Improving the hemocompatibility of centrifugal mechanical circulatory support devices at low flow rates
Abstract
Background
An innovative solution has been recently proposed for the treatment of heart failure with preserved ejection fraction (HFpEF), using a centrifugal mechanical circulatory support (MCS) device. We sought firstly to assess the hemocompatibility of the proposed device. HFpEF treatment requires the blood pump to operate at low blood flow rate (0.05–0.5 L/min). Given high blood trauma expected in these conditions, we sought secondly to investigate design improvements in order to reduce this risk.
Method
Computational fluid dynamics was used to analyze the blood fluid filled within the centrifugal pump and to estimate the blood trauma due to its operation. This assessment is based on the computation of integrated quantities such as the hemolysis index and the turbulent dissipation energy.
Results
The hemolysis index associated with the present device is comparable to the figures from HVAD. With the proposed pump, for instance, an index of 0.0036 is obtained at 1 L/min and 3000 rpm. Using thinner blades for the impeller allows a 12% reduction of the hemolysis index in average, while reducing its diameter leads to an index 2.8 folds lower at 0.5 L/min.
Conclusion
The present investigation shows the promising hemocompatibility of our centrifugal pump. Concerns about very high hemolysis generated at low flow rates could be overcome by reducing the impeller diameter. Experimental validations are planned to support our findings.
1 INTRODUCTION
Since the early 2000s, the number of patients diagnosed with heart failure (HF) is constantly increasing, now affecting 30 million people worldwide.1, 2 Firstly in a context of lack of heart donors, mechanical circulatory support (MCS) devices have been developed to help the biological pump with delivering oxygenated blood to the organs. For end-stage HF, implantation of a left ventricular assist device (LVAD) is lifesaving and provides a significant improvement in quality of life.3 Such devices are designed to operate at the typical flow condition of 5 L/min and 80 mm Hg. However, there are pathologies which require lower level of assistance or flow rates. LVAD can be used, for instance, for mild HF which pump flow rate as low as 1 L/min.4, 5 A previous study has highlighted the increase of adverse events with centrifugal pumps, for which a sixfold increase of hemolysis at low flow compared to high flow has been observed.6 This study focuses on the treatment of a specific type of HF, namely heart failure with preserved ejection fraction (HFpEF), which requires as well low blood flow.
HFpEF is a form of heart failure characterized by an increased rigidity of the heart muscle, the myocardium, hindering its proper relaxation during diastole. In other words, the heart cannot be filled appropriately during diastole, the level of filling being limited by the maximum diastolic pressure (the preload) and the passive stiffness of the ventricle. Despite the diastolic dysfunction associated with HFpEF, the ejection fraction (EF) remains almost unaffected. This preservation is attributed to the heart muscle's stiffness, which helps maintain a heightened end-diastolic volume. Clinically, HF with reduced left ventricular ejection fraction is differentiated from HF with preserved ejection fraction.7, 8 The prevalence of HFpEF continues to rise and accounts for approximately 50% of heart failure cases, affecting around 500 000 individuals in France. However, unlike heart failure with reduced ejection fraction, pharmaceutical treatments for HFpEF have yielded disappointing results and shown no significant improvement in survival rates.9-11 Consequently, HFpEF carries a high mortality rate, with 29% mortality within a year and 65% within 5 years.12 Thus, this prevalent and critical condition must be addressed, exploring interventional therapies being a potential successful solution.
One of the main options entails the installation of a discharge system, such as a centrifugal MCS device. The main benefit of this device lies in its compactness, of comparable size with a battery or pacemaker.13, 14 This apparatus can effectively discharge a reduced blood volume, ranging from 0.05 to 0.50 liters per minute. Nevertheless, the efficacy of this particular device in the context of HFpEF treatment remains relatively uncharted. Design and analysis studies need to be performed to thoroughly assess its clinical, physiological, and hemodynamic impacts, ensuring the reliability of this medical equipment.3, 15, 16 In the case of opting for a centrifugal pump, it is important to acknowledge that it will operate under off-design conditions, significantly deviating from its nominal operational point due to the low flow rate requirement. Similarly, commercial LVAD is designed to achieve the standard cardiac output of a healthy person, and not for mild assistance. Using these centrifugal MCS outside their optimal range can increase the potential of both hemolysis and thrombosis generation.17
The present paper assesses the hemocompatibility of the proposed centrifugal pump designed for the solution to HFpEF and allows to identify the best designs. A preliminary research was performed in Abbasnezhad et al.1 with a simplified hemocompatibility study focusing on scalar shear stress and vorticity. Although this study demonstrated the capabilities of the pump to reach the specific operating conditions for HFpEF, it could not clearly conclude on the hemolysis generation. In the present work, blood trauma is explicitly assessed with the computation of the hemolysis index. The first innovative contribution of this paper lies in the first hemocompatibility study of a centrifugal discharge pump, specifically designed for the treatment of HFpEF, while previous studies focused on assistance devices designed for higher flow rates in the context of HFrEF. Although the influences of diverse geometric parameters on the hemolysis risk for centrifugal pump have been widely studied in the literature, there is no precise study regarding the optimum between angular speed and impeller diameter for hemocompatibility. The second innovative contribution of the paper is thus to propose a preliminary answer to this question. Finally, limitations of the standard hemolysis index to accurately assess blood trauma have been recently demonstrated.18, 19 This issue is however very scarcely accounted for in the literature for blood pumps design. In this context, we present here an investigation on the influence of the impeller diameter on blood trauma based on turbulent energy dissipation.
2 MATERIALS AND METHODS
2.1 HFpEF treatment
The innovative solution presented here for the solution to HFpEF is based on a small centrifugal blood pump, designed to discharge the left atrium, and offering a minimally invasive implantation method without the risk of serious side effects. The implantation of the developed pump is shown in Figure 1. The discharge pump will be implanted under the clavicula. The inlet cannulae will be connected to the left atrium for the pump to capture a small fraction of blood, which will be re-injected efficiently in the systemic circulation. As for the outlet cannulae, it will be connected to the subclavian artery. This will allow the pump to deliver the blood directly in the patient arm, thus reducing the pressure in the left atrium. Batteries and external controllers will be designed as paracorporeal systems and connected to the pump using percutaneous drivelines. In order to guarantee the efficacy of these connections and avoid suction risks or flow obstruction, a mock-loop of the cardiovascular system involving the pump, as well as a lumped numerical model, has been developed. In vitro tests will then allow to assess the pump operation, and the results will be presented in a future paper. The amount of blood discharged can be adjusted over time to eventually compensate for a worsening of the disease. In the present configuration, 0.5 L/mim should however not be exceeded to avoid unphysiological flow in the subclavian artery.

2.2 Pumps design
The different pump parts were designed with an in-house software dedicated to single-stage centrifugal pumps, which are based on reverse engineering. More details about this design method and the software are available in Asuaje et al.20 Once the geometrical features were fixed, the solid geometries were obtained with the commercial software CATIA. In the present study, two designs of the pump's impeller are considered. The first impeller geometry is directly obtained from the in-house design software for the targeted operating conditions, which represent an atrial unloading of 0.5 L/min at 80 mm Hg. The second geometry is based on a modification of the first one with the idea of reducing the contact area between the rotor and the blood. The aim is to reduce the overall wall shear stress by this mean and thus hemolysis generation (as explained in the next section). The design modification lies in the blade thickness. While the thickness is kept constant within the blade width for the first design (impeller A), it is reduced from hub to shroud for the second design (impeller B), as shown in Figure 2. Manufacturing concerns regarding biocompatibility may rise for extremely thin blades, with specific attention required for surface polishing or design strategies to avoid touchdown during operation. This will be treated in our next studies. The main geometric characteristics of the two impellers are given in Table 1. Both with 5 blades, the two impellers have a constant width of 9 mm, with only 2 mm allocated specifically to the blade width, while the remaining part will contain the different magnets necessary for electric motorization and magnetic levitation.
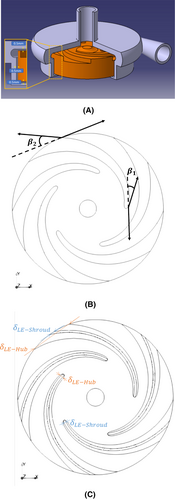
| Variable | Impeller A | Impeller B |
|---|---|---|
| [mm] | 1.00 | 1.00 |
| [mm] | 3.68 | 3.68 |
| [mm] | 1.00 | 0.5 |
| [mm] | 3.68 | 0.5 |
| 15 | 15 | |
| 24 | 24 | |
| 5 | 5 | |
| [mm] | 17.5 | 17.5 |
| [mm] | 35 | 35 |
2.3 Physical and computational model
The 3D numerical simulations are performed with the open-source library OpenFOAM library (−v8). Although the characteristic size of the present problems is small, the Reynolds numbers remain relatively large (above ), justifying the assumption of turbulent flows. Turbulence is modeled with a RANS (Reynolds Averaged Navier–Stokes) approach, based on the k-omegaSST model. The latter has shown to perform well in comparison with other turbulence models for the particular application of blood pump, as highlighted by Al-Azawy et al.21 Blood is a well-known non-Newtonian fluid, exhibiting a shear-thinning behavior. However, it can be approximated by a Newtonian model, given the relatively high shear rates, above 100 , obtained in blood pumps.22 Blood's properties are thus taken at and for its density and its dynamic viscosity, respectively. The influence of the zones of low shear rates, where the non-Newtonian behavior would appear, has been found to remain negligible.23 CFD calculations are performed with a steady-state formulation, using a multiple-reference approach. Information and variables between the rotating and static zones are transferred by an Arbitrary Mesh Interface (AMI). Gil et al.24 have observed that the influence of transient phenomena on the hemodynamic is relatively low for the HVAD device and that the hypothesis of quasi-stationary flow is assumed to be valid for HeartMate3. Similarly to the latter, the design of our impeller presents channels. Hirschhorn et al.25 have shown that such geometries could lead to sudden spike of fluid forces (more than 10 N at 1000 rpm with potential touchdown detected), invalidating the assumption of quasi-stationary flow and requiring a special care to magnet design.
Regarding the mesh, the cut-cell technique is employed in order to obtain a grid of quasi-perfect orthogonal quality, mainly based on hexahedral elements (>90%). Refinements in the three space directions are made in the gaps, close to the walls, and near the rotor-stator interface. Finally, two inflation layers are added for both fixed and moving walls. From a mesh independence study based on integrated quantities for both hemodynamic (pressure head) and hemocompatibility (hemolysis index), a grid of approximately 4.4 million elements is selected. This final mesh satisfies the following criteria: maximum aspect ratio below 25, maximum non-orthogonality below 65 (with average close to 9), and maximum skewness below 5. Due to the small dimension of the pump, the first layer of cells lies easily in the viscous sub-layer with the selected grid for every computation. In the worst-case scenario, the maximum is, respectively, below 2 for the volute wall and below 1 for the rotor wall.
2.4 Evaluation of blood damage
A transport equation of the hemolysis index has been implemented in OpenFOAM library and validated by comparison against two Couette flow benchmarks from Hariharan et al.32 The hemolysis index is advected with an already converged steady velocity field. Our results are in good agreement with theoretical data from Hariharan et al.32 with a relative error of 0.02% and 2.96%, respectively, for the standard Couette flow and inclined Couette flow, in terms of flow rate averaged hemolysis index at the outlet of the domain.
3 RESULTS AND DISCUSSION
The present numerical model of the hemodynamic behavior of the pumps was firstly validated against equivalent experimental data. The details of the manufacturing process of the pumps as well as the complete description of the experimental test rig are provided in Abbasnezhad et al.1 A good agreement was found, in terms of pressure head, for both impeller designs, especially near the designed operating point. CFD simulations lead to a slight overestimation of the head at low flow rates, and on the contrary, to a slight underestimation at high flow rates, with a relative maximum difference below 6% and 10% for impellers A and B, respectively. Similar levels of confidence are found in the literature for this type of centrifugal devices.23, 24
3.1 Hemodynamic performance
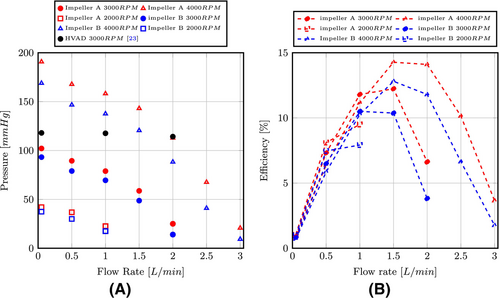
The hemodynamic performances of the two prototypes of impellers are shown in Figure 3 for different rotational speeds. The two impellers allow to achieve the specified atrial unloading, which is approximately 80 mm Hg near 0.5 L/min, for an angular speed of 3000 rpm. The efficiency of this type of mini-centrifugal pump is relatively small, between 10 and 15%. The best efficiency point lies around 1.5 L/min. For the sake of comparison, CFD simulations of the HVAD are also performed at such very low cardiac output, as they are not available in the literature. A similar procedure is used for the meshing stage, which ends up to a 14.6 million cells grid, due to the thinner clearance gap in this device. The same blood properties, modeling parameters, and boundary conditions are also considered for the HVAD. Details of this computational model are available in Marcel et al.,33 where it was used for higher flow rates. The manometric height obtained is shown along the height of the discharge pump in Figure 3. A difference of approximately 4% is obtained in comparison with the results of Gil et al.23 at 2 L/min. One can see that the different targeted designs for the HVAD allow this pump to achieve a similar pressure head to our pump model at 2 L/min, only with 3000 rpm instead of the 4000 rpm required by the present prototypes. Finally, regarding the impeller geometry of the proposed pump, it appears clearly that the thinner blades of impeller B lead to lower pressure head and efficiency. The pressure offset is approximately constant over the entire flow rate range.
The pump internal flow is represented in Figure 4 and compared for both impellers. Figure 4A,B show specifically contours and vectors of the relative velocity on a section perpendicular of the axis of rotation located at the blade half-width. No recirculations are visible for either impeller, as opposed to what was observed in the inter-blade passages for the Heartware pump at all flow rates.24, 34 Further, the velocity distribution is relatively homogeneous with respect to the axis of rotation. However, a strong stator-rotor interaction for the inter-blade passage facing the volute tongue leads to strong velocity gradients in this area. This strong interaction shrinks as the flow rate increases. Paths of decelerated flow are also noticed on the blade's pressure side for the two impeller designs. Figure 4C,D compares the contours of absolute velocity and associated streamlines for both impellers at Q = 1.0 L/min on a plane that includes the axis of rotation. Discrepancies in terms of flow patterns are relatively small between both impellers. The only noticeable differences are (i) the size of the recirculation zone at the tip of the two blades, which appear smaller for impeller B, and (ii) the absence of the tiny recirculation bubbles at the entrance of the rotor part of impeller B. Although their specific influence cannot be quantified separately, both are probably beneficial for reducing shear level and thus hemolysis generation. At lower flow rates, similar observations can be drawn for the flow within the rotor part. Finally, heterogeneity of the velocity distribution within the leakage gap highlights small fluctuations. All these flow characteristics were detailed in Abbasnezhad et al.1

3.2 Hemocompatibility
The blood damage due to the pump operation is firstly assessed thanks to an index of hemolysis. Figure 5 highlights the different zones of significant hemolysis generation. The areas with the highest risks of hemolysis are shown on the 3D view. They lie firstly below the blade's trailing edge in the clearance gap and secondly within the hub gap. For both zones, the risk intensity is increasing with the radial distance to the center of rotation, and the blood damage is thus the most pronounced on the periphery of the impeller where the centrifugal velocity is the highest. With a closest view on the internal fluid domain (see the 2D view on a cross-section plane located at the blades half-width in Figure 5B), other areas prone to hemolysis are noticed, such as the inter-blade passage and the volute tongue, in particular. They are indeed subjected to rapid variation of velocity direction and magnitude, as shown before in Figure 4. The wake behind the blade's trailing edge and the fluid layers within the volute, where the radial distance between the rotor and the stator is the thinnest, are also zones leading to hemolysis generation. With increasing flow rates, the fluid volumes where blood is damaged remain sensibly similar. The main difference is in the vicinity of the volute tongue, where hemolysis generation is reduced, although still present. A lower surface area would a priory imply less hemolysis if the new geometry does not enhance too much recirculation structures. The second impeller design (impeller B) with less surface area is exhibiting the same zones of high hemolysis generation. The main difference between both design is the extent of the hemolysis generation areas in the clearance gap within the vicinity of the blade's trailing edge. Because of its thinner blades, impeller B leads to smaller size for such zones.

In order to conclude on the global hemocompatibility of both impellers at the flow rate considered in this study, the hemolysis index is estimated as a mass-flow-average at the outlet of the pump fluid domain. Results are shown in Figure 6A. The model predicts well the typical -like curve for the index evolution with the flow rate.22-24 The very high hemolysis levels obtained at the lowest flow rate, i.e., Q = 0.05 L/min are of concern for the use of this type of devices and for the operating conditions considered. As expected, the index increases with the angular speed and its sensitivity to this parameter is similar to what is found for HVAD.24 More precisely, going from 3000 to 4000 rpm approximately doubles the hemolysis index at BEP for both the HVAD and the pump designed proposed here. Finally, the present pump, especially with impeller B, performs better than the Heartware device in terms of hemocompatibility for the lowest flow rate. This comparison does not stand for higher flow rate, given the different operating conditions between HVAD and the present pump. As published studies usually use relative index of hemolysis, it is cumbersome to make quantitative comparison of the hemocompatibility of different devices. Nonetheless, Bourque et al. have obtained a hemolysis index of 0.0089 for the HeartMate3 at 5.4 L/min for 65 mm Hg.35 In this study, impeller B leads to an index of about 0.0036 at 1 L/min for a close manometric height, highlighting its superior performance. The greater hemolysis index obtained with HeartMate3, especially given the higher flow rate considered, can also be attributed to the different empirical constants used for Equation (1). While the study of Bourque et al. employs the constants of Giersiepen et al.,26 the present model is based on the ones proposed by Heuser and Opitz.27
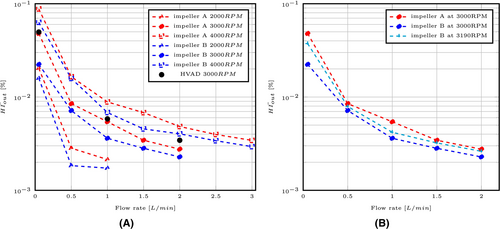
For a consistent comparison between both impellers, we must look at the same operating conditions (flow, head). A value of 3190 rpm for impeller B was thus determined by scaling the speed with similarity coefficients, based on pressure-flow values obtained for impeller A at 3000 rpm. Due to the higher rotational speed, the corrected hemolysis index obtained for impeller B is thus increasing. Nonetheless, its level remains lower in comparison with impeller A for the whole range of flow rate, as depicted in Figure 6B. In the light of these results, impeller B is a more hemocompatible design at low cardiac outputs, such as the ones typically required for HFpEF. Results from Abbasnezhad et al.1 highlighted a slightly higher level of vorticity and scalar shear stress obtained with impeller B, while fluid volumes exposed to different shear stress thresholds remain sensibly similar. The authors concluded for a probably better hemocompatibility of impeller A. As presented here, the precise resolution of the transport equation of hemolysis index shows the opposite, as impeller B leads to an index 12% lower to the one obtained with impeller A in average for the whole range of flow rates considered here.
3.3 Influence of the impeller's diameter
The aim of this section is to investigate the influence of the impeller diameter on the hemocompatibility of the pump. A lower diameter allows to reduce the area of the moving surfaces generating high shear stress, as well as to increase the gaps between the rotor and the stator parts. This reduction will benefit the hemolysis issue. A lower diameter however reduces the hemodynamic performance of the centrifugal pump. To overcome this reduction, higher angular speed must be used to achieve similar pressure head, to the detriment of hemolysis risk this time. For these reasons, it appears interesting to find out the predominant phenomenon, and if an optimum diameter can be found. To the author's knowledge, this issue has not yet been treated in the literature.
The size reduction is considered only in the radial direction, while all other dimensions are kept identical. Similarly, the volute dimensions are not changed. The influence of the clearance gaps is thus not taken into account in this study. This issue was studied for the HVAD in Gil et al.,24 where the authors showed that reduced gap clearance yields to an increase of hemolysis. Table 2 highlights the influence of the impeller's size on the risks of hemolysis at Q = 0.5 L/min. As the diameter shrinks, higher angular speed is required to achieve the targeted 80 mm Hg. Despite this increasing rotational speed, hemolysis exhibits a reduction trend, with the lower index obtained with the smallest diameter. The index shrinks indeed from 0.00716 to 0.00250 between 35 and 15 mm. This evolution is yet not monotonic. The overall reduction trend is supported by an analysis on the blood volumes exposed within the pump to the different shear stress thresholds. These volumes are listed as fraction of the total pump volume (without inflow and outflow grafts) in Table 2. The volume exposed to the lowest threshold of <9 Pa increases with a decrease of the impeller diameter, while the volumes exposed to both high shear stress thresholds (>50 and >150 Pa) increase. These three thresholds correspond, respectively, to von Willebrand factor cleavage, platelet activation, and finally high hemolysis risks, as explained in Thamsen et al.36 While only 47% of the blood is exposed to low shear for D = 35 mm, it goes up to almost 89% for D = 15 mm. On the contrary, 2.6% of the blood experiences shear stress above 150 Pa for the widest impeller, while it represents only 0.3% for the smallest one. This shows that reducing the impeller size can mitigate the hemolysis risks for the same targeted operating conditions, i.e., even with higher angular speed. This result can explain why the design chosen for HeartMate3 has a much smaller impeller diameter compared to its volute, than the former HVAD.23
| Diameter [mm] | 35 | 30 | 25 | 20 | 15 |
| Angular speed required to achieve 80 mm Hg [rpm] | 3000 | 3500 | 4400 | 5700 | 7650 |
| [%] | 0.00716 | 0.00558 | 0.00797 | 0.00597 | 0.00250 |
| [m2/s3] | 3.52 | 4.78 | 6.22 | 0.31 | 0.80 |
| Blood volume fractions subjected to specific shear stress thresholds [%] | |||||
| 47.1 | 64.5 | 74.3 | 83.5 | 88.7 | |
| 8.03 | 3.32 | 1.73 | 1.35 | 0.91 | |
| 2.58 | 1.28 | 0.69 | 0.42 | 0.28 |
Hemolysis generation based on power law models such as in Equation (1) have been developed for laminar regimes involving relatively constant shear stress level. Concerns have been raised regarding the use of Reynolds stresses with RANS turbulence modeling to account for the effective instantaneous stress experienced by the red blood cell.18 Mantegazza et al. have shown that this assumption leads to an excessive overestimation of hemolysis.19 On the contrary, energy dissipation is the most appropriate metric to quantity blood trauma.18 Estimation of blood trauma with this technique for MCS is however scarce in the literature.37
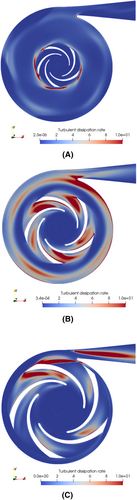
Figure 7 shows the contour of for three different impeller diameter in a cross-section perpendicular to the axis of rotation, at half-blade width. Apart from the volute tongue and both pressure and suction sides of the blades, the locations of high turbulent energy dissipation rate differ for each impeller. For the smallest diameter, energy dissipation is concentrated near the blade's trailing edges, while it remains very low in the blades' passages. Higher rotational speed and thinner passage lead to a more directed flow. On the contrary, for both wider impellers, is high in this area. Qualitatively, the level of turbulent energy dissipation is the highest for an impeller diameter of 25 mm (Figure 7B). Indeed, for this diameter, accumulation of turbulent dissipation energy appears in the volute canal. This is supported by the volume average of this dissipation energy, , within the pump, whose variations with the impeller diameter are also listed in Table 2. The evolution is not monotonic and a maximum appears for D = 25 mm. This may explain the local maximum obtained for the hemolysis index at this specific diameter as well. Further, a transition occurs between D = 25 and 20 mm. Below this size, two flow features reduce significantly the average turbulent energy dissipation: (i) a strongly guided flow in the inter-blade passages and (ii) a much lower competition between the volute and the rotor flows. increases again from D = 15 to 10 mm, mainly because of the higher angular speed required to achieve the targeted operating conditions.
4 CONCLUSION
Hemolysis risks in centrifugal MCS are expected to be higher at low flow rates, as the blood experiences high shear stress for a longer time span before being washed out. In this paper, the hemocompatibility of a centrifugal mini pump designed for these low flows (as in the context of heart failure with preserved ejection fraction) is qualitatively and quantitatively assessed. The estimation of blood trauma is based on the resolution of the transport equation of hemolysis based on a Eulerian approach. Further, the level of turbulent dissipation energy within the mini pump is analyzed to gain insight on the turbulent stress experienced by the blood cells. Different geometric configurations are considered for the pump. For a similar operating condition, the impeller configuration with thinner blades allows to reduce the hemolysis level of more than 10% in average and more than 20% at the lowest output of 0.05 L/min. Besides, the influence of the impeller size is investigated. While a maximum of hemolysis is observed for a diameter of 25 mm, shrinking the impeller diameter from the initial configuration of 35–15 mm leads to a reduction of the hemolysis index from 0.00716 to 0.00250 (for 80 mm Hg at 0.5 L/min). This improvement is also underlined by the lower level of turbulent energy dissipation obtained for the smallest impellers, despite the higher rotational speeds required to achieve the same targeted operating condition. Experimental measurements of blood trauma under the operation of the proposed pumps are on-going within our team in order to extend the present numerical study. Furthermore, our next investigations will focus also on (i) the manufacturing issues related to biocompatibility and (ii) the pump motorization to achieve stable targeted operating conditions.
AUTHOR CONTRIBUTIONS
All authors were involved in the Concept/design; Data analysis/interpretation: Mathieu Specklin, Farid Bakir; Drafting Article: Mathieu Specklin; Cricical revision of article: Mathieu Specklin, Farid Bakir, All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the Arts et Métiers High Performance Computing Center Cassiopee for providing computing resources required to conduct the research reported in this paper.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest with the contents of this article.




