Effects of fishmeal replacement with eight protein sources on growth performance, blood biochemistry and stress resistance in Opsariichthys bidens
Abstract
Nine experimental diets were formulated to study the effects of fishmeal replacement on Opsariichthys bidens. Weight gain rate, protein efficiency rate and apparent digestibility coefficient of fish in fishmeal (FM), maggot meal (MM) and soybean meal (SM) groups were significantly the highest. There was no significant difference in intestinal amylase activity between FM and SM groups. Principal component analysis showed that SM group and wild fish (WF) individual had similar essential amino acid compositions in muscle. The lowest value of serum malondialdehyde and the highest value of total antioxidant capacity were found in SM group, but fish in MM group did the opposite. Fish in MM group had the higher whole-body lipid, serum triglycerides, glucose, complement C3, C4 and immunoglobulin contents and lysozyme activity. After ammonia exposure, cumulative mortality of fish in FM and SM groups was significantly the lowest. Liver carbamyl phosphate synthetase activity of fish in FM group was significantly the highest, but the highest values of glutamine synthetase and glutamate dehydrogenase were found in SM group. This study suggests that fishmeal replacement with soybean meal is acceptable, and muscle amino acid compositions of fish in SM group were more similar to wild individual.
1 INTRODUCTION
The fishmeal (FM) is a primary dietary protein source for most farmed fish due to its palatability and digestibility (Moreira et al., 2008). However, the shortage of fishmeal resource has been recognized worldwide, and it is necessary to evaluate different alternative protein sources to meet the protein requirement of farmed fish and reduce production cost (Teves & Ragaza, 2016). So far, the plant proteins from soy, corn and peanut have been widely used to fishmeal replacement, which showed the advantages of high protein content and low cost (Gatlin et al., 2007), but presence of anti-nutritional factor, poorer digestibility and amino acid profile imbalance are also a cause of concern (Francis et al., 2001). Under this scenario, the by-product from terrestrial vertebrates may provide a low-cost and sustainable alternative; however, the nutrient composition as feed ingredients has been unstable, because it is highly dependent on raw material supply and processing technology, such as blood meal, meat and bone meal and chellocken meal (Moutinho et al., 2017). In addition, insect meals such as maggots, yellow mealworm and black soldier fly have long been considered a potential substitute to fishmeal (Van Huis, 2013). A study showed that the insect meal has the same biological values as fishmeal, does not contain the anti-nutritional factors commonly found in plant proteins and has balanced amino acid profiles, but the high fat content is a disadvantage (Kamarudin et al., 2021). In general, a comprehensive understanding of how different protein resources affect growth performance of fish is critical to designing diet formulation.
The Opsariichthys bidens (Cyprinidae) is a small economic fish widely distributed in mountain streams, lakes and rivers across East Asia, due to its delicious taste and high nutritional value, with widely cultured in the southeast of China (Lin et al., 2021). For a long time, this species has been considered a carnivorous fish because a large number of insect limbs have been found in the intestines of wild individuals. In recent years, ammonia pollution has caused more and more serious economic losses to O. bidens farming. Improving stress resistance of animal is an important indicator to evaluate the feed quality, but it has to be based on understanding of physiological mechanisms of this species. To our knowledge, the main strategies for ammonia detoxification in fish include urea synthesis and glutamine synthesis (Ip & Chew, 2018). The urea cycle is an efficient ammonia detoxification pathway, which is regulated by carbamoylphosphate synthetase, ornithine carbamoyltransferase, argininosuccinate synthetase, argininosuccinate lyase and arginase (Takiguchi & Mori, 1995). In general, the herbivority fish detoxify ammonia mainly through urea cycle pathway, such as grass carp Ctenopharyngodon idella (Xing et al., 2016), and the carnivorous fish must depend on glutamine synthesis due to urea cycle defect, including the vast majority of cold-water fish (Clark et al., 2019; Zhu et al., 2020). A previous study reported that dolly varden char Salvelinus malma was injected with ammonium acetate caused carbamoylphosphate synthetas, ornithine carbamoyltransferase and arginase activities decreased, but glutamine synthetase and glutamate dehydrogenase activities increased, suggest that ammonia detoxification of this species depends on glutamine synthesis (Zhu et al., 2020). So far, the detoxification mechanism of ammonia in O. bidens is poorly known and need to be elucidated.
The present study investigated the effects of fishmeal replacement with eight protein sources on growth performance, serum chemistry parameter, digestive and antioxidant enzyme activities, immunity and ammonia tolerance in O. bidens. The findings of this study could be useful for future research on the development of feeding strategies in O. bidens farming.
2 MATERIALS AND METHODS
2.1 Experimental diets
Nine experimental diets were formulated, and the diets formulation and amino acid compositions are presented in Tables 1 and 2. The diet with 600 g/kg fishmeal was considered as the control, and the other eight proteins were used to replace 180 g/kg of fishmeal in the FM group, separately. The eight diets were coded as blood meal (BM), chellocken meal (CM), meat and bone meal (MBM), maggot meal (MM), soybean meal (SM), corn gluten meal (CGM), peanut meal (PM) and soy protein concentrate (SPC). In addition, 0.10% yttrium oxide was added as an indicator to determine apparent digestibility coefficients (ADC). All the ingredients are thoroughly mixed and processed into (2.00 × 2.00) mm pellets using an F-26II feed mill (South China University of Technology) and dried at room temperature to about 10.00% moisture, then stored at −20°C until use.
| Item | FM | BM | CM | MBM | MM | SM | CGM | PM | SPC |
|---|---|---|---|---|---|---|---|---|---|
| Ingredients | |||||||||
| Fish meal | 600.00 | 420.00 | 420.00 | 420.00 | 420.00 | 420.00 | 420.00 | 420.00 | 420.00 |
| Blood meal | 180.00 | ||||||||
| Chellocken meal | 180.00 | ||||||||
| Meat and bone meal | 180.00 | ||||||||
| Maggot meal | 180.00 | ||||||||
| Soybean meal | 180.00 | ||||||||
| Corn gluten meal | 180.00 | ||||||||
| Peanut meal | 180.00 | ||||||||
| Soy protein concentrate | 180.00 | ||||||||
| Casein | 80.00 | 0.00 | 70.00 | 110.00 | 90.00 | 130.00 | 110.00 | 130.00 | 110.00 |
| Wheat meal | 130.00 | 130.00 | 130.00 | 130.00 | 130.00 | 130.00 | 130.00 | 130.00 | 130.00 |
| Fish oil | 20.00 | 25.00 | 20.00 | 20.00 | 20.00 | 25.00 | 25.00 | 25.00 | 25.00 |
| Soybean oil | 20.00 | 25.00 | 20.00 | 20.00 | 20.00 | 25.00 | 25.00 | 25.00 | 25.00 |
| Soybean lecithin | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Vitamin premixa | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Mineral premixb | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 |
| Calcium biphosphate | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 |
| Choline chloride | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Cellulose | 68.00 | 138.00 | 78.00 | 38.00 | 58.00 | 8.00 | 28.00 | 8.00 | 28.00 |
| Sodium carboxymethylcellulose | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 |
| Ethoxyquin | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yttrium oxide | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Total | 1000.00 | 1000.00 | 1000.00 | 1000.00 | 1000.00 | 1000.00 | 1000.00 | 1000.00 | 1000.00 |
| Analysed nutrients compositions (g/kg) | |||||||||
| Dry matter | 890.54 | 890.33 | 888.81 | 886.54 | 887.96 | 885.98 | 890.12 | 889.54 | 889.12 |
| Crude protein | 482.16 | 484.66 | 483.45 | 492.15 | 497.25 | 496.72 | 499.98 | 499.78 | 492.69 |
| Crude lipid | 103.40 | 107.44 | 111.15 | 105.29 | 107.69 | 103.08 | 102.15 | 101.55 | 107.19 |
| Ash | 100.12 | 111.54 | 105.16 | 103.46 | 110.12 | 105.34 | 106.15 | 103.22 | 109.59 |
- Abbreviations: BM, blood meal; CGM, corn gluten meal; CM, chellocken meal; FM, fish meal; MBM, meat and bone meal; MM, maggot meal; PM, peanut meal; SM, soybean meal; SPC, soy protein concentrate.
- aVitamin premix (/kg diet): Vitamin A, 0.032 g; Vitamin D, 0.005 g; Vitamin E, 0.24 g; Vitamin K, 0.01 g; Vitamin B1, 0.025 g; Vitamin B2, 0.045 g; Nicotinic acid, 0.2 g; Vitamin B6, 0.02 g; Biotin, 0.06 g; Inosito, 1.8 g; Calcium pantothenate, 0.06 g; Folic acid, 0.02 g; Vitamin B12, 0.01 g; Vitamin C, 2 g; Microcrystalline cellulose, 6.29 g.
- bVitamin and mineral premix (/kg diet): CuSO4•5H2O, 0.01 g; Na2SeO3, 0.02 g; MnSO4•H2O, 0.045 g; CoCl2•6H2O, 0.05 g; ZnSO4•H2O, 0.5 g; Ca(IO3)2, 0.06 g; FeSO4•H2O, 0.08 g; MgSO4•7H2O, 1.2 g; Zeolite powder, 18.485 g.
| Item | FM | BM | CM | MBM | MM | SM | CGM | PM | SPC |
|---|---|---|---|---|---|---|---|---|---|
| Essential amino acids (EAA) | |||||||||
| Methionine | 14.70 | 10.80 | 12.90 | 12.10 | 13.80 | 13.50 | 12.40 | 11.50 | 11.50 |
| Threonine | 22.10 | 20.30 | 21.10 | 19.50 | 20.80 | 22.50 | 20.60 | 18.60 | 20.40 |
| Valine | 28.20 | 28.40 | 26.90 | 25.40 | 27.30 | 29.70 | 26.80 | 25.20 | 26.30 |
| Isoleucine | 22.70 | 20.10 | 22.60 | 20.70 | 22.50 | 25.50 | 22.90 | 21.00 | 23.40 |
| Leucine | 41.50 | 42.60 | 39.60 | 37.50 | 38.70 | 44.40 | 52.00 | 37.20 | 39.70 |
| Phenylalanine | 23.30 | 24.80 | 22.50 | 21.20 | 28.80 | 25.90 | 25.50 | 22.20 | 24.20 |
| Histidine | 14.50 | 17.70 | 12.80 | 12.40 | 13.90 | 15.60 | 12.90 | 12.80 | 14.50 |
| Lysine | 42.20 | 39.30 | 38.80 | 36.80 | 39.70 | 41.90 | 33.30 | 37.80 | 37.90 |
| Arginine | 27.10 | 24.80 | 27.90 | 24.90 | 24.80 | 29.40 | 23.20 | 28.60 | 30.60 |
| Non-essential amino acids (NEAA) | |||||||||
| Asparticacid | 47.40 | 43.50 | 45.30 | 41.50 | 45.80 | 51.00 | 42.40 | 44.30 | 50.10 |
| Glutamine | 81.50 | 63.60 | 78.70 | 78.10 | 80.80 | 93.40 | 92.20 | 81.90 | 84.30 |
| Serine | 21.00 | 17.50 | 20.40 | 20.20 | 20.40 | 23.70 | 22.20 | 20.10 | 21.50 |
| Glycine | 27.40 | 23.60 | 30.50 | 29.60 | 23.60 | 25.20 | 22.70 | 23.70 | 25.10 |
| Alanine | 30.10 | 33.00 | 29.90 | 27.50 | 26.80 | 27.80 | 32.60 | 24.10 | 26.70 |
| Tryptophan | 19.50 | 16.00 | 18.60 | 17.90 | 24.40 | 22.20 | 21.70 | 19.40 | 18.80 |
- Abbreviations: FM, fish meal; BM, blood meal; CM, chellocken meal; MBM, meat and bone meal; MM, maggot meal; SM, soybean meal; CGM, corn gluten meal; PM, peanut meal; SPC, soy protein concentrate.
2.2 Animal and sample collection
Juvenile O. bidens were obtained from Fengyu Agricultural Development Co. LTD. Fish were acclimated to experimental conditions for 4 weeks with control diet (FM). The experiment fish (7.51 ± 0.34) g were randomly selected and stocked in twenty-seven 500 L cylindrical plastic bucket (diameter of 0.8 m) with 50 fish per bucket in triplicate. Fish were hand-fed with experimental diets twice daily (05:00 h and 17:00 h) to apparent satiation for 70 d. The amount of diet consumed each day was recorded. During the feeding trial, the water temperature ranged from 28°C to 31°C, dissolved oxygen >6.80 mg/L, nitrate<0.1 mg/L. Photoperiod was maintained at 12-h light and 12-h dark.
Sampling was carried out in accordance with standard operation procedures of the Ningbo university guidelines for the use of experimental animals. All animal care was approved by the institutional animal care and use committee of Ningbo university. The faeces were collected with a collection column affixed to the bottom of the bucket every morning before feed, then were precipitated, filtered and frozen at −20°C for the apparent digestibility coefficient analysis. Twenty-four hours after the last feeding, all the experimental fish were anaesthetized with 20 mg/L eugenol for counting, weighing and measurement. Three fish per bucket were randomly sampled and stored at −20°C. Blood samples (three fish from each bucket) were drawn from the caudal vein with 100 IU/ml heparinized tuberculin syringe, then were centrifuged at 500 × g for 10 min at 4°C to obtain serum and stored at −20°C. Other three fish per bucket were randomly selected, livers were removed and individually weighed, and intestines and muscle were removed and stored at −20°C. In addition, in the analysis of amino acid content, the three wild O. bidens (35.55 ± 6.12) g were considered as the wild control, which were caught in the Lishui section of the Ou River.
2.3 Chemical analysis
The diets and fish samples were analysed for proximate composition following the standard methods (AOAC, 1995) as described in our previous study (Li et al., 2016).
Serum chemical analysis was performed following the standard methods of clinical immunology and inspection (Wu & Shang, 2006) using the automatic chemistry analyser (7600–110, Hitachi Ltd).
Serum superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and lysozyme activities, total antioxidant capacity (T-AOC) and malondialdehyde (MDA) content were determined by the colorimetric method using a PT-3502C microplate reader (Beijing Putian Xinqiao Technology Co., LTD) with commercial kits (Nanjing Jiancheng Bioengineering Institute). Serum complement C3 (C3), complement C4 (C4) and total immunoglobulin (Ig) contents were determined by the immunoturbidimetry using a microplate reader with commercial kits (Zhejiang Elikan Biological Technology Co., Ltd).
The frozen intestinal tissues were homogenized at 24,000 × g for 20 s in ice-cold sodium phosphate buffer; then, the homogenates were centrifuged at 12,000 × g for 15 min at 4°C, and the supernatant was collected for digestive enzyme activity analysis. The pepsase, amylase and lipase activities were determined by the colorimetric method using a microplate reader with commercial kits (Nanjing Jiancheng Bioengineering Institute).
The frozen liver tissues were homogenized 24,000 × g for 20 s in ice-cold phosphate buffer saline; then, the supernatant was obtained by centrifugation (10,000 × g, 15 min, 4°C), and the supernatant was collected for ammonia metabolism enzyme activity analysis. Liver carbamyl phosphate synthetase (CPS), arginase (ARG), glutamine synthetase (GS) and glutamate dehydrogena (GDH) activities were determined by the enzyme-linked immunosorbent assay method using a microplate reader (PT-3502C, Beijing Putian Xinqiao Technology Co., LTD) with ELISA kits (Nanjing Jiancheng Bioengineering Institute).
2.4 Ammonia stress
To determine the 96-h lethal concentration of 50% (LC50) of total ammonia nitrogen (T-AN), the O. bidens (34.11 ± 5.25 g, N = 10) were held in fifteen 100 L cylindrical plastic bucket (diameter of 0.5 m) in triplicate for 96 h, and the target ammonia concentrations were set as 40.00, 50.00, 60.00, 70.00 and 80.00 mg/L T-AN. The required ammonia concentration can be obtained by adding a solution of 10 g/L NH4Cl through an electromagnetic metering pump (Iwaki Co., Ltd), as previously described (Li et al., 2020). Dead fish were removed immediately upon detection. At the end of the 96-h period, all surviving fish were kept in the system for an additional seven days while being supplied with ammonia-free water to check for delayed mortality. During the trial, the water temperature ranged from 28°C to 30°C, dissolved oxygen >6.55 mg/L, nitrite<0.1 mg/L, and fish were stopped feeding and exposed to a natural photoperiod. The estimated 96-h LC50 based on linear interpolation of cumulative mortality (y = 2.70 × −104, R2 = 0.9492 where y = % mortality and x = ammonia concentration) was 57.00 mg/L T-AN.
After feeding for 70 days, thirty fish were randomly selected from each bucket and were exposed to LC50 ammonia. Mortality was recorded daily during the period of 96-h trial. At the end of acute ammonia exposure, liver samples were collected from three randomly chosen live fish per bucket and stored at −20°C.
2.5 Statistical analysis
Results of different groups were analysed using a one-way ANOVA and Tukey's multiple range test using SPSS 18.0.0 software. Differences among the groups were considered statistically significant at p < .05.
3 RESULTS
The fishmeal replacement did not lead to any death of experimental fish during the feeding trial (p > .05) (Table 3). The final body weight (FBW) of fish in FM, MM and SM groups was significantly the highest, followed by PM group, then CGM and SPC groups, after that CM group, and BM and MBM groups had the lowest (p < .05). Similarly, weight gain rate (WGR) of fish in FM, MM and SM groups was the highest (p < .05). The variation of specific growth rate (SGR) followed the similar pattern with FBW and WG, and numerically highest value was observed in FM, MM, SM and PM groups (p < .05). There was no significant difference in condition factor (CF) (p > .05). The hepatosomatic index (HSI) of fish in MM group was significantly higher than other groups (p < .05). The daily feed intake (DFI) of fish in SM group was the highest (p < .05). The feed conversion rate (FCR) of fish in BM group was significantly the highest and that of fish in FM, MM, SM and PM groups was the lowest (p < .05). The protein efficiency rate (PER) of fish in FM, MM, SM and PM groups was significantly the highest, followed by CGM group, then CM and SPC groups, after that MBM group, and BM group had the lowest (p < .05). The variation of ADC followed the similar pattern with PER, and numerically highest value was observed in FM, MM and SM groups (p < .05).
| Item | FM | BM | CM | MBM | MM | SM | CGM | PM | SPC |
|---|---|---|---|---|---|---|---|---|---|
| SR (%) | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| IBW (g) | 7.58 ± 0.41 | 7.65 ± 0.31 | 7.73 ± 0.48 | 7.91 ± 0.13 | 7.48 ± 0.17 | 7.33 ± 0.21 | 7.58 ± 0.43 | 7.14 ± 0.18 | 7.76 ± 0.33 |
| FBW (g) | 36.65 ± 1.12e | 28.51 ± 0.82a | 30.99 ± 0.97b | 29.44 ± 1.10a | 36.64 ± 1.47e | 37.74 ± 0.49e | 33.69 ± 1.03c | 35.58 ± 0.46d | 33.13 ± 0.96c |
| WGR (%) | 384.62 ± 29.47d | 272.93 ± 10.49a | 324.28 ± 23.96b | 345.54 ± 35.54b | 398.77 ± 17.03d | 414.77 ± 8.63d | 272.04 ± 14.07a | 390.14 ± 28.62c | 336.15 ± 25.35b |
| SGR | 2.25 ± 0.89d | 1.88 ± 0.04a | 2.06 ± 0.08b | 1.88 ± 0.05a | 2.27 ± 0.08d | 2.34 ± 0.02d | 2.13 ± 0.11c | 2.30 ± 0.05d | 2.10 ± 0.08b |
| CF | 0.84 ± 0.06 | 0.88 ± 0.03 | 0.86 ± 0.03 | 0.88 ± 0.10 | 0.94 ± 0.06 | 0.83 ± 0.09 | 0.85 ± 0.04 | 0.89 ± 0.06 | 0.98 ± 0.07 |
| HSI (%) | 0.98 ± 0.10c | 1.45 ± 0.15d | 1.08 ± 0.12c | 1.32 ± 0.05d | 1.63 ± 0.17e | 1.40 ± 0.07d | 0.96 ± 0.04c | 0.91 ± 0.06b | 1.01 ± 0.06c |
| DFI | 0.65 ± 0.04a | 0.63 ± 0.02a | 0.67 ± 0.02a | 0.65 ± 0.01a | 0.62 ± 0.04a | 0.78 ± 0.03b | 0.62 ± 0.01a | 0.62 ± 0.03a | 0.61 ± 0.05a |
| FCR | 1.38 ± 0.11a | 1.89 ± 0.08e | 1.63 ± 0.04c | 1.76 ± 0.12d | 1.33 ± 0.13a | 1.31 ± 0.04a | 1.49 ± 0.08b | 1.36 ± 0.02a | 1.58 ± 0.14c |
| PER (%) | 151.10 ± 11.56e | 109.10 ± 4.47a | 126.82 ± 3.20c | 113.96 ± 7.69b | 152.41 ± 14.43e | 154.22 ± 4.85e | 136.34 ± 7.75d | 147.81 ± 2.34e | 128.99 ± 11.95c |
| ADC (%) | 75.81 ± 0.42e | 67.15 ± 0.46a | 69.26 ± 0.35b | 67.31 ± 0.51a | 75.33 ± 0.56e | 75.88 ± 0.19e | 70.10 ± 0.21c | 72.91 ± 0.69d | 70.57 ± 0.49c |
Note
- Data are means of triplicates (N = 3). Means in the same row sharing the same superscript letter are not significantly different as determined by Tukey's test (p > .05). Survival rate (SR, %) = 100 × (final fish number)/(initial fish number); IBW (g): initial body weight; FBW(g): final body weight; Weight gain rate (WGR, %) =100 × [final weight (g) – initial weight (g)] /initial weight (g); Specific growth rate (SGR) = [Ln final body weight (g) − Ln initial body weight (g)] ×100/days; Condition factor (CF) =100 × body weight/body length3; Hepatosomatic index (HSI, %) = liver mass (g) ×100/body mass (g); Daily feed intake (DFI) = dry feed intake/ [(final body weight +initial body weight)/2] ×100/days; Feed conversion rate (FCR) = dry feed fed (g)/wet weight gain (g); Protein efficiency rate (PER, %) = 100 × weight gain (g)/protein ingested; Apparent digestibility coefficient (ADC, %) = 100 × [1 − (Y2O3 in dry feed)/Y2O3 in dry faeces].
- Abbreviations: BM, blood meal; CGM, corn gluten meal; CM, chellocken meal; FM, fish meal; MBM, meat and bone meal; MM, maggot meal; PM, peanut meal; SM, soybean meal; SPC, soy protein concentrate.
The crude protein in whole body of fish in SM group was significantly the highest, followed by FM group, then MM group, after that CGM, PM and SPC groups, and BM, CM and MBM groups had the lowest (p < .05; Table 4). The crude lipid in whole body of fish in MM group was significantly higher than other groups (p < .05). There were no significant differences in moisture and ash (p > .05).
| Item | FM | BM | CM | MBM | MM | SM | CGM | PM | SPC |
|---|---|---|---|---|---|---|---|---|---|
| Crude protein | 190.10 ± 1.40d | 166.10 ± 2.40a | 169.10 ± 2.10a | 167.70 ± 2.30a | 183.40 ± ± 1.70c | 201.20 ± 3.90e | 174.60 ± 0.60b | 175.80 ± 3.70b | 173.60 ± 3.30b |
| Crude lipid | 73.00 ± 0.80d | 89.40 ± 3.00e | 73.70 ± 3.40d | 87.20 ± 0.80e | 90.70 ± 1.70f | 89.00 ± 3.80e | 65.00 ± 0.70b | 61.40 ± 2.70a | 69.40 ± 2.40c |
| Moisture | 706.60 ± 0.20 | 699.80 ± 0.40 | 714.10 ± 0.90 | 722.60 ± 0.40 | 701.40 ± 0.40 | 703.50 ± 0.80 | 700.10 ± 0.50 | 713.90 ± 0.30 | 706.00 ± 0.40 |
| Ash | 33.90 ± 0.40 | 35.40 ± 1.30 | 33.80 ± 0.80 | 32.90 ± 1.10 | 33.50 ± 0.90 | 33.10 ± 0.40 | 35.20 ± 0.60 | 35.30 ± 1.40 | 33.10 ± 0.90 |
Note
- Data are means of triplicates (N = 3). Means in the same row sharing the same superscript letter are not significantly different as determined by Tukey's test (p > .05).
- Abbreviations: BM, blood meal; CGM, corn gluten meal; CM, chellocken meal; FM, fish meal; MBM, meat and bone meal; MM, maggot meal; PM, peanut meal; SM, soybean meal; SPC, soy protein concentrate.
The intestinal pepsase and lipase activities of fish in FM group were the highest (p < .05; Table 5). The amylase activity of fish in FM and SM groups was significantly the highest, followed by MM and PM groups, then CGM and SPC groups, after that CM group, and BM and MBM groups had the lowest (p < .05).
| Item | FM | BM | CM | MBM | MM | SM | CGM | PM | SPC |
|---|---|---|---|---|---|---|---|---|---|
| Pepsase | 313.27 ± 2.81g | 152.40 ± 1.51a | 202.30 ± 1.73c | 183.50 ± 2.21b | 289.80 ± 4.50f | 301.60 ± 6.76f | 259.40 ± 5.67e | 289.27 ± 4.01f | 242.97 ± 7.30d |
| Amylase | 72.66 ± 1.19e | 43.81 ± 0.43a | 49.50 ± 1.48b | 46.73 ± 1.01a | 67.76 ± 1.64d | 70.24 ± 0.62e | 55.85 ± 1.51c | 65.49 ± 0.42d | 55.44 ± 0.46c |
| Lipase | 21.64 ± 0.60e | 10.27 ± 0.22a | 14.67 ± 0.17c | 11.86 ± 0.13b | 20.26 ± 0.92d | 20.06 ± 0.25d | 15.01 ± 0.55c | 18.77 ± 0.73d | 15.32 ± 0.34c |
Note
- Data are means of triplicates (N = 3). Means in the same row sharing the same superscript letter are not significantly different as determined by Tukey's test (p > .05).
- Abbreviations: BM, blood meal; CGM, corn gluten meal; CM, chellocken meal; FM, fish meal; MBM, meat and bone meal; MM, maggot meal; PM, peanut meal; SM, soybean meal; SPC, soy protein concentrate.
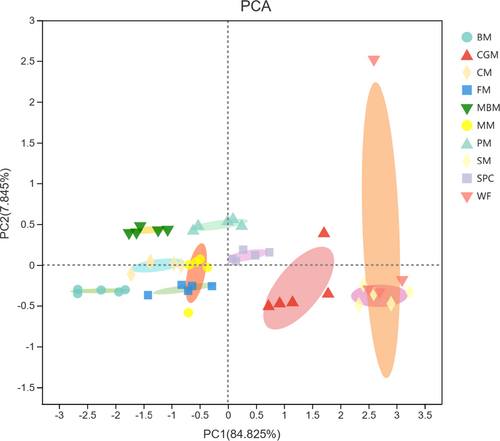
The essential amino acids in muscle were analysed by principal component analysis (PCA), including methionine, threonine, valine, isoleucine, leucine, phenylalanine, histidine, lysine and arginine (Figure 1). The PCA showed that the essential amino acid compositions were different obviously among BM, CGM, CM, FM, MBM, MM, PM, SM and SPC groups; however, SM group and wild fish (WF) individual had similar amino acid compositions in muscle.
The serum total protein, high-density lipoprotein and low density lipoprotein of fish in FM, MM and SM groups were significantly higher than other groups (p<0.05; Table 6). The total cholesterol of fish in FM, CM, MM, SM, CGM, PM and SPC groups was higher than that of fish in BM and MBM groups (p < .05). The MM group obtained significantly the highest triglycerides and glucose (p < .05). The alanine transaminase and aspartate aminotransferase of fish in BM, CM, MBM and MM groups were significantly the highest (p < .05). The alkaline phosphatase of fish in SM group was the highest (p < .05).
| Item | FM | BM | CM | MBM | MM | SM | CGM | PM | SPC |
|---|---|---|---|---|---|---|---|---|---|
| TP (g/L) | 1.05 ± 0.01d | 0.75 ± 0.01a | 0.85 ± 0.01b | 0.85 ± 0.02b | 1.15 ± 0.02d | 1.17 ± 0.01d | 0.95 ± 0.03c | 0.87 ± 0.01b | 0.98 ± 0.01c |
| TC (mmol/L) | 18.18 ± 0.37b | 16.79 ± 0.44a | 17.54 ± 0.40b | 16.84 ± 0.46a | 18.17 ± 0.15b | 18.49 ± 0.42b | 17.62 ± 0.07b | 17.30 ± 0.88b | 17.78 ± 0.28b |
| TG (mmol/L) | 2.64 ± 0.48a | 2.32 ± 0.10a | 2.78 ± 0.07a | 2.30 ± 0.07a | 4.10 ± 0.09b | 2.36 ± 0.12a | 2.73 ± 0.13a | 2.16 ± 0.09a | 2.33 ± 0.53a |
| Glu (mmol/L) | 6.32 ± 0.10a | 6.48 ± 0.13a | 6.27 ± 0.22a | 6.23 ± 0.25a | 7.67 ± 0.42b | 6.84 ± 0.14a | 6.61 ± 0.07a | 6.36 ± 0.16a | 6.49 ± 0.31a |
| HDL (mmol/L) | 10.43 ± 0.20c | 8.79 ± 0.18a | 8.85 ± 0.26a | 8.69 ± 0.27a | 10.79 ± 0.22c | 10.85 ± 0.34c | 9.56 ± 0.28b | 9.23 ± 0.31a | 9.66 ± 0.05b |
| LDL (mmol/L) | 26.54 ± 0.70b | 24.42 ± 0.40a | 24.65 ± 0.45a | 24.63 ± 0.23a | 26.78 ± 0.51b | 26.52 ± 0.50b | 24.46 ± 0.45a | 24.17 ± 0.53a | 24.78 ± 0.43a |
| ALT (mmol/L) | 288.63 ± 5.07a | 345.06 ± 3.94c | 340.74 ± 3.28c | 346.26 ± 3.75c | 340.10 ± 4.12c | 327.93 ± 1.90b | 337.14 ± 5.21b | 334.77 ± 3.56b | 334.69 ± 3.46b |
| AST (mmol/L) | 133.10 ± 1.14a | 160.90 ± 2.41b | 164.45 ± 2.35b | 160.06 ± 0.86b | 160.63 ± 1.36b | 133.44 ± 0.21a | 131.05 ± 0.70a | 131.93 ± 2.37a | 135.61 ± 2.22a |
| AKP (mmol/L) | 5.34 ± 0.04d | 4.10 ± 0.06a | 4.58 ± 0.16b | 4.34 ± 0.21b | 5.53 ± 0.14d | 5.96 ± 0.07e | 5.00 ± 0.05c | 4.92 ± 0.08c | 5.20 ± 0.18c |
Note
- Data are means of triplicates (N = 3). Means in the same row sharing the same superscript letter are not significantly different as determined by Tukey's test (p > .05).
- Abbreviations: AKP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate aminotransferase; BM, blood meal; CGM, corn gluten meal; CM, chellocken meal; FM, fish meal; Glu, glucose; HDL, high-density lipoprotein; LDL, low density lipoprotein; MBM, meat and bone meal; MM, maggot meal; PM, peanut meal; SM, soybean meal; SPC, soy protein concentrate; TC, total cholesterol; TG, triglycerides; TP, total protein.
The serum superoxide dismutase, catalase and glutathione peroxidase activities of fish in FM, SM, CGM, PM and SPC groups were significantly the highest, followed by BM, CM and MBM groups, and MM group had the lowest (p < .05) (Table 7). Fish in SM group had the lowest malondialdehyde content and the highest total antioxidant capacity, but fish in MM group did the opposite (p < .05). The serum lysozyme activity, complement C3, C4 and total immunoglobulin contents of fish in MM group was significantly higher than those of fish in other groups (p < .05).
| Item | FM | BM | CM | MBM | MM | SM | CGM | PM | SPC |
|---|---|---|---|---|---|---|---|---|---|
| SOD (U/ml) | 584.14 ± 9.57c | 485.48 ± 9.83b | 491.09 ± 6.78b | 481.60 ± 6.86b | 468.80 ± 9.31a | 582.88 ± 6.01c | 582.22 ± 11.84c | 586.18 ± 6.61c | 586.17 ± 3.91c |
| CAT (U/ml) | 14.05 ± 0.10c | 11.41 ± 0.15b | 11.31 ± 0.04b | 11.55 ± 0.51b | 9.61 ± 0.08a | 13.96 ± 0.07c | 13.56 ± 0.09c | 13.29 ± 0.41c | 13.85 ± 0.04c |
| GPX (U/ml) | 143.45 ± 4.66c | 86.39 ± 0.26b | 83.61 ± 1.23b | 85.19 ± 1.50b | 75.13 ± 3.87a | 141.57 ± 1.11c | 142.07 ± 3.10c | 141.84 ± 3.93c | 149.27 ± 0.92c |
| MDA (nmol/ml) | 2.59 ± 0.06c | 4.58 ± 0.02d | 4.54 ± 0.14d | 4.56 ± 0.16d | 5.05 ± 0.25e | 1.61 ± 0.05a | 2.04 ± 0.01b | 1.99 ± 0.07b | 2.07 ± 0.03b |
| T-AOC (U/ml) | 1.52 ± 0.02c | 1.25 ± 0.11b | 1.27 ± 0.01b | 1.26 ± 0.01b | 0.63 ± 0.05a | 2.51 ± 0.08d | 1.48 ± 0.07c | 1.43 ± 0.04c | 1.50 ± 0.02c |
| LYZ (U/ml) | 595.11 ± 10.14a | 593.54 ± 4.49a | 591.85 ± 9.02a | 596.48 ± 7.27a | 630.04 ± 9.86b | 594.96 ± 6.37a | 591.87 ± 12.54a | 592.91 ± 7.00a | 593.93 ± 9.09a |
| C3 (mg/ml) | 0.91 ± 0.02a | 0.95 ± 0.03a | 0.93 ± 0.03a | 0.95 ± 0.03a | 1.12 ± 0.01b | 0.97 ± 0.01a | 1.02 ± 0.05a | 0.94 ± 0.03a | 0.92 ± 0.09a |
| C4 (mg/ml) | 0.96 ± 0.05a | 0.98 ± 0.02a | 0.93 ± 0.02a | 0.98 ± 0.02a | 1.15 ± 0.03b | 0.98 ± 0.01a | 0.97 ± 0.07a | 0.95 ± 0.01a | 0.96 ± 0.02a |
| Ig (mg/ml) | 0.95 ± 0.03a | 0.97 ± 0.03a | 0.97 ± 0.03a | 0.97 ± 0.01a | 1.23 ± 0.02b | 0.96 ± 0.01a | 0.94 ± 0.03a | 0.98 ± 0.01a | 0.95 ± 0.03a |
Note
- Data are means of triplicates (N = 3). Means in the same row sharing the same superscript letter are not significantly different as determined by Tukey's test (p > .05).
- Abbreviations: BM, blood meal; C3, complement C3; C4, complement C4; CAT, catalase; CGM, corn gluten meal; CM, chellocken meal; FM, fish meal; GPX, glutathione peroxidase; Ig, total immunoglobulin; LYZ, lysozyme; MBM, meat and bone meal; MDA, malondialdehyde; MM, maggot meal; PM, peanut meal; SM, soybean meal; SOD, superoxide dismutase; SPC, soy protein concentrate; T-AOC: total antioxidant capacity.
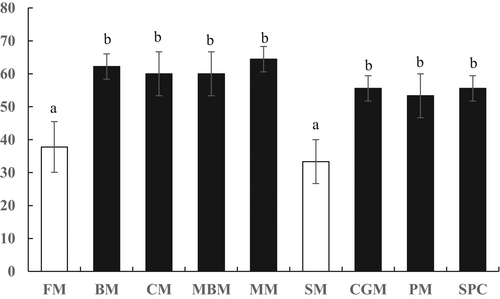
Fish were exposed to LC50 ammonia (57.00 mg/L T-AN) at hour 96, and the cumulative mortalities of fish in FM and SM groups were lower than that of fish in BM, CM, MBM, MM, CGM, PM and SPC groups (p < .05) (Figure 2). The liver carbamyl phosphate synthetase activity of fish in FM groups was significantly the highest (p < .05; Table 8). The arginase activity of fish in FM and SM groups was significantly the highest, followed by CGM group, then PM and SPC groups, and the lowest values appeared in BM, CM, MBM and MM groups (p < .05). The glutamine synthetase and glutamate dehydrogenase activities of fish in SM group were significantly higher than other groups (p < .05).
| Item | FM | BM | CM | MBM | MM | SM | CGM | PM | SPC |
|---|---|---|---|---|---|---|---|---|---|
| CPS | 652.34 ± 3.71f | 529.28 ± 8.82b | 511.08 ± 8.61a | 540.63 ± 5.32b | 570.44 ± 2.41d | 620.98 ± 2.48e | 558.11 ± 9.11c | 548.11 ± 3.89c | 550.97 ± 4.54c |
| ARG | 1230.49 ± 16.84d | 940.66 ± 5.64a | 949.49 ± 4.23a | 932.31 ± 7.36a | 922.57 ± 4.43a | 1208.48 ± 7.43d | 1109.18 ± 7.30c | 1074.42 ± 10.50b | 1074.52 ± 22.27b |
| GS | 1632.17 ± 9.42b | 1461.82 ± 9.30a | 1466.59 ± 8.74a | 1491.60 ± 6.80a | 1471.58 ± 16.53a | 1740.74 ± 24.80c | 1440.39 ± 22.45a | 1445.03 ± 11.00a | 1468.70 ± 3.45a |
| GDH | 738.66 ± 7.85c | 702.07 ± 3.62a | 712.85 ± 2.29a | 709.73 ± 3.75a | 712.85 ± 4.21a | 756.74 ± 4.48d | 729.01 ± 3.52b | 721.88 ± 6.68b | 722.38 ± 7.07b |
Note
- Data are means of triplicates (N = 3). Means in the same row sharing the same superscript letter are not significantly different as determined by Tukey's test (p > .05).
- Abbreviations: ARG, arginase; BM, blood meal; CGM, corn gluten meal; CM, chellocken meal; CPS, carbamyl phosphate synthetase; FM, fish meal; GDH, glutamate dehydrogenase; GS, glutamine synthetase; MBM, meat and bone meal; MM, maggot meal; PM, peanut meal; SM, soybean meal; SPC, soy protein concentrate.
4 DISCUSSION
NRC (2011) suggested that the soybean meal in aquafeed is about 20%, and a further increase is limited by the anti-nutritional factors, such as phytates, saponins and lectins. However, the recent studies found that the fishmeal replacement with 25% soybean meal (or soy protein concentrate) is suitable in burbot Lota lota maculosa diets and long-term feeding of soy-based proteins does not impair innate immune and disease resistance (Timothy et al., 2021); and 30% fishmeal could be replaced by soybean meal, soy protein concentrate and corn gluten meal in red sea bream Pagrus major diets (Kader & Koshio, 2012); and based on the growth performance and feed utilization, about 40% substitution level of fishmeal by soybean meal was suggested in crucian carp C. auratus gibelio ♀ × Cyprinus carpio ♂ the diets (Liu et al., 2020). In the present study, the fishmeal replaced with 30% soybean meal did not affect the growth performance, which may be highly tolerance of O. bidens for plant-based protein. To demonstrate the hypothesis, the yttrium oxide is added to experiment diets as an indicator to analyse the apparent digestibility coefficient. The result shows that O. bidens has favourable protein digestibility for plant-based proteins, including soybean meal, corn gluten meal, peanut meal and soy protein concentrate. Soy protein concentrate, is also a soy-based protein source, can be used to fully replace fish meal for burbot culture (Walker et al., 2010), and 50% fish meal could be replaced was suitable for growth of juvenile Atlantic cod Gadus morhua (Colburn et al., 2012). However, in this study, the fishmeal replacement with 30% soy protein concentrate resulted in a significant decrease in the weight gain rate and protein efficiency rate of O. bidens compared with soybean meal group. Decreased body weight gain is not the only production metric of importance, as the body composition and organosomatic indices also impacts quality and quantity of aquaculture species (Aksnes et al., 2006). In the present study, the SM group displayed a relatively elevated hepatosomatic index and whole-body lipid content. Although SM diet was the top-performing diet, the composition may relate to differences in energy allocation for the nutrients or play a role in altering the lipid metabolism. Therefore, a further evaluation of the lipid requirement for O. bidens would provide the ability to formulate diets with soy-based protein ingredients. In general, the apparent digestibility coefficient of fishmeal is higher than that of animal-based proteins, but some studies have reported higher apparent digestibility coefficients of animal by-products, for example, in silver perch Bidyanus bidyanus, the apparent digestibility coefficients of blood meal, poultry meal and hydrolysed feather meal were similar to that of fishmeal (Allan et al., 2000); in mulloway Argyrosomus japonicus, the apparent digestibility coefficient of meat and bone meal (ovine and bovine) was also similar to that of fishmeal (Booth et al., 2013). This study evaluated that the apparent digestibility coefficients of four animal-based proteins and found that the apparent digestibility coefficients of blood meal, chellochen meal and meat and bone meal were significantly lower than that of fishmeal, which is the direct reason for the low growth performance of O. bidens in BM, CM and MBM groups. Nevertheless, there was no significant difference in apparent digestibility coefficients between FM and MM groups. The replacement of fishmeal with insect meal in aquafeed has been widely reported, such as barramundi Lates calcarifer (Katya et al., 2017) and lemon fin barb hybrid Hypsibarbus wetmorei ×Barbonymus gonionotus (Kamarudin et al., 2021). Some studies have suggested that the maggot meal is of similar biological value to fishmeal and it does not contain anti-nutritional factors which are commonly found in plant protein, and maggot meal has balanced amino acid profiles in many protein sources (Kamarudin et al., 2021; Ogunji et al., 2011). In contrast, other studies reported that the lower weight gain of fish fed with maggot meal may be related to the chitin content, which has been proven as a causing factor for the declining protein utilization (Kroeckel et al., 2012; Ng et al., 2001). In the present study, the WGR, FCR, PER and ADC have not much affected by 30% maggot meal replacement, which may be due to the tolerance of O. bidens for chitin. It is a pity that the fishmeal replacement with 30% maggot meal resulted in a significant increase in hepatosomatic index and whole-body lipid content of O. bidens, which is consistent with the result of previous study in barramundi (Katya et al., 2017). The results suggest that before replacing fish meal with insect protein, degreasing may achieve better results.
Digestive enzyme activity is closely related to digestive function of animal, but it is affected by food quality. The previous studies reported that the increase of dietary soybean protein level will lead to the decrease of digestive enzyme activity in fish, such as Atlantic cod (Lemieux et al., 1999), Atlantic salmon Salmo salar (Krogdahl et al., 2003) and Japanese seabass Lateolabrax japonicus (Li et al., 2014). The presence of anti-nutritional factors in plant proteins was main factor to reduce the food digestibility (NRC, 2011). However, in the present study, differences in intestinal digestive enzymes activities of O. bidens were minor between FM and SM groups, and the values of digestive enzymes further demonstrate the reason for the high growth performance and apparent digestibility coefficient in plant protein (SM, CGM, PM and SPC) groups. The soybean meal is recommended as an alternative source of protein to replace fishmeal based on digestive enzyme activity.
For a long time, O. bidens has been considered a carnivorous fish because of the large number of insect limbs found in the gut of wild individual. However, this study found that the growth performance of fish in SM group was the higher among all experiment groups, which is very confusing for us. Mohammadi et al. (2020) reported that the muscle essential amino acid contents of fish were closely associated with amino acid composition in food. Therefore, this study also analysed the muscle amino acid compositions of wild O. bidens; then, the essential amino acids of different fish types were analysed by principal component analysis. Evidence from principal component analysis shown that the WF individual and SM group were clustered together but visibly separated from other groups, suggesting that the amino acid compositions of soybean meal may be more suitable for the growth of O. bidens. In order to improve the dietary formulation, it is necessary to carry out a comprehensive survey on the feeding habits of O. bidens.
Serum biochemical parameter serves as reliable indicators for the physiological health status of animal (Kader et al., 2010). In the present study, differences in most of the hemochemical parameters were minor between FM and SM groups, which indicated that replacing 30% fishmeal with soybean meal did not cause visible negative effects on O. bidens health. In addition, although the growth performance has not much affected by maggot meal replacement, the serum triglycerides and glucose contents were significantly increased, which may be related to the high fat content in maggot meal, perhaps a defatted maggot meal would be an effective way to avoid the adverse effects. Serum aspartate aminotransferase and alanine aminotransferase were usually used as indicators of hepatocyte injury in animals (Takagi et al., 2006). In the present study, compared with the plant protein groups, the significantly higher values of aspartate aminotransferase and alanine aminotransferase were found in BM, CM and MBM groups, which suggests that replacing fishmeal with animal by-products may increase the risk of blood deterioration in O. bidens.
The effect of fishmeal replacement with different protein sources on antioxidant enzyme activity in several fish species has been reported. Jiang et al. (2018) reported that intestinal SOD and GPx activities of yellow catfish were not affected by dietary replacement of fishmeal by soybean meal; Abasubong et al. (2018) found that feeding on diet containing rice protein concentrate as fishmeal substitute did not affect liver MDA content and CAT and GPx activities in blunt snout bream Megalobrama amblycephala; Mohammadi et al. (2020) reported that replacing fishmeal with processed canola meal did not cause negative effects on antioxidant system of Nile tilapia Oreochromis niloticus. Similarly, in the present study, the replacing fish meal by plant protein sources in the diets of O. bidens did not affect the antioxidant enzyme activity, even the MDA content decreased significantly. In contrast, this study also found fishmeal replacement with animal protein sources resulted in antioxidant enzymes activities were inhibited and MDA accumulation, especially MM group, which may be related to the high content of saturated fatty acids in insect proteins or animal by-products, and it may be improved by degrease or adding antioxidant additives. In immunity, a previous study found that feeding the Nile tilapia with diets containing graded levels of processed canola meal did not suppress the innate immunity (Mohammadi et al., 2020). This study also found that replacing fishmeal did not affect the immune response of O. bidens, except for maggot meal. The effect of insect meal on fish immunity has not received enough attention. Some studies indicate that insect meal has anti-inflammatory and immunostimulating effects, which may be related to the abundance of chitin in insect exoskeletons, but so far there has been no in-depth study (Henry et al., 2018).
After the completion of the growth performance trial, O. bidens were exposed to LC50 ammonia environment. The aim of this trial was to distinguish any diet-related changes to O. bidens survival against ammonia stress, because dietary factor has been found to influence the ammonia tolerance in fish (Ip & Chew, 2018). In the present study, the fishmeal was replaced with soybean meal did not affect the cumulative mortality of O. bidens exposure to ammonia at hour 96. Fish ammonia detoxification mainly depends on urea synthesis and glutamine synthesis (Clark et al., 2019; Xing et al., 2016). This study found that the replacement of fishmeal with plant proteins enhanced activities of CPS, ARG, GS and GDH, especially the soybean meal. The results indicated that the improvement of anti-ammonia ability of O. bidens by replacing fishmeal with plant proteins was obviously better than that of animal proteins.
5 CONCLUSION
This study evaluated the potential for eight protein sources to be included in O. bidens diet formulations. Based on findings of growth performance, haematologic status, antioxidant capacity, immunity and ammonia tolerance, we conclude that fishmeal replacement with soybean meal is acceptable in this juvenile life stage; the muscle amino acid compositions of O. bidens in SM group were more similar to wild individual; the fishmeal was replaced with plant proteins was obviously better than animal proteins for O. bidens.
ACKNOWLEDGEMENTS
This work was supported by the Key Research and Development Program of Zhejiang Province (2019C02049); the Natural Science Research Fund (Special-post) of Guizhou University (2021(26)); the Key Research and Development Program of Lishui City (2019ZDYS13); the National Natural Science Foundation of China (32072948); the Fundamental Research Funds for the Provincial Universities of Zhejiang (SJLY2020009); the Natural Science Foundation of Ningbo City (202003N412); the Natural Science Foundation of Guizhou Province of China (20191114); and the Open Project of Key Lab of Freshwater Biodiversity Conservation, Ministry of Agriculture and Rural Affairs of China (LFBC1010).
CONFLICT OF INTEREST
The authors declare no competing or financial interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.




