Effects of replacing soybean meal protein with cottonseed protein concentrate on the growth condition and intestinal health of Nile tilapia (Oreochromis niloticus)
Abstract
With the development of processing technology, cottonseed protein concentrate (CPC) has been widely used as a new protein source to replace fish meal in aquaculture. But whether CPC can replace soybean meal still needs investigation. A ten-week experiment was designed to evaluate the impact of replacing soybean meal (SBM) protein with CPC at different levels (CPC0 [0%], CPC15 [15%], CPC30 [30%], CPC45 [45%], CPC75 [75%] and CPC100 [100%]) on Nile tilapia (Oreochromis niloticus). The results showed that 45% or higher CPC substitution significantly retarded the growth performance. Increased intestinal permeability was found in the CPC75 and CPC100 treatments. To investigate whether the residual gossypol is account for the intestinal dysfunction, an 8-week feeding trial including a control diet (CON), a control diet supplemented with 150 mg/kg gossypol (ML) and 300 mg/kg gossypol (MH) treatments, was conducted. The results showed that the transepithelial resistance (TER) was significantly decreased in MH treatment. In conclusion, the replacement of 30% SBM protein by CPC can be used for Nile tilapia's dietary needs, while 45% or higher CPC substitution of SBM influenced the intestinal health and growth condition of Nile tilapia.
1 INTRODUCTION
Aquaculture has been one of the fastest growing food production systems in the world over the past 30 years (Cooney et al., 2021; Hua et al., 2019). The booming aquaculture industry led to the increasing demand for protein sources including fish meal (FM) and soybean meal (SBM), and as a result, the prices of FM and SBM increased rapidly (Hao et al., 2020; Jiang et al., 2012; Li et al., 2021). Considering the need for sustainable development of the aquaculture industry and the demand for low-cost dietary formulas, increasing efforts were directed to identify and evaluate new protein sources (Fasakin et al., 2001; Wang, Zhang, et al., 2020).
Cotton is an important textile raw material and is widely grown in the world (Dai et al., 2017). Cottonseed protein concentrate (CPC), a high-protein product obtained from the processing of cottonseed flakes, is available in large quantities (Shen et al., 2020) and generally less expensive per unit of protein than FM and SBM (Wang et al., 2020; Zhou & Liu, 2007). CPC was mainly used to replace FM in aquaculture. For example, studies have found that it can replace 24% FM without affecting the growth of juvenile golden pompano Trachinotus ovatus (Shen et al., 2020). However, significant decreases in crude protein were found when using CPC to replace 36% FM in the feed of juvenile golden pompano Trachinotus ovatus (Shen et al., 2020), and using CPC to replace 50% FM caused significant weight loss in juvenile red drum Sciaenops ocellatus (Wang, Clark, et al., 2020). The mechanism of these negative effects of using CPC is not clear yet (Deng et al., 2015; Lim & Lee, 2009; Wang, Zhang, et al., 2020). It has been reported that replacing 45% of FM with CPC in diets caused intestinal inflammation (Yin et al., 2020) and residues of gossypol may account for the negative effects of CPC (Shen et al., 2020; Wang, Clark, et al., 2020). Gossypol is a toxic substance of cottonseed protein (Sun et al., 2015), but whether gossypol is related to the negative impact of CPC substitution remains unclear.
Nile tilapia is an omnivorous fish (Yue & Zhou, 2008) and has become the most important species in freshwater aquaculture (Joshi et al., 2021). Currently, SBM is the main protein source in commercial feeds for Nile tilapia, and it remains unclear whether CPC can effectively replace SBM in commercial feeds for Nile tilapia. In order to identify the impact of SBM protein replaced by CPC on the growth of Nile tilapia and to identify the possible mechanisms, Nile tilapia were fed with different diets formed by replacing 0%, 15%, 30%, 45%, 75% and 100% SBM protein with CPC (referred to as CPC0, CPC15, CPC30, CPC45, CPC75 and CPC100, respectively) for 10 weeks. The growth condition and intestinal health of Nile tilapia in each treatment were detected, and the influence of gossypol on fish health was also characterized.
2 MATERIALS AND METHODS
All experiments were carried out under the Guide for the Care and Use of Laboratory Animals in China. This research was approved by the Committee on the Ethics of Animal Experiments of East China Normal University (ECNU) (No. F20201002).
2.1 Experimental design
Juvenile male Nile tilapia were purchased from Tianfa fry Co., Ltd. In the first trial, six experimental diets were formulated with different inclusion levels of cottonseed protein concentrate to replace 0%, 15%, 30%, 45%, 75% and 100% of the soybean meal protein in the diet (referred to as CPC0, CPC15, CPC30, CPC45, CPC75 and CPC100, respectively) (Table 1). Each treatment contained three 256-L tanks and each tank contained 15 individuals. The initial weight of the fish for each tank was 70.82 ± 0.36 g (n = 15). The fish were fed at 3% of their average body weight per day for 10 weeks.
| Ingredients (g/kg) | CPC0 | CPC15 | CPC30 | CPC45 | CPC75 | CPC100 |
|---|---|---|---|---|---|---|
| Soybean meala | 400 | 340 | 280 | 220 | 100 | 0 |
| Cottonseed protein concentrateb | 0 | 45 | 90 | 135 | 225 | 300 |
| Casein | 85 | 85 | 85 | 85 | 85 | 85 |
| Wheat flour | 400 | 400 | 400 | 400 | 400 | 400 |
| Soybean oil | 52 | 51 | 51 | 50 | 50 | 49 |
| 1% premixc | 10 | 10 | 10 | 10 | 10 | 10 |
| Carboxyl methyl cellulose | 25 | 25 | 25 | 25 | 25 | 25 |
| Cellulose | 5.35 | 21.35 | 36.35 | 52.35 | 82.35 | 108.35 |
| Butylated hydroxytoluene | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Ca (H2PO4)2 | 15 | 15 | 15 | 15 | 15 | 15 |
| Choline chloride | 5 | 5 | 5 | 5 | 5 | 5 |
| DMPTd | 2 | 2 | 2 | 2 | 2 | 2 |
| Yttrium oxide | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Proximate composition (%) | ||||||
| Crude protein | 32.46 | 32.39 | 32.49 | 32.78 | 32.61 | 32.68 |
| Crude lipid | 4.76 | 4.79 | 4.77 | 4.63 | 4.62 | 4.54 |
| Dry matter | 94.95 | 95.99 | 94.80 | 94.79 | 93.51 | 93.99 |
| Crude ash | 4.95 | 4.94 | 4.84 | 4.91 | 4.86 | 5.04 |
| Gossypol content (mg/kg) | 0 | 24.89 | 37.21 | 65.33 | 109.05 | 143.45 |
- a Qingdao Bohai Agricultural Development Co. LTD; proximate composition (% dry matter): moisture, 10.14; crude protein, 46.05; crude lipid, 1.03; crude ash, 6.15.
- b Xinjiang Jinlan Plant Protein Co., Ltd.; proximate composition (% dry matter): moisture, 5.25; crude protein, 61.51; crude lipid, 2.36.
- c 1% premix, (per kg mixture): 60 g Vitamin C phosphate; 30 g Vitamin E; 15 mg Inositol; 8 g Nicotinamide; 4 g Calcium pantothenate; 2 g Vitamin A; 2 g Vitamin K3; 1.5 g Vitamin B2; 1.5 g Vitamin B6; 1 g Vitamin D3; 1 g Vitamin B1; 1 g Folic acid; 0.8 g Vitamin B12; 0.2 g Biotin; 22 g Wheat middling; 36.2 g Zeolite powder; 30 g FeSO4·H2O; 20 g ZnSO4·7H2O; 10 g NaCl; 2.5 g MnSO4·H2O; 0.5 g CoCl2·6H2O; 0.5 g Na2SeO3; 0.3 g Potassium Iodate; 90 g Magnesium sulphate; 5 g Compound antioxidant; 20 g Mildewcide; 635 g Zeolite powder.
- d DMPT, Dimethyl-beta-propiothetin.
In the second trial, the control diet was supplemented with free gossypol (purity ≥ 98%) (Ci Yuan Biotechnology Co., Ltd). Nile tilapia (average weight 3.38 ± 0.03 g) were divided into three treatments: control diet (CON), control diet supplemented with 150 mg/kg gossypol (ML) and control diet supplemented with 300 mg/kg gossypol (MH) (Table 2). Phloroglucinol spectrophotometric method was used to analyse the gossypol content in diets as previously described (Ao & Qu, 2007; Zhu et al., 2017). Each treatment contained three 100-L tanks, and each tank contained 20 individuals. The fish were fed at 4% of their average body weight per day for 8 weeks.
| Ingredients (g/kg) | CON | ML | MH |
|---|---|---|---|
| Casein | 320 | 320 | 320 |
| Gelatin | 80 | 80 | 80 |
| Soybean oil | 60 | 60 | 60 |
| Corn starch | 300 | 300 | 300 |
| Vitamin Premixa | 12 | 12 | 12 |
| Mineral Premixb | 12 | 12 | 12 |
| Ca (H2PO4)2 | 10 | 10 | 10 |
| Carboxyl methyl cellulose | 30 | 30 | 30 |
| Cellulose | 169.75 | 169.60 | 169.45 |
| Choline chloride | 5 | 5 | 5 |
| DMPTc | 1 | 1 | 1 |
| Butylated hydroxytoluene | 0.25 | 0.25 | 0.25 |
| Gossypol | 0 | 0.15 | 0.30 |
| Proximate composition (%) | |||
| Crude protein | 34.46 | 34.77 | 34.65 |
| Crude lipid | 5.15 | 5.13 | 5.05 |
| Dry matter | 92.01 | 92.85 | 88.98 |
| Crude ash | 2.71 | 2.83 | 2.73 |
| Gossypol content (mg/kg) | 0 | 125.86 | 302.04 |
- a Vitamin Premix, (per kg mixture): 500,000 I.U. (international units) Vitamin A, 50,000 I.U. Vitamin D3, 2500 mg Vitamin E, 1000 mg Vitamin K3, 5000 mg Vitamin B1, 5000 mg Vitamin B2, 5000 mg Vitamin B6, 5000 μg Vitamin B12, 25,000 mg Inositol, 10,000 mg Pantothenic acid, 100,000 mg Choline, 25,000 mg Niacin, 1000 mg Folic acid, 250 mg Biotin, 10,000 mg Vitamin C.
- b Mineral Premix, (per kg mixture): 314.0 g CaCO3; 469.3 g KH2PO4; 147.4 g MgSO4·7H2O; 49.8 g NaCl; 10.9 g Fe (II) gluconate; 3.12 g MnSO4·H2O; 4.67 g ZnSO4·7H2O; 0.62 g CuSO4·5H2O; 0.16 g KI; 0.08 g CoCl2·6H2O; 0.06 g NH4 molybdate; 0.02 g NaSeO3.
- c DMPT, Dimethyl-beta-propiothetin.
In both experiments, indoor recirculating systems in East China Normal University Experimental Station were used. The feed consumption in each tank was recorded daily, and the weight of fish in each tank was recorded every other week. All the experimental fish were fed at 9:00, 14:00 and 19:00, and all feeds were eaten up every day. Water temperature was kept at 27 ± 1°C by heating rods. A 12 h/12 h light/dark cycle was used. pH was ranged from 7.5 to 7.9, and ammonia nitrogen was less than 0.02 mg/L. In the first trial, dissolved oxygen was maintained higher than 6.0 mg/L, and in the second trial, dissolved oxygen was maintained higher than 4.0 mg/L.
2.2 Feed processing
The dietary ingredients (Tables 1 and 2) were mixed with oil and distilled water to form a paste, and then, a Twin-Screw Extruder (TSE65; Yanggong Machine) was used to produce particles (5-mm diameter particles for the first trial and 2-mm diameter particles for the second trial). The prepared diets were dried at room temperature and stored at −20°C.
2.3 Sample collection
At the end of the trial, fish were fasted for 12 h before sampling. In both trials, nine fish from each treatment (three individuals from each tank) were sampled and stored at −20°C for the whole fish composition analysis. Six fish from each treatment (two individuals from each tank) were used for blood collection and subsequent biochemical analysis. Fish were euthanized with 120 mg/L MS-222 (Sigma). The body weight and length were measured. The blood was collected by caudal venipuncture using 2 ml syringes and then centrifuged at 4°C by 900 g for 20 min. The serum samples were stored at −80°C for the following biochemical analysis. The viscera mass and the liver of nine fish per treatment were weighed. We used liquid nitrogen to freeze the liver and intestine samples before storage at −80°C for the following biochemical analysis.
2.4 Growth performance
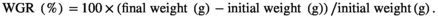
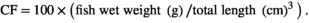
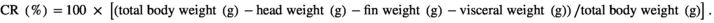
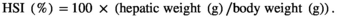
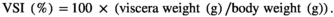
2.5 Biochemical analysis
Serum ALT, AST, intestinal amylase, lipase and hepatic TG, AKP and ACP were assessed by commercial kits (Nanjing Jiancheng Biotech Co., Ltd.) (kit number: C009-2-1, C010-2-1, C016-1-1, A054-2-1, A110-1-1, A059-2-2 and A060-2-2, respectively). Serum lipopolysaccharide (LPS) and intestinal trypsin were measured by Elisa kit (Shanghai Hengyuan Biological Technology Co., Ltd.) (kit number: HB794-QT and HB927X-QT). The lipid of fish and feed was extracted by using chloroform/methanol (2:1, v/v) (Folch et al., 1957). Crude protein of fish and feed was measured by Kjeltec™ 8200 (FOSS). The dry matter was analysed by drying the sample at 105°C to a constant weight. The ash content of the feed was measured after combustion in a muffle furnace at 550°C for 6 h. Amino acid compositions in diets were determined by using an L-8900 High-speed Amino Acid Analyzer (Hitachi) equipped with the packed column (Hitachi ion-exchange resin 2622).
2.6 Intestinal permeability
In both trials, six individuals from each treatment (two individuals from each tank) were collected for Ussing chamber analysis. Ussing chamber was used to measure the transepithelial electrical resistance (TER) in vitro, and the method was according to the previous report (Zhang et al., 2020) with some modifications. Briefly, the fresh tilapia intestines were cut open, and around 0.01 cm2 of foregut tissue was rinsed in Krebs–Ringer solution (CaCl2·2H2O, 2.5 mM; NaCl, 117 mM; NaHCO3, 25 mM; KCl, 4.7 mM; NaH2PO4·2H2O, 1.2 mM; MgCl2·2H2O, 1.2 mM; glucose, 11 mM; pH 7.8) and then mounted on P2306 clamps. After 10 min of equilibration, transepithelial electrical resistance (TER) was automatically recorded every 1 min over a 10-min period.
2.7 Histological analysis
In both trials, liver and foregut were taken from two fish in each tank and processed for haematoxylin–eosin (H & E) analysis according to the previous report (Limbu et al., 2018). The stained samples were observed under a microscope (Nikon Ds-Ri2). Villus height (100×) and villus width (100×) were measured from at least 24 segments in each treatment by using imaging software (Nis-Elements F package version 4.60).
2.8 RNA isolation and real-time quantitative PCR
In both trials, foregut samples were collected from six fish in each treatment (two individual from each tank), and the total RNA from the foregut was isolated according to the instructions of Tri Pure Reagent (Aidlab). The quality and quantity of RNA were tested by NANODROP 2000 Spectrophotometer (Thermo). A260/A280 of RNA ranged from 1.9 to 2.1. RNA integrity was confirmed by 1% agarose gel electrophoresis. cDNA was synthesized by FastQuant RT Kit with gDNase (TIANGEN) by S1000™ Thermal Cycler (BioRad). The primers for quantitative PCR (qPCR) were designed in NCBI (Table 3). The qPCR was carried out in a QuantStudio® 5 Real-Time PCR Instrument (Thermo Scientific) with the reaction programme: 94°C for 2 min, 40 cycles of 94°C for 10 s, 60°C for 15 s and 72°C for 20 s. The relative mRNA expression was estimated by the 2 −ΔΔCt method.
| Primers | qPCR primers, forward/reverse (5′ to 3′) | Gene Bank NO. |
|---|---|---|
| il-8 | F: CTGTGAAGGCATGGGTGTGGAG | NM_001279704.1 |
| R: TCGCAGTGGGAGTTGGGAAGAA | ||
| il-1β | F: GAGCACAGAATTCCAGGATGAAAG | XM_019365842.2 |
| R: TGAACTGAGGTGGTCCAGCTGT | ||
| il-6 | F: ACAGAGGAGGCGGAGATG | XM_003453898.2 |
| R: GCAGTGCTTCGGGATAGAG | ||
| tgfβ | F: AAGAGGAGGAGGAATACTTTGCCA | NM_001311325.1 |
| R: GAAGCTCATTGAGATGACTTTGGG | ||
| claudin-1 | F: GAGGAGTCAGTCGGAGTCT | XM_003448981.5 |
| R: CAGCACCGTCTTGAACTTG | ||
| occludin-X1 | F: GTGTTGCTGCTTTCTTCGCT | XM_003445131.5 |
| R: GTGTTGCTGCTTTCTTCGCT | ||
| zo-1 | F: CCGCAGATCAGTCCCTCTTC | XM_013270540.3 |
| R: GTACGGAGTTAGCATCGCCA | ||
| ire1 | F: CCGACTCCATCTGTCCCAAC | XM_025900066.1 |
| R: GATGGGCCATTTTGTGCGTG | ||
| perk | F: GATGTTTCAGGGGCAGCTCT | XM_003447769.5 |
| R: CGTCGTGGGAGAACTTGTCA | ||
| atf6 | F: GTGGTATGAGAGGTCGCTGG | XM_003440029.5 |
| R: ATCTGGAACACCGTTGGCAT | ||
| ef1α | F: ATCAAGAAGATCGGCTACAACCCT | KJ123689.1 |
| R: ATCCCTTGAACCAGCTCATCTTGT | ||
| β-actin | F: AGCCTTCCTTCCTTGGTATGGAAT | KJ126772.1 |
| R: TGTTGGCGTACAGGTCCTTACG |
2.9 Statistical analysis
All data were presented as mean ± SEM (n = 6 or 9). Normal distribution was confirmed by Shapiro–Wilk test. Then one-way ANOVA and Duncan's multiple range tests were conducted to test for the differences among the means (p < .05). All data were analysed by using the SPSS Statistics 25.0 software (IBM).
3 RESULTS
3.1 Effects of replacing soybean meal with cottonseed protein concentrate on the growth condition of Nile tilapia
The survival rates of the six treatments CPC0, CPC15, CPC30, CPC45, CPC75 and CPC100 were 91%, 98%, 100%, 93%, 93%, 91% and 98% respectively, and there was no significant difference among treatments (p > .05). As shown in Table 4, when the ratio of CPC substitution to SBM protein was 15% and 30%, WGR did not change significantly (p > .05). But when the ratio reached 45%, WGR decreased significantly compared with CPC0 treatment (p < .05). No significant differences were observed in CF and carcass ratio among all treatments (p > .05). The HSI and VSI value in CPC45 treatment were significantly lower than those of CPC0 treatment (p < .05). The total protein of fish was less affected by different CPC inclusion levels (p > .05). The results showed that replacing 30% SBM protein with CPC had no significant effect on the growth index and body composition of Nile tilapia.
| Treatment | CPC0 | CPC15 | CPC30 | CPC45 | CPC75 | CPC100 |
|---|---|---|---|---|---|---|
| WGR (%) | 211.92 ± 13.20a | 217.33 ± 22.42a | 218.08 ± 9.36a | 163.68 ± 5.76b | 167.31 ± 16.83b | 169.49 ± 13.56b |
| CF (g/cm3) | 3.74 ± 0.08 | 3.91 ± 0.09 | 3.97 ± 0.08 | 3.78 ± 0.08 | 3.74 ± 0.07 | 3.97 ± 0.13 |
| CR (%) | 58.23 ± 0.80 | 57.67 ± 0.53 | 57.01 ± 0.64 | 58.26 ± 0.39 | 58.09 ± 0.44 | 56.07 ± 0.59 |
| HSI (%) | 3.00 ± 0.11a | 3.08 ± 0.16a | 2.72 ± 0.12ab | 2.47 ± 0.10b | 2.77 ± 0.17ab | 2.77 ± 0.12ab |
| VSI (%) | 11.01 ± 0.33ab | 11.87 ± 0.48a | 10.38 ± 0.58b | 8.61 ± 0.49c | 9.93 ± 0.25b | 11.15 ± 0.34ab |
| Crude protein (%) | 52.11 ± 1.10ab | 52.94 ± 1.66a | 52.20 ± 0.90b | 53.07 ± 1.26c | 50.74 ± 1.27b | 49.49 ± 0.97ab |
| Crude lipid (%) | 25.46 ± 0.62ab | 22.40 ± 1.04b | 24.05 ± 1.16ab | 26.91 ± 0.55a | 26.08 ± 1.16a | 26.51 ± 1.94a |
Note
- Values are mean ± SEM (n = 3). Means with different subscripts are significantly different (p < .05).
- Abbreviations: CF, condition factor; CR, carcass ratio; HIS, hepatosomatic index; VSI, visceral somatic index; WGR, weight gain rate.
3.2 Effects of replacing soybean meal protein with cottonseed protein concentrate on the amino acids composition of diet and intestinal digestive enzyme activity of Nile tilapia
Amino acid composition of the diets was detected. The content of essential amino acids including Lys, Ile, Leu and Thr differed significantly among diets (p < .05). Non-essential amino acids including Ser, Asp and Arg showed significant difference among diets (Table 5).
| Amino acids | CPC0 | CPC15 | CPC30 | CPC45 | CPC75 | CPC100 |
|---|---|---|---|---|---|---|
| Phe | 1.71 ± 0.01bc | 1.68 ± 0.00c | 1.69 ± 0.01c | 1.69 ± 0.00c | 1.71 ± 0.00b | 1.77 ± 0.00a |
| Met | 0.48 ± 0.01a | 0.46 ± 0.00ab | 0.44 ± 0.01b | 0.41 ± 0.00c | 0.45 ± 0.01b | 0.48 ± 0.01a |
| Lys | 1.97 ± 0.01a | 1.89 ± 0.00b | 1.82 ± 0.01c | 1.74 ± 0.00d | 1.64 ± 0.00e | 1.56 ± 0.00f |
| Ile | 1.53 ± 0.00a | 1.46 ± 0.00b | 1.38 ± 0.01c | 1.35 ± 0.00d | 1.28 ± 0.00e | 1.22 ± 0.00f |
| Val | 1.73 ± 0.01a | 1.70 ± 0.00b | 1.66 ± 0.01c | 1.67 ± 0.00c | 1.64 ± 0.00c | 1.64 ± 0.00c |
| Leu | 2.68 ± 0.01a | 2.58 ± 0.00b | 2.46 ± 0.02c | 2.43 ± 0.00c | 2.33 ± 0.01d | 2.26 ± 0.01e |
| Thr | 1.29 ± 0.01a | 1.24 ± 0.00b | 1.22 ± 0.01c | 1.18 ± 0.00d | 1.14 ± 0.00e | 1.11 ± 0.00f |
| EAA | 11.39 ± 0.04a | 11.02 ± 0.01b | 10.66 ± 0.00c | 10.44 ± 0.01d | 10.19 ± 0.01e | 10.03 ± 0.03f |
| Ser | 1.70 ± 0.01a | 1.64 ± 0.00b | 1.62 ± 0.01c | 1.59 ± 0.00d | 1.56 ± 0.01e | 1.54 ± 0.00f |
| Gly | 1.21 ± 0.01a | 1.19 ± 0.00b | 1.18 ± 0.01bc | 1.17 ± 0.00c | 1.16 ± 0.00c | 1.17 ± 0.01c |
| Asp | 3.08 ± 0.01a | 2.96 ± 0.00b | 2.90 ± 0.02c | 2.81 ± 0.01d | 2.71 ± 0.01e | 2.64 ± 0.01f |
| Cys | 0.27 ± 0.01b | 0.29 ± 0.00a | 0.27 ± 0.01b | 0.27 ± 0.00b | 0.28 ± 0.00ab | 0.29 ± 0.01a |
| His | 0.85 ± 0.00b | 0.84 ± 0.00b | 0.84 ± 0.01b | 0.85 ± 0.00b | 0.86 ± 0.00b | 0.88 ± 0.00a |
| Glu | 7.17 ± 0.03c | 7.11 ± 0.01c | 7.13 ± 0.05c | 7.16 ± 0.02c | 7.32 ± 0.01b | 7.50 ± 0.02a |
| Ala | 1.30 ± 0.00a | 1.26 ± 0.00b | 1.24 ± 0.01c | 1.22 ± 0.00d | 1.20 ± 0.00e | 1.18 ± 0.00e |
| Tyr | 1.32 ± 0.01a | 1.38 ± 0.00b | 1.25 ± 0.02b | 1.22 ± 0.00c | 1.20 ± 0.01c | 1.19 ± 0.00c |
| Arg | 1.95 ± 0.01f | 2.07 ± 0.00e | 2.18 ± 0.02d | 2.37 ± 0.00c | 2.71 ± 0.01b | 3.04 ± 0.02a |
| NEAA | 18.85 ± 0.05b | 18.65 ± 0.00c | 18.63 ± 0.10c | 18.65 ± 0.00c | 19.00 ± 0.00b | 19.42 ± 0.03a |
| FAA | 12.76 ± 0.06a | 12.52 ± 0.00b | 12.45 ± 0.09bc | 12.35 ± 0.01c | 12.39 ± 0.00bc | 12.49 ± 0.01bc |
Note
- Values are mean ± SEM (n = 3). Means with different subscripts are significantly different (p < .05).
- Abbreviations: EAA: Essential amino acids are the sum of Phe, Met, Lys, Ile, Val, Leu and Thr; FAA: Flavour amino acids are the sum of Asp, Glu, Gly and Ala; NEAA: Non-Essential amino acids are the sum of Asp, Ser, Glu, Gly, Ala, Cys, Tyr, His and Arg.
Considering the close relationship between the digestive enzymes and the growth condition of fish (Carneiro et al., 2020), the activity of intestinal digestive enzymes was detected. The results indicated that the activity of intestinal amylase was not significantly different among dietary treatments (p > .05) (Figure 1a). Compared with CPC0 treatment, there was no significant difference in intestinal lipase activity in other treatments (p > .05) (Figure 1b). The activity of intestinal trypsin was markedly decreased compared with CPC0 treatment when the CPC substitution exceeded 45%, including CPC45, CPC75 and CPC100 (p < .05) (Figure 1c).
3.3 Effects of replacing soybean meal protein with cottonseed protein concentrate on the gut of Nile tilapia
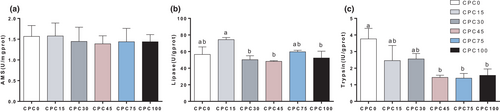
Because the intestine is the main tissue for nutrients absorption, the influence of CPC on the intestinal structure and function was detected. According to the intestinal H&E staining slice, the villi height was decreased when the replacement ratio surpassed 30% (p < .05) (Figure 2a,b). The intestinal permeability was detected in vitro and the results showed that when the replacement ratio reached 75% and above, the intestinal TER decreased significantly (p < .05) (Figure 2d and Table S1). Accordingly, LPS in serum showed a significant increase in the CPC100 treatment compared with other treatments (p < .05) (Figure 2e). The expression level of tight junction protein 1 (zo-1) was not significantly influenced by the replacement (p > .05) (Figure 2f). The mRNA expression of claudin-1 was significantly higher in the CPC100 treatment than that in the CPC0, CPC15 and CPC30 treatments (p < .05). The mRNA expression of occluding-X1 was dramatically increased in CPC75 and CPC100 (p < .05). These findings suggested that higher replacement ratios (75% and 100%) caused the change in the structure and permeability of the intestine.
3.4 Effects of replacing soybean meal protein with cottonseed protein concentrate on the intestinal health of Nile tilapia
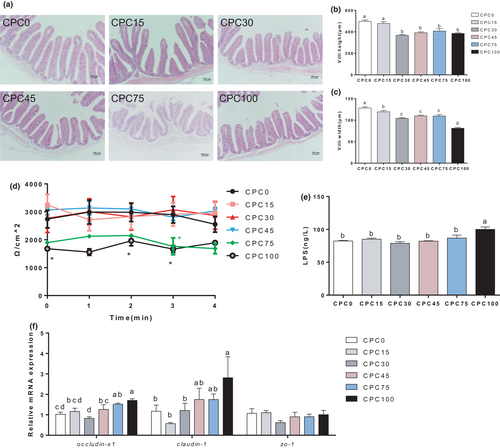
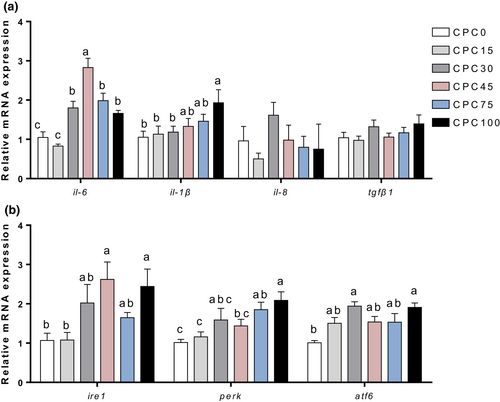
To further identify the inflammatory status of the intestinal tissue, the expression level of genes related to inflammation was tested. As shown in Figure 3a, the mRNA expression of intestinal proinflammatory cytokine gene interleukin-6 (il-6) and interleukin-1β (il-1β) was markedly increased (p < .05) when the replacement ratio exceeded 45% and 75% respectively, but interleukin-8 (il-8) and anti-inflammatory cytokine transforming growth factor-β (tgfβ1) gene expression showed no significant difference among treatments (p > .05). It has been shown that endoplasmic reticulum (ER) stress is involved in the induction of inflammation, so we examined the expression of genes related to the ER stress signalling pathway. The significant increase in mRNA expression of ER stress gene (ire1, perk and atf6) indicated that ER stress occurred in the higher substitution treatments (CPC45, CPC75 and CPC100) (p < .05) (Figure 3b). From these results, we can find that higher substitution (more than 45%) may cause intestinal inflammation and ER stress.
3.5 Effects of gossypol on the intestinal barrier structure of Nile tilapia
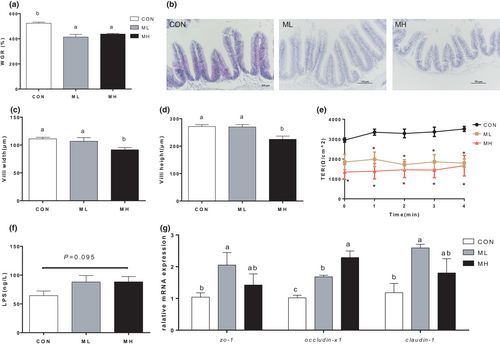
With the increased replacement ratio, the gossypol content in the feed also increased proportionally (Table 2). To identify whether the gossypol residue in CPC was the main cause of the weight loss and the increased intestinal permeability in Nile tilapia, two concentrations (150 and 300 mg/kg) of gossypol were added in the diets and the actual content of the gossypol in each treatment was 125.86 and 302.04 mg/kg. The survival rates of the CON, ML and MH treatments were 93%, 86% and 86% respectively, and no significant difference was found (p > .05). WGR of ML and MH treatments was significantly lower than that of CON treatment (p < .05) (Figure 4a). The width and height of intestinal villi in the MH treatment were significantly higher than those in the CON treatment (p < .05) (Figure 4b-d). Intestinal permeability was also detected in vitro, and the results showed that the TER values of ML treatment and MH treatment were remarkably lower than that of CON treatment (p < .05) (Figure 4e), implying a significant increase in the intestinal permeability in gossypol addition treatments. Serum LPS was also detected, and the result showed an increasing trend with the addition of gossypol (p > .05) (Figure 4f). Relative mRNA expression levels of intestinal barrier gene (occluding-X1, claudin-1 and zo-1) were significantly increased in ML treatment and MH treatment (p < .05) (Figure 4g). These results implied that gossypol in CPC played an important role in causing structural damage to the intestine and increased the intestinal permeability.
4 DISCUSSION
Studies in recent years have shown that cottonseed protein concentrate (CPC) can partially replace traditional protein sources, especially fish meal, but when substitution ratios overpassed 50%, CPC often caused many problems, including lower crude protein (Wang, Clark, et al., 2020), reduced feed efficiency (Liu, Dong, et al., 2020) and higher inflammation (Ye et al., 2020). It has been documented that when CPC replaced 48% FM, the weight gain rate of silver sillago decreased significantly (Liu, Dong, et al., 2020), and the growth of juvenile golden pompano Trachinotus ovatus significantly decreased when the CPC level in the diet exceeded 360 g/kg (Shen et al., 2020). The results of the present study showed that the growth condition and the survival rate of Nile tilapia were not significantly affected by the 30% CPC substitution for SBM protein, but substitution rates of 45% and above significantly affected the weight. At present, there are some possible factors for the negative impact of CPC, including that the gossypol in CPC impacted the palatability (Alford et al., 1996; Anderson et al., 2016) and CPC had an imbalance in amino acid levels (Wilson et al., 1981). In the present study, we used quantitative feeding to feed Nile tilapia to avoid the adverse effect of CPC caused by palatability. Amino acids composition in the diets was detected, and we found that the content of essential amino acids such as Lys, Ile, Leu and Thr differed significantly among the feeding diets (Table 5). However, the decline of these essential amino acids in the CPC15 and CPC30 treatments did not significantly affect the growth parameters. The decrease in these essential amino acids may be the reason for the decrease in growth performance in the higher substitution treatments (CPC45, CPC75 and CPC100). Previous studies have found that the enzymatic activity of digestive enzymes was significantly changed after the use of CPC in juvenile cobia Rachycentron canadum and juvenile crucian carp (Chou et al., 2004; Liu, Han, et al., 2020), suggesting that CPC impacted intestinal digestive enzyme activity (Liu, Dong, et al., 2020). The activity of intestinal trypsin decreased significantly compared with CPC0 treatment when the replacement ratio reached 45% and above, but the activity of intestinal amylase and lipase did not change significantly with increasing CPC substitution.
The intestine is the main site for nutrient absorption, and intestinal villi height is an important indicator for the absorption capacity of aquatic animals (Khosravi et al., 2015; Shi et al., 2019). Changes in villi caused by plant protein sources have been found in other fish species, including gilthead sea bream Sparus aurata L. and largemouth bass Micropterus salmoides (Bonaldo et al., 2008; Li et al., 2020). Our results suggested that excessive substitution ratio (more than 30%) can shorten intestinal villi height in fish and consistently, and we found lipopolysaccharide (LPS) was significantly increased in the serum, suggesting the intestinal barrier is damaged (Dong et al., 2017; Khan et al., 2012). Expression level of genes related to the intestinal tight junction was usually detected to show the intestinal integrity, but the change of these genes was inconsistent when the intestinal barrier was impaired (Barekatain et al., 2019; Humam et al., 2021). For example, it was found that downregulation of tight junction protein expression usually occurs in response to intestinal damage (Kuo et al., 2019; Sharma et al., 2018), but the upregulation of tight junction protein claudin-1 expression was found during the development of enteritis (Gowrikumar et al., 2019; Pope et al., 2014). In the present study, we found the mRNA expression of intestinal barrier gene (occluding-X1 and claudin-1) was significantly upregulated with the increased CPC substitution. To investigate the intestinal barrier, Ussing chamber which is an in vitro system for measuring the permeability on excised tissue segments was used (Sjögren et al., 2016; Ungell et al., 1998). This method provides a clearer picture for the state of the intestinal barrier as lower intestinal transepithelial resistance (TER) values of the intestinal epithelium indicate higher permeability and impaired integrity of the intestinal barrier (Genser et al., 2018). Our data showed that there was a significant increase in the intestinal permeability in both CPC75 and CPC100 treatments compared with CPC0 treatment, suggesting the impaired intestinal barrier in these treatments.
Due to the presence of anti-nutritional factors, some plant protein sources often cause intestinal damage (Barekatain et al., 2019; Hossain et al., 2018; Zhou et al., 2020). The negative impact of the substitution of cottonseed meal was speculated to be attributed to gossypol, which is considered to be the main anti-nutritional factor in cottonseed protein (Yue & Zhou, 2008), but the underlying mechanism has not been studied. In the present study, we found that the addition of gossypol caused significant weight loss and induced the damage to the structure and the function of the intestinal tract, which mirrored the harmful effect of substitution of CPC. It should be noticed that the addition of equivalent gossypol showed less severe damage to the intestine of Nile tilapia, suggesting some other anti-nutritional factors exist in CPC may also account for the damaged intestinal health. Compared with cotton meal or cottonseed meal, CPC has lower gossypol but the content of gossypol in CPC varied in different studies, which may be caused by the different cottonseed and processing technology (Alford et al., 1996; Lee et al., 2006; Rinchard et al., 2003). How to reduce the gossypol residue and other anti-nutritional factors in CPC is still a bottle neck for the wide application of CPC.
In conclusion, this experiment investigated the effect of replacing SBM with CPC on Nile tilapia (Oreochromis niloticus). Less than 30% SBM protein replaced by CPC did not result in a significant decrease in growth condition and intestinal health of Nile tilapia. However, high CPC replacement level diet (above 45%) caused significant weight loss, intestinal damage, inflammation and endoplasmic reticulum stress and gossypol played a crucial role in causing these intestinal barrier damage.
ACKNOWLEDGEMENTS
This work was supported by the National Key R&D programme (grant number 2019YFD0900200), and the National Natural Science Foundation of China (grant number 31972798).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




