Cold atmospheric plasma therapy as a novel treatment for Berlin Heart EXCOR pediatric cannula infections
Abstract
Background
Cold atmospheric plasma (CAP) therapy has been recognized as effective treatment option for reducing bacterial load in chronic wounds, such as adult ventricular assist device (VAD) driveline exit-site infections. Currently, there have been no reports on the safety and efficacy of CAP therapy for pediatric cannula infections and inflammations in paracorporeal pulsatile VADs.
Methods
The mechanical strength of Berlin Heart EXCOR cannulas were tested both before and after CAP treatment (SteriPlas, Adtec Healthcare Limited, UK) to prove material safety. A ring tensile test of 20 untreated and 20 CAP-treated (5 min) EXCOR cannulas (Ø12mm), assessed the force at the breaking point of the cannulas (Fmax), at 25% (F25%) and 50% (F50%) of the maximum displacement. Additionally, the scanning electron microscope (SEM) micrographs for both groups examined any surface changes. Finally, the case of a 13-year-old male EXCOR patient with cannula infections, treated with CAP over 100 days, is presented.
Results
The in vitro measurements revealed no statistically significant differences in mechanical strength between the control and CAP group for F25% (8.18 ± 0.36 N, vs. 8.02 ± 0.43 N, p = 0.21), F50% (16.87 ± 1.07 N vs. 16.38 ± 1.32 N, p = 0.21), and FMAX (44.55 ± 3.24 N vs. 42.83 ± 4.32 N, p = 0.16). No surface structure alterations were identified in the SEM micrographs. The patient's cannula exit-sites showed a visible improvement in DESTINE wound staging, reduction in bacterial load and inflammatory parameters after CAP treatment without any side effects.
Conclusion
Overall, CAP therapy proved to be a safe and effective for treating EXCOR cannula exit-site wound healing disorders in one pediatric patient, but further studies should investigate this therapy in more detail.
1 INTRODUCTION
Due to a lack of donor organs for pediatric patients,1 ventricular assist devices (VADs) are a valuable treatment option to bridge the waiting time until heart transplantation (HTx) and help decrease the waiting list mortality of pediatric heart failure patients.2 Paracorporeal pulsatile devices, such as the Berlin Heart EXCOR (EXCOR pediatric, Berlin Heart GmbH, Berlin, Germany), account for a large portion of VADs implanted in pediatric patients.3-7 Up to the age of 6 years, about 87.2% of all implanted devices are paracorporeal pulsatile devices.3 Most common adverse events in the early stage (within the first 3 months) of pediatric mechanical circulatory support (MCS) are bleeding, device malfunction, infection, and neurological dysfunction.4 In later stages (after 3 months of support), infection becomes the most prevalent adverse event for pediatric MCS patients.3-7 According to the sixth annual Pediatric Interagency Registry for MCS (Pedimacs) report, patients supported with paracorporeal pulsatile MCS devices experienced an infection rate of 9% in the early period (within the first 2 weeks), which subsequently increased to 22% (after 2 weeks).3 Independent of device type, 67% of Pedimacs patients remained infection-free within the first year following implantation, while 18% experienced one infection, 8% had two infections, and another 8% encountered three infections.3 Notably, individuals with one or two infections showed a tendency toward poorer survival rates at 3 and 6 months post-implantation.3
Infections at the driveline8, 9 or cannula exit-sites10 are primarily caused by bacterial colonization and, less commonly, by fungal presence along the external components. Current best practices for treating exit-site infections in VAD patients include more frequent dressing changes, the use of bacteriostatic silver wound dressings,11 antibiotic therapy, surgical debridement, vacuum-assisted closure therapy,10 and, in the worst case, pump replacement.12 Cold atmospheric plasma (CAP) therapy offers a novel approach in the field of MCS for treating infections at percutaneous exit-sites of drivelines or cannulas.13, 14 CAP refers to energized gases15 and can be considered as the fourth state of matter,16 constituting multicomponent systems that include electromagnetic fields, thermal radiation, electrons and ions, visible light, reactive oxygen and nitrogen species (RONS), and ultraviolet radiation.17 The temperature of CAP is comparable to that of the human body (≤40°C).15 In recent years, CAP has become a standard medical procedure for dermatological applications, particularly in wound treatment.16, 18 Clinical studies show improvement of chronic wound healing by reducing bacterial burden and alleviating local pain.19-21 CAP has no adverse effects on human skin cells and keratinocyte proliferation, migration, and apoptotic mechanisms.21-25 Recent studies have confirmed the effectiveness, biological safety, and painlessness of CAP therapy for driveline exit-site infections in long-term adult VAD patients, resulting in a reduction of bacterial load irrespective of the specific pathogen present.13, 14, 26 Long-term CAP therapy also proved to be more cost-efficient than conventional treatment options.13 Even the prophylactic use of CAP treatment has been proven to be beneficial, as it improves freedom from driveline infection for up to 6 months.27 Although infection is a major adverse event in paracorporeal pulsatile devices,3-7, 28 there is currently no evidence of pediatric patients with cannula exit-site infections being treated with CAP therapy. This is because the SteriPlas CAP device (Adtec Healthcare Limited, Twickenham, United Kingdom) is currently only CE mark-certified as adult Class IIb medical device.29 The SteriPlas has been tested for biological safety, meeting the standards of international regulatory bodies, to ensure its suitability for clinical trials.30 Currently, no reports of material fatigue of VAD drivelines or cannulas due to CAP therapy exist when used for treatment in the adult population.13, 19, 27 Controversially, between January 2019 and July 2023, a total of 3148 EXCOR cannulas were implanted worldwide, with 17 cases of non-CAP-related cannula tears reported.31 A significant breach in an EXCOR cannula can lead to massive blood loss or air embolism, both of which could be fatal.31
As the loss of material strength in EXCOR cannulas might be a potential complication of CAP use, we investigated whether EXCOR cannulas treated with CAP show any signs of material fatigue, aiming to better understand the safe application of this therapeutic option. To exclude the potential negative impact of CAP treatment on the mechanical strength of EXCOR pediatric cannulas, an in vitro ring tensile test was performed. In addition, micrographs of the cannula samples were taken, to inspect for any visible surface changes. Finally, the outcomes of CAP therapy in one pediatric patient with an EXCOR device were observed.
2 MATERIALS AND METHODS
A total of 42 samples were collected from 3 new EXCOR cannulas (Ø12 mm). Forty samples were evenly distributed and measured within both a control group and a CAP group. Two additional samples, one CAP-treated and one control sample, were prepared for capturing micrographs of the cannula surface.
2.1 Sample preparation
The samples were obtained from the silicone cannulas using a custom-made procedure (see Figure 1A). Therefore, a cannula was pulled over a mandrel with a matching diameter, clamped into a lathe, and coated in soap to minimize friction for a clean cut. Subsequently, the lathe rotated at 400 rpm, and the cannulas were sliced into 1 mm rings using a scalpel blade mounted in a specially designed holder. Following the cutting process, the samples were rinsed with water, and their thickness was verified using a Vernier caliper. The dimensions of the final cannula samples are depicted in Figure 1B.
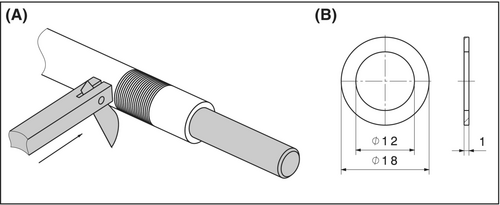
The rings in the CAP group were treated for 5 min with CAP generated from argon gas using the Adtec SteriPlas (SteriPlas, Adtec Healthcare Limited, Twickenham, United Kingdom) (see Figure 2A). The cannula samples in the control group were left untreated.

2.2 Ring tensile testing
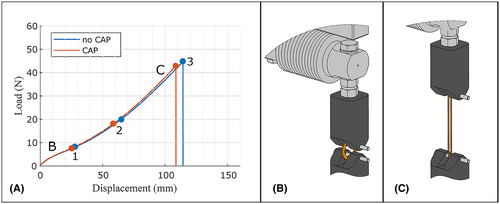
The tensile test was conducted 24 hrs after the CAP treatment, following the guideline ISO 3732 for the determination of tensile strain properties in rubber, on an uniaxial universal testing machine (Beta 10–2.5; Messphysik Materials Testing GmbH, Fürstenfeld, Austria). To mount the samples, a custom-made fixture was used (see Figure 3B,C). Dowel pins with a diameter of 3 mm were used to load the ring-shaped specimens in circumferential directions. The initial distance between the pins (l = 12 mm) matched the inner diameter of the sample rings. The fixtures were pulled apart at a testing speed of 100 mm/min until the cannula ring ruptured. The upper part of the fixture was connected to a load cell (Z6 Bending Beam Load Cell, Range: 50 N, Hottinger Brüel & Kjær GmbH, Darmstadt, Germany), enabling the continuous measurement of the maximal force at the breaking point of the cannulas (Fmax) and the forces at 25% (F25%) and 50% (F50%) of the maximal displacement were evaluated. An example of a tensile test curve is shown in Figure 3A.
2.3 Statistical analysis
Statistical analysis was carried out using SPSS (IBM SPSS Statistics, Version 29.0, SPSS Inc., Chicago, IL, USA). The statistical significance level was set at p < 0.05. Normally distributed variables are presented as mean ± standard deviation, while non-normally distributed data are reported as median (interquartile range [IQR]). The measured forces (F25%, F50%, and Fmax) were tested for normal distribution using the Shapiro–Wilk test. An unpaired t-test was employed for comparing normally distributed continuous variables (forces) between the control group and CAP group. In cases of non-normally distributed data, the Mann–Whitney U test was applied.
2.4 Scanning electron microscope
To identify potential changes caused by CAP therapy, the surfaces of the cannula samples were first sputtered with gold using a Q150R ES sputter coater (Quorum Technologies Ltd., United Kingdom) and then imaged by scanning electron microscopy (SEM) at an acceleration voltage of 10 kV (Zeiss EVO MA10, Oberkochen, Germany).
2.5 Review board application and informed consent
The Institutional Review Board approved the retrospective analysis of routinely assessed clinical data (identification number: EK Nr: 1839/2022, with a waiver for informed consent). The child's parents were informed about the risks, benefits, and alternatives to CAP. After discussing these details, they provided their informed consent for the use of this innovative treatment.
2.6 Case study
The case study involves a 13-year-old male patient (42.7 kg, 140 cm, BSA 1.29 m2), with hypoplastic left heart syndrome after stage 3 palliation and undergoing total cavopulmonary connection at the age of 4 years. As detailed elsewhere,33 8 years post-Fontan surgery, the patient experienced subaortic and subpulmonary failure, leading to presentation in Pedimacs Level II. The failing ventricle was supported by a standard EXCOR 50 mL ventricle (12 mm apex and staged 12/9 mm arterial cannula); additionally, the novel EXCOR Venous Cannula (14/18 mm) facilitated subpulmonary support (staged 12/9 mm outflow cannula to the pulmonary artery) with a 50 mL ventricle in the Fontan circulation.33
Due to “Stage 2b – local infection” according to the DESTINE staging proposal,12 which included purulent drainage and was treated with more frequent dressing changes, targeted systemic antibiotic therapy and a bacteriostatic dressing for 125 days starting from postoperative day (POD) 196 (leukocytes: 3.89 g/L, C-reactive protein (CRP): 3.97 mg/dL, and fibrinogen: 284 mg/dL) but did not improve, and thus, adjuvant CAP therapy was initiated. The treatment was administered off-label because the SteriPlas CAP device used is currently only approved for adult patients.29 The wound was cleaned using a wound irrigation solution (Granudacyn®, Mölnlycke Health Care AB, Göteborg, Sweden) and covered with an absorbent adhesive dressing (Cosmopor® E, Paul Hartmann AG, Heidenheim, Germany). Depending on the amount of exudate, two different sizes of bacteriostatic silver wound dressings were applied (Aquacel™ AG+ Extra, ConvaTec Limited, Deeside, United Kingdom) (Mepilex® Transfer Ag, Mölnlycke Health Care AB, Göteborg, Sweden). CAP therapy was initiated 321 days after device implantation and continued until the day of HTx (see Figure 2B). In total, the patient underwent treatment 28 times every 3–4 days over a period of 100 days. Initially, the right and left cannula exit-sites were treated for 5 min in the first seven therapy sessions. The CAP treatment time was reduced from 5 to 4 min per session once the amount of exudate decreased. The treatment times were determined based on the manufacturer's recommendations and recent studies. These studies have shown that CAP therapy is effective for significant bacterial load reduction after just 2 min treatment,34 while 4 min of CAP treatment destroyed the cell structures of Escherichia coli.21 Each cannula exit-site received a total CAP treatment time of 119 min. Weekly laboratory tests, bacterial culture swab analyses (quantitative cultures categorized as sparse, moderate, and plentiful) from the cannula exit-sites, and DESTINE wound stage classification were conducted throughout the CAP treatment period.
3 RESULTS
3.1 Ring tensile testing
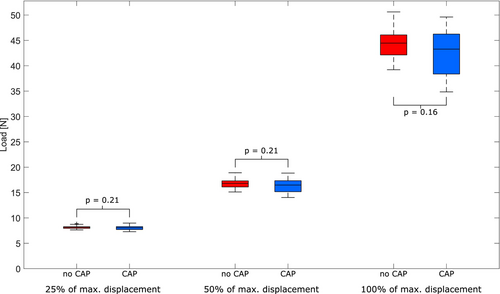
The in vitro measurements revealed no statistically significant differences in mechanical strength between the control and CAP group for F25% (control: 8.18 ± 0.36 N, vs. CAP: 8.02 ± 0.43 N, p = 0.21), F50% (control: 16.87 ± 1.07 N vs. CAP: 16.38 ± 1.32 N, p = 0.21), and Fmax (control: 44.55 ± 3.24 N vs. CAP: 42.83 ± 4.32 N, p = 0.16). Figure 4 illustrates boxplots visualizing the ring tensile measurement results.
3.2 Scanning electron microscope
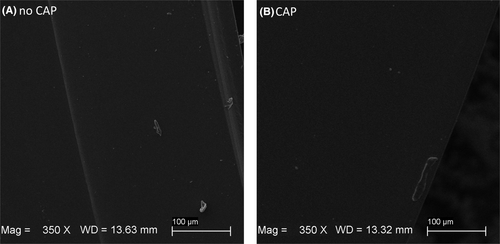
When examining the micrographs captured with the SEM (see Figure 5), there was no apparent alteration (subjective assessment) in the surface structure caused by CAP therapy.
3.3 Clinical CAP treatment
On day 1 of CAP treatment, corresponding to POD 321, the patients cannula exit-sites were classified as infection “Stage 2b – local infection” according to the DESTINE staging proposal.12 A local infection of the cannula exit-sites was evident, characterized by erythema, swelling, and pruritus, purulent drainage, pyrexia, and positive blood culture. In addition, a low albumin level was detected, and bacterial culture analysis revealed Escherichia coli, micrococcus luteus, coagulase-negative staphylococci, and Staphylococcus epidermis at the cannula exit-sites. Throughout the CAP treatment period, a visible improvement of the infection sites was observed (see Figure 6), without any signs of systemic illness. Particularly, the right ventricular cannula exit-site exhibited significant improvement, with almost no erythema and swelling remaining at the end of the treatment period. Therefore, 40 days after starting CAP therapy, the oral systemic antibiotics were replaced with local fusidic acid cream at the cannula exit-sites. On day 98, the day of the last CAP treatment before HTx (POD 421), the wound sizes were reduced, with cannula exit-sites classified as DESTINE ‘Stage 1a – local wound healing disorder’. In addition, all infection-relevant laboratory parameters were within a normal range (leukocytes: 9.33 g/L, CRP: 0.35 mg/dL, and fibrinogen: 266 mg/dL). The bacterial load in the swab analysis had initially decreased, with only Escherichia coli remaining at the exit-sites on days 39 and 67 of the treatment process, but it began to rise again before the HTx. No side effects or pump malfunctions were observed during the 28 CAP treatment sessions. We observed that the material strength of the cannula was maintained throughout the patient's treatment, with no signs of weakness, indicating that its properties remained unaffected (see Figure 6).

4 DISCUSSION
In light of the scarcity of donor organs for pediatric patients,1, 35 consequently long VAD support times are common among pediatric heart failure patients.10 As infections are major adverse events in all phases of pediatric VAD therapy,3-7 appropriate infection treatment8, 12 is crucial to prevent deep wound healing disorders or even lethal sepsis.36 Therefore, the management of cannula exit-site infections and the stabilization or reduction of wound size until HTx are of great importance. To avoid more invasive procedures such as vacuum-assisted closure therapy10 or pump exchange,12 CAP treatment emerges as a new option, which has recently been investigated as an additional therapy accompanying conventional treatment of driveline infection in adult VAD patients,13, 14, 26 regardless of the bacterial species.37
To the best of our knowledge, this study is the first to document the clinical utilization of the SteriPlas CAP for treating cannula exit-site infection of a pediatric Berlin Heart EXCOR patient. In addition, the in vitro investigation delved into the mechanical properties of the cannulas before and after CAP treatment. Although no evidence of material fatigue due to CAP therapy has been reported in previous clinical VAD recipient applications,13, 14, 26 we conducted these tests in our study to provide evidence of both biological and material safety before considering off-label use in a pediatric patient. We also aimed to demonstrate that, in the unlikely event of a cannula rupture31 in this EXCOR patient, CAP therapy could be ruled out as the cause.
The in vitro analysis revealed that CAP does not adversely affect the material properties of the EXCOR cannulas. No significant differences were observed at 25% of maximum displacement (F25%), at 50% of maximum displacement (F50%) or breakpoint (Fmax) regions, as assessed in the tensile force measurements between untreated and CAP-treated samples (Figure 4), with p-values of 0.21, 0.21, and 0.16, respectively. Consistent with the ring tensile test,32 which is used to evaluate the ductility of welded joints and detect surface defects in tubes, subjectively, no apparent alterations of surface structure caused by CAP therapy were observed in the SEM micrographs (Figure 5). As the CAP treatment was performed on the already cut rings, the cannulas were exposed to CAP from the outside as well as the cross section, resulting in much higher exposure than in a clinical environment. This suggests that CAP therapy does not compromise the structural integrity of the material, a finding that is critical for ensuring the safety and efficacy of the cannulas in clinical use.
This aligns with the clinical observations, where no cannula damage or pump malfunctions were observed even after 28 CAP treatment sessions (119 min in total). The presented case demonstrates that CAP can be administered safely and easily in a pediatric patient (13 years of age). The treatment time of 100 days is in accordance with the duration reported by Kremer et al.13 for the treatment of VAD driveline infections, with a median treatment time of 42 days (ranging from 1 to 117 days). Consistent with previous findings in adult VAD patients, which identified decreased albumin as a predictor for DLI,9, 38 our patient had an albumin level of only 40.9 g/L at the beginning of CAP therapy. Inflammation increases capillary permeability and the escape of serum albumin, leading to hypoalbuminemia.39 Therefore, the normalization of albumin levels (47.9 g/L) 11 days after the start of therapy (Figure 6) may be attributed to the accelerated wound healing induced by CAP. However, given the numerous factors that influence wound healing, a comprehensive study is necessary to thoroughly investigate the implications of this case study. This is particularly important because pediatric skin differs markedly from adult skin, not only anatomically but also physiologically.40 Before CAP treatment, a thorough assessment of the child's skin, considering factors, such as age, specific skin thickness, and condition, is recommended. As the distance to the target tissue is fixed at 2 cm by replaceable plastic spacers on the CAP device29 and the CAP's power remains constant, application time is the only adjustable parameter for smaller infants with thinner skin. In this case, a 13-year-old patient underwent a CAP application time similar to that used in adults (4–5 min per session21). However, previous studies indicate that a minimum application time of 2 min is sufficient for effectively killing bacteria in infected wounds,34 allowing for individualized treatment plans in smaller pediatric patients. Although children's skin barriers are less developed, have higher water content, and are more prone to dryness and dehydration compared to adults,40 no eczema or dry skin was observed during CAP treatment (Figure 6). This aligns with a previous study, indicating that CAP is well-tolerated, does not damage the skin barrier, and does not cause skin dryness.41
In the presented case report, the CAP therapy resulted in a clinically relevant reduction in pathogens detected in the patient's cannula exit-site wound swab and in a visible improvement in the wound stage. Escherichia coli was the only pathogen that repeatedly reappeared at all four cannula exit-sites. However, studies evaluating the efficacy of CAP treatment have shown that this therapy is capable of significantly reducing Escherichia coli loads with a lasting effect.22 Therefore, after consultation with the medical personnel treating the patient, it was concluded that Escherichia coli was most likely transmitted anew by the patient repeatedly touching the cannula exit-sites. This could also be due to the abdominal position of the cannula exit-sites, which are not expected to be sterile as they are exposed to the outside. The CAP treatment was continued for prophylactic purposes despite the presence of non-eradicable contamination. The patient reported no pain during the CAP therapy, and no skin damage or any other adverse effects due to CAP were detected – which is consistent with studies of adult VAD patients treated with CAP.13 Furthermore, compared to conventional treatments like surgical debridement, CAP has proven to be more cost-efficient for adult VAD patients with exit-site infections.13 The cost of a SteriPlas CAP device is 70 000€. Additionally, a consumable sensor module, costing 5€ per treatment, is required for each patient.
Complete healing could not be achieved in this case due to HTx occurring. Therefore, further research is needed to evaluate the long-term efficacy of CAP therapy in the pediatric VAD population. Our study is limited to a single case report, and the observed benefits of CAP therapy may be partially due to the increased vigilance it entails. Additionally, mechanical tensile force measurement of EXCOR cannulas after an even longer CAP treatment period might be beneficial in the future.
5 CONCLUSION
The in vitro measurements revealed no significant changes in the mechanical strength or surface structure of the EXCOR cannulas after CAP treatment. Consistent with studies in the adult population, CAP appeared to be effective and safe as an adjuvant treatment of EXCOR cannula exit-site infection in a pediatric patient, but further studies should investigate this therapy in more detail.
AUTHOR CONTRIBUTIONS
Concept and design: TS, MSt; Data collection and analysis: JS, MK, MSo, CG, TA, DW; Funding: TS, DZ; Drafting article: JS; Conduction of the Study: MSo, TS; All authors performed critical revision of the article and approved the final version.
ACKNOWLEDGMENTS
We would like to express our gratitude to Berlin Heart GmbH for kindly providing us with samples of their EXCOR cannulas to conduct the in vitro analyses.
CONFLICT OF INTEREST STATEMENT
Daniel Zimpfer: research grants, travel support, and lecture honoraria (Medtronic Inc., Abbott Inc., Berlin Heart GmbH, Edwards Lifesciences Corporation, Abiomed Europe GmbH, Corcym S.r.l., and AtriCure Inc.) and consultant (Medtronic Inc., Abbott Inc., and Berlin Heart GmbH). Dominik Wiedemann: proctor and consultant (Abbott Inc.), and advisor (Xenios/Fresenius Medical Care). Thomas Schlöglhofer: consultant, advisor, and research grants (Medtronic Inc., Abbott Inc., CorWave SA, Berlin Heart GmbH, and BiVACOR, Inc.). All other authors have nothing to disclose.




