure to high mesopic BGL illumination, these OPs are unrecordable allowing the three later OPs (O3. O4, O5) indicated by numbers in the second row tracing in the right column to dominate. Thus, the adult light-exposed rat retina shows three OPs compared to the five OPs in the dark-adapted rat ERG. However, the ERGs of all baby rats (Fig. 2A tracings in the left and middle columns) showed five OPs during DA as well as during exposure to this BGL (indicated by numbers in the top and second row tracings), although the amplitude of each individual OP was greatly diminished.
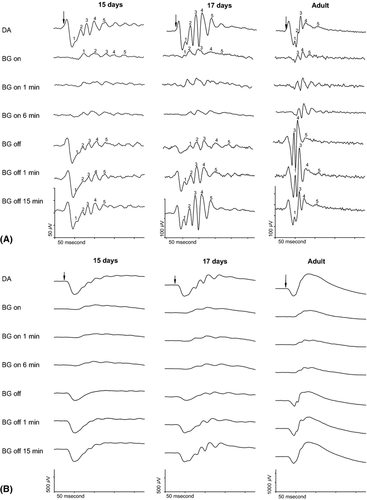
After the BGL was turned off, O1, one of the small OPs in DA reappeared at the first minute in the dark and was significantly augmented in amplitude compared to its DA value in the adult rat. O2 remained indiscernible during the time observed. However, in the baby rats, all five OPs remained after the BGL was turned off as well as during the following time in the dark during which the amplitudes gradually increased.
Oscillatory response
At once as the BGL were turned on, the summed amplitudes of the OPs (SOP) dropped significantly in all animals regardless of age (Fig. 3B, Table 2). The decrease was about 70% in the adult rats and approximately 80% in the baby rats. In the adult rats, the SOP slightly but significantly increased within the first minute of BGL exposure and showed no tendency to decrease during the following time in BGL illumination. In the ERGs of the immature rats, the decrease of the SOP remained during exposure to this BGL.
| ERG | Age | DA | BG on | 1 min | 6 min | BG off | 1 min | 2 min | 3 min | 4 min | 5 min | 6 min | 15 min |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SOP | 15 days | 263.6 ± 62.4 | 49.7a ± 7.3 | 63.5a ± 11.6 | 59.8a ± 11.0 | 61.4a ± 17.2 | 144.5a ± 28.2 | 171.2a ± 46.1 | 208.8a ± 47.1 | 215.4a ± 55.6 | 222.4a ± 59.6 | 228.6 ± 57.4 | 260.7 ± 72.0 |
| 17 days | 331.4 ± 57.3 | 66.8a ± 20.0 | 84.1a ± 25.5 | 71.3a ± 20.3 | 86.3a ± 26.1 | 328.9 ± 88.7 | 414.7a ± 97.6 | 427.1a ± 99.7 | 453.7a ± 112.2 | 485.3a ± 122.7 | 469.3a ± 102.9 | 431.0a ± 61.3 | |
| Adult | 399.9 ± 15.2 | 130.1a ± 10.7 | 163.2a ± 8.8 | 181.6a ± 20.4 | 715.1a ± 82.8 | 875.6a ± 65.7 | 825.8a ± 75.8 | 786.9a ± 56.5 | 766.4a ± 53.1 | 742.0a ± 43.9 | 746.4a ± 33.2 | 648.4a ± 52.6 | |
| a-wave | 15 days | 378.2 ± 28.1 | 11.3a ± 2.7 | 14.3a ± 3.4 | 15.2a ± 3.5 | 116.6a ± 22.5 | 278.3a ± 21.5 | 294.2a ± 22.3 | 299.5a ± 23.9 | 301.5a ± 23.1 | 302.1a ± 23.0 | 305.9a ± 25.3 | 277.0a ± 31.6 |
| 17 days | 410.3 ± 82.3 | 12.8a ± 4.2 | 21.3a ± 3.7 | 16.8a ± 3.0 | 168.1a ± 39.3 | 321.6a ± 70.8 | 339.5a ± 69.7 | 357.2a ± 75.3 | 363.8a ± 77.3 | 370.7a ± 75.2 | 372.5a ± 75.9 | 377.6a ± 79.0 | |
| Adult | 751.5 ± 42.4 | 24.0a ± 5.7 | 22.2a ± 4.5 | 27.3a ± 7.9 | 455.0a ± 43.7 | 570.4a ± 49.4 | 558.4a ± 43.0 | 553.2a ± 49.3 | 565.2a ± 43.4 | 572.8a ± 46.6 | 559.0a ± 45.5 | 548.2a ± 49.0 | |
| b-wave | 15 days | 598.3 ± 54.1 | 128.8a ± 22.1 | 116.8a ± 19.5 | 125.9a ± 17.8 | 224.9a ± 27.2 | 405.6a ± 32.4 | 474.4a ± 37.6 | 492.8a ± 40.3 | 509.3a ± 41.7 | 517.0a ± 41.1 | 517.7a ± 34.3 | 532.0a ± 50.8 |
| 17 days | 765.7 ± 145.8 | 162.9a ± 38.4 | 131.2a ± 23.7 | 149.8a ± 31.9 | 282.4a ± 64.3 | 572.6a ± 125.8 | 621.8a ± 135.3 | 654.4a ± 147.2 | 664.2a ± 154.8 | 697.2 ± 163.5 | 724.0 ± 165.6 | 778.8 ± 157.5 | |
| Adult | 1841.9 ± 95.0 | 398.6a ± 56.4 | 317.1a ± 41.4 | 356.3a ± 37.8 | 1102.1a ± 113.1 | 1480.9 ± 133.2 | 1547.6 ± 138.0 | 1586.3 ± 138.3 | 1617.1 ± 141.5 | 1634.8 ± 143.2 | 1646.6 ± 144.1 | 1694.2 ± 149.6 |
- a Indicates a significant change of the amplitudes of the SOP, the a- and b-waves of the ERG compared to values in DA (p < 0.05).
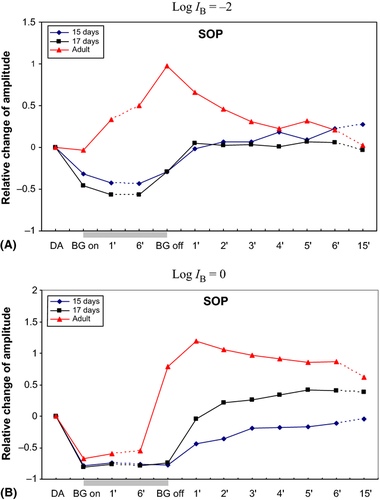
Immediately as the BGL was turned off, the amplitude of the SOP in the recordings from the adult rats increased to almost twice its previous level in DA. A further significant increase continued at a slower rate up to more than double its level in DA during the first minute in darkness. Thereafter, the SOP decreased but had not returned to its DA value after 15 min in the dark.
In the immature rats, the recovery of the SOP differed from that of the adult ones. In the 17-days-old rats, the SOP decreased by previous BGL exposure, increased to reach its DA level after 1 min. During the following time in darkness, the SOP continued to increase at a slower, gradual pace. After 15 min in darkness, the SOP was still elevated compared to previous size in DA. In the 15-days-old rats, the SOP increased to reach about 50% of DA levels after 1 min in the dark and obtained DA size after 6 min in darkness.
a-wave
In all animals regardless of age, the amplitudes of the a-waves dropped immediately and significantly to below 5% of their DA sizes as the BGL was turned on, and remained depressed during the complete period of BGL exposure (Fig. 2B, Table 2). As the BGL was switched off, the amplitude of the a-wave in the adult animals increased instantly to about 60% and in the immature rats the increase reached 30–40% of previous DA value. One minute after BG off, the a-wave had reached about 75% of its DA values in all animals. There was no further significant increase regardless of age during the following time in dark.
b-wave
The change of amplitude of the b-wave in this BGL showed similarities to that of the a-wave in all rats regardless of age (Fig. 2B, Table 2). Immediately as the BGL was turned on, the b-wave amplitude decreased about 80% and remained depressed during the following time in BGL illumination.
Instantly at BG off, the reduced b-wave amplitude enhanced in the adult animals to about 60% of its previous value in DA, a value to be completed within the first minute in the dark. At BG off, the b-wave amplitude in the immature rats enhanced to about 40% of its DA value and reached approximately 70% within the first minute. In the rats aged 17 days, the b-wave amplitude had returned to its DA value at 5 min. At the scheduled end of recordings, that is, 15 min after the BGL was turned off, the b-wave was significantly depressed in the ERG of the 15-days-old rats.
Discussion
In comparison to the adult rat, when exposed to mesopic background illumination, we found the immature rat retina to lack a similar ability to handle light as well as recovery during the subsequent period in the dark.
During light exposure, the difference was found to be conspicuous; the oscillatory response of the baby rats decreased significantly to about half of its DA value, whereas it increased about 50% in the mature ones starting at 1 min in low mesopic background illumination. The presence of this light adaptational misbehaviour of the SOP in the baby rats indicates an immature function of neuronal inhibitory feed-back circuits in the inner retina as well as an immaturity of the interacting balance between rod- and cone activity (Wachtmeister & Dowling 1978; Heckenlively et al. 1983; Lachapelle et al. 1983; Heynen et al. 1985; Peachey et al. 1987; Friedburg et al. 2004; Lei et al. 2006; Lundström et al. 2007). In the immature inner retina of the rat, the rapid fine-tuned reorganization of the microcircuits from a mixed rod/cone activity to a relatively more cone-activated function seems to be insufficient.
Regarding recovery in the dark, that is, the results after the BGL was extinguished, the behaviour of the oscillatory response in the baby rats was also found to differ from that of the mature ones. In the young rats, there was no rapid enhancement above DA level of the SOP at both mesopic BG off as was evident in the adults. Thus, there were also signs of immaturity in the process of readaptation to dark. This observation indicates an imbalance between the ON- and OFF-systems related to the rapid oscillatory response to light stimulus postulated to underlie the OPs. A part of the oscillatory activity (intermediate and later OPs) has been suggested to mainly be generated by synaptic interactions in third order neurons in the ON-pathway in the inner retina (Dong et al. 2004). Moreover, the OPs recorded in response to the termination of the light stimuli, that is, the OFF-response of the ERG, has been reported to be time-locked to stimulus turnoff, presumably generated by OFF-elements described in single cell recordings (Kojima & Zrenner 1978). Our study was neither designed to separate the ON- and OFF-components of the ERG nor the OFF-contribution to the OP response. Future studies are needed for such analysis which will require, for example, long-lasting stimulus light, pharmacological dissections to identify the ON- and OFF-components of the OPs (Kojima & Zrenner 1978; Wachtmeister 1981b, 1998; Shirato et al. 2008; Raghuram et al. 2013).
We found that after the BGL was turned off, the oscillatory response in the immature retina, having been depressed on BGL exposure, augmented at a modest pace in the subsequent time in the dark. In the mature retina the SOP, having expanded during mesopic illumination, and surged beyond BG on and DA control level at BG off, successively decreased further in the dark. Thus, in the baby rats, partly due to different points of start, the continuing process of recovery of the neuronal adaptive function and activity of the inner retina advanced in opposite direction compared to the adult ones. Furthermore, the immature process of readaptation was less forceful and more restrained compared to that of the adult rat. We also found differences in pattern of recovery after exposure to high mesopic BGL comparing the 15- and 17-days-old rats. The relatively younger baby rats showed a slower tempo of recovery than the 17-days-old, rats. These findings sensitively disclose the immaturity of the neuronal adaptive system and further indicate an ongoing postnatal maturation process of this system at this age.
Our findings of a relatively late refinement of the function of the inner retinal circuits underlying the OPs seem to concur with the morphological time table of the neuronal development of the IPL in rodents. Dopaminergic inhibitory neurons are likely to underlie the OPs (Wachtmeister & Dowling 1978; Wachtmeister 1981a, 1998; Perry & George 2007). The postnatal development and maturation of dopaminergic neurons and receptors in the IPL occur late and dopaminergic neurons are not fully responsive until Day 25 in the rat (Cohen & Neff 1982; Koulen 1999). In rat retina, inhibitory connections in the IPL are also established comparatively later postnatally than the excitatory pathways (Rörig & Grantyn 1993). Moreover, Johansson et al. (2000) reported that the terminals of the cone bipolar cells in IPL do not develop mature characteristics until 25 days of age in the rat.
In the report of Fulton & Hansen (2003), the kinetics of rod cell recovery in infant (18–19-days old) and adult rats is described to be similar to one another. The a-wave findings in our study comply at large with their results as the adaptive behaviour of the a-wave in the baby rats was similar to that of the adult ones. Thus, the BGL and readaptation properties of the neuronal adaptive system in terms of the OPs seem to be relatively more immature than those of the photoreceptor response as reflected in the a-wave. These findings correspond with previous reports that the OPs mature later than the photoreceptor response in rats as well as in humans (el Azazi & Wachtmeister 1990; Moskowitz et al. 2005).
The developmental feature of immaturity of the neuronal background and readaptation systems as reflected in the OPs was also found to differ to that of the b-wave. Background light adaptation of the b-wave was adult-like in the baby rats, whereas the early phase of recovery in the dark of the b-wave was slightly slower compared to that of the mature rats. The b-wave of the rod-dominated rat retina also reflects cone activity during mesopic BGL exposure and during early recovery in the dark in the present study. The mixed (rod/cone-driven) b-wave is known to have its origin in the inner retina; mostly from the ON-bipolar cells (Robson & Frishman 1995, 1996). Moreover, the cone-mediated b-wave in human infants has been described to be relatively more mature than rod-mediated parameters (Fulton & Hansen 2000; Hansen & Fulton 2005). In our study, the slight initial retardation of recovery of the b-wave in the dark observed in the baby rats may tentatively reflect a late maturation of the relatively more rod-driven DA b-wave reported to occur in the rat (el Azazi & Wachtmeister 1990). Thus, our results indicate that the maturation of the very fine-tuned function of reorganization of the retinal microcircuitry occurring in background illumination and readaptation in the dark, reflected in the OPs, seems to be delayed also compared to that of the bipolar function.
Although anatomically and physiologically different in several ways, the mixed rod/cone retina of the human and the rod-dominated rat retina show functional similarities of the inner retina (el Azazi & Wachtmeister 1990, 1991a,b, 1993; Moskowitz et al. 2005; Akula et al. 2007). At about 15 days of age, that is, after eye-opening, the ERG of the immature postnatal rat shows OPs of very low amplitudes as does the ERG of preterm infants (el Azazi & Wachtmeister 1990; Mactier et al. 2013). Thus, a rather extensive refinement of the inner retinal circuits seems to be underway during a relatively long time during development enclosing both pre-and postnatal periods in humans as well as in the rat presuming its first weeks alive with anatomically closed eyelids allegedly equates time of gestation. In the present study, we chose to study the first five OPs that were present in the adult as well as in the baby rat. However, O5 in the adult rat may not be the same as O5 in the baby rat. Future studies will be required to establish whether the individual OPs in the baby rat correspond to those of the adult ones, especially as each OP may have separate origins and also may reflect different functions (Wachtmeister & Dowling 1978; Wachtmeister 1998; Lundström et al. 2007).
Consequently, we conclude that maturation and refinement of the functions of the inner retinal circuitry required for coping with BGL adaptation and readaptation as reflected in the oscillatory response still progresses postnatally. The development of these functions lags behind that of the photoreceptor response and seems to be delayed also to that of the bipolar response, indicating that a prerequisite for their maturation seems to be relatively mature photoreceptor and bipolar cell functions. The late development of these neuronal functions of the inner microcircuits implies their more complex maturation process compared to that of the rod-and cone-driven more direct pathways, reflected in the a- and b-waves.