Use of rice protein concentrates in Oreochromis niloticus diets and its effect on growth, intestinal morphology, biochemical indices and ghrelin gene expression
Funding information
This work was financially supported by grant from Executive Program of the agreement on scientific cooperation between the Academy of Scientific Research and Technology (ASRT) and the National Research Council of Italy (CNR)
Abstract
Fish meal (FM) is a significant source of protein, but the high cost has limited its use in feeds. Hence, alternative, economic and available FM substitutes should be found. In this study, the consequences of replacing FM with rice protein concentrate (RPC) on the growth parameters, proximate body composition, digestive and absorptive capabilities, biochemical parameters and the expression of appetite-related gene (ghrelin) in Nile tilapia, Oreochromis niloticus, were investigated. Fish (27.8 ± 0.1 g) were randomly organized into four groups and fed on experimental diets having four replacement percentages of FM with RPC: 0%, 25%, 50% and 75% (RPC0, RPC25, RPC50 and RPC75) for 5 months. The results cleared that RPC25 group exhibited linearly and quadratically the highest values for growth parameters, growth hormone level, and activities of protease, amylase as well as proximal intestinal villus length, width, the goblet cells count and ghrelin gene expression among all groups. From overall results, RPC could be used as an economically and beneficial ingredient to replace up to 25% of dietary FM in Nile tilapia diet with enhancing the growth performance, digestive and absorptive capability, and health status, but with the supplementation of synthetic amino acids.
1 INTRODUCTION
Globally, the aquaculture industry has rapidly flourished during last years to meet high demand of human consumption and reduce poverty. As a result of growth in aquaculture sector, the production of aquafeeds increased. The substantial targets of fish industry are to improve nutrient digestibility, growth, survival and lowering feed cost ( Abdel Rahman et al., 2019; Alagawany et al., 2021; El-Saadony et al., 2021; FAO, 2020). Fish survival, growth and flesh quality mainly depend on feeds quality that were introduced. However, the increasing cost of feed constituents is a major problem facing fish industry, where fish feeds resemble about 65–70% of the fish culture cost (Ogueji et al., 2020; Soliman et al., 2018).
The fundamental source of protein in commercial feeds is fish meal (FM) owing to its content of high-quality protein and essential amino acid, but its high price limited its uses in feeds (Tacon & Metian, 2008). Hence, alternative FM substitutes should be found with many characteristics as economic and available with good digestibility and high protein content of balanced amino acid (Sánchez-Lozano et al., 2009).
Numerous researches have been conducted on a different fish species by using a wide variety of FM substitutes at different percentages, such as vegetable ingredients (wheat and corn gluten, rice, soybean, sunflower and pea) (Hernandez et al., 2021; Nasr et al., 2021; Reda et al., 2021) and animal meal (blood meal, bone meal, meat meal and poultry meal) (Li et al., 2021; Twahirwa et al., 2021). However, the search for different high-quality FM alternatives in fish diets is still needed. Plant protein meals have devoted a worthy attention as a result of low price, wide availability and safety to human consumption (Daniel, 2018; Hua et al., 2019). Rice protein concentrate (RPC) is one candidate of the plant protein sources that has received considerable interest because of its relatively high protein and lipid contents and appropriate amino acid profile than many other plants (Oujifard et al., 2015). Previously, RPC was used as a replacement of FM in different aquatic species such as gilthead seabream, Sparus aurata (Sánchez-Lozano et al., 2009), white shrimp, Litopenaeus vannamei (Oujifard et al., 2012 and Oujifard et al., 2015), Chinese soft-shelled turtle, Pelodiscus sinensis (Sun et al., 2018), and blunt snout bream, Megalobrama amblycephala (Abasubong et al., 2019).
A freshwater fish species, Nile tilapia, Oreochromis niloticus (L.), is one of the most important species around the world. The culture of Nile tilapia has increased significantly in recent years due to many factors belonging to this fish species such as its ability to consume different protein sources, easy culture, adapt different environmental conditions and resist variable diseases with its high palatability for consumer (El-Sayed, 2019).
There are few reports on using different levels of RPC as FM replacer on Nile tilapia diets. Data regarding the use of RPC in Oreochromis niloticus diets and its effect on ghrelin gene expression, appetite and intestinal morphometric parameters are scarce. Consequently, the current study was performed to assess the effects of partial FM replacement by RPC on growth rate, digestive and absorptive capacity as well as general health status of O. niloticus.
2 MATERIALS AND METHODS
2.1 Animal ethics
This study was conducted under approval of Animal Research Ethics Committee of Zagazig University, Egypt (permit number: ZU-IACUC/2/F/46/2021). In experimental procedures, the NIH-approved ethical guidelines for the treatment and use of laboratory animals in scientific investigations were followed.
2.2 Diet preparations and analysis
Four isonitrogenous and isoenergetic diets were formulated according to the National Research Council (NRC, 2011) to fulfil the nutrient requirements of O. niloticus (El-Sayed & Teshima, 1992) as shown in Table 1. The basal diet as control (RPC0) consisted of 20% FM and another plant protein sources such as soybean meal, corn grain, wheat and wheat bran. In the other three experimental diets, FM was gradually replaced with RPC at 25, 50 and 75% (RPC25, RPC50 and RPC75) replacement levels with addition of synthetic amino acids, such as methionine and lysine and dicalcium phosphate to maintain constant levels as basal diet. The chemical analysis of the RPC is illustrated in Table 2 according to AOAC (2000). Amino acids were determined using an automated amino acid analyser after hydrolysing the samples with 6 M HCl at 110°C for 24 h, and the sulphur amino acids (Met+Cys) were oxidized using performic acid before the acid hydrolysis (Bassler & Buchholz, 1993).
| Ingredient (g/kg) | Rice protein concentrate (RPC) levels as substitute for fishmeal (FM) | |||
|---|---|---|---|---|
| RPC0 | RPC25 | RPC50 | RPC75 | |
| Fish meal | 200 | 150 | 100 | 50.0 |
| Soybean meal | 319 | 319 | 316.5 | 310 |
| Rice protein concentrate | 0.00 | 50 | 100 | 150 |
| Corn | 206 | 206 | 200 | 197 |
| Wheat bran | 90.0 | 90 | 90 | 90 |
| Wheat | 100 | 100 | 100 | 100 |
| Fish oil | 70.0 | 70.0 | 70.0 | 70.0 |
| Di-calcium P | 0.00 | 0.00 | 3.50 | 9.50 |
| Limestone | 0.00 | 0.00 | 3.50 | 6.00 |
| Lysine | 0.00 | 0.00 | 2.00 | 3.00 |
| Methionine | 5.00 | 4.60 | 4.50 | 4.50 |
| Premix1 | 10.00 | 10.00 | 10.00 | 10.00 |
| Calculated composition (%)2 | ||||
| Crude protein | 315.7 | 315.9 | 315 | 314.2 |
| Crude fiber | 43.6 | 43.1 | 42.1 | 41 |
| NFE3 | 400.7 | 399.5 | 398.5 | 392 |
| Crude lipids | 99.1 | 101.1 | 103.1 | 104.8 |
| Calcium | 8.90 | 8.90 | 9.20 | 11.60 |
| Available phosphorus | 8.20 | 8.20 | 8.60 | 9.70 |
| DE (kcal/kg) 4 | 2909 | 2923 | 2933 | 2928 |
| Amino acids profile (%)5 | ||||
| Lysine | 18.9 | 18.8 | 18.6 | 18.9 |
| Methionine | 8.00 | 8.00 | 7.90 | 7.80 |
| Cysteine | 4.20 | 4.10 | 4.30 | 4.30 |
| Threonine | 12.3 | 12.4 | 12.4 | 12.5 |
| Arginine | 22.6 | 22.9 | 23 | 23 |
| Hisitidine | 7.40 | 7.80 | 8.00 | 8.20 |
| Isoleucine | 13.3 | 14 | 14.3 | 14.5 |
| Leucine | 24.3 | 25.2 | 25.6 | 26.1 |
| Tyrosine | 10.9 | 11 | 11.1 | 11.3 |
| Tryptophan | 3.90 | 4.20 | 4.30 | 4.40 |
| Valine | 16.0 | 16.9 | 17.3 | 17.6 |
- RPC0 (control group) = fish fed normal base diet. RPC25, RPC50 and RPC75= fish fed diet, with fishmeal replaced by 25, 50, and 75% RPC, respectively.
- 1 Premix: each 1kg of premix contain: vit A 550000 IU, vit D 110000 IU, vit E 11000 mg, vit K 484 mg, vit C 50 gm, vit B1 440 mg, vit B2 660 mg, vit B3 132oo mg, vit B5 1100 mg, vit B6 1045 mg, vit B9 55 mg, Choline 110000 mg, Biotin 6.6 mg, iron 6.6 gm, copper 330 mg, Mn 1320 mg, Zn 6.6 gm, Se 44 mg, iodine 110 mg.
- 2 All compositions calculated according to NRC (2011).
- 3 NEF "Nitrogen free extract" = 100 − (crude protein + crude lipids + ash + crude fiber).
- 4 Digestible energy (DE) was calculated by applying the coefficient of 0.75 to convert gross energy to digestible energy according to Hepher et al. (1983).
- 5 Analyzed according to Bassler and Buchholz (1993).
| Item | (g/kg) |
|---|---|
| Crude protein | 670 |
| Ether extract | 45 |
| Ash | 25 |
| Moisture | 80 |
| Organic matter | 975 |
| Dry matter | 920 |
| Amino acid profile2 | |
| Lysine | 22 |
| Methionine | 20 |
| Cysteine | 24.4 |
| Threonine | 22.7 |
| Arginine | 57.3 |
| Hisitidine | 17.4 |
| Isoleucine | 27.4 |
| Leucine | 52.8 |
| Phenylalanine | 36.5 |
| Tyrosine | 36.5 |
| Glycine | 29.8 |
| Valine | 45.3 |
| Aspartic acid | 58.2 |
| Serine | 28.2 |
| Alanine | 44.5 |
| Proline | 30.5 |
| Glutamic acid | 126.6 |
- 1 According to AOAC (2000).
- 2 Analyzed according to Bassler and Buchholz (1993).
2.3 Fish rearing and experimental protocol
Fingerlings of Nile tilapia with an average body weight of 27.8 ± 0.1 g were provided from a local fish hatchery (El-Abbassa, Sharki a Governorate, Egypt). Fish were acclimated by feeding a basal diet before the trial for two weeks. External investigations were done for random samples of fish to ensure they were apparently healthy according to Austin and Austin (2007). A total of 240 fish were distributed into 12 hapas (20 fish per hapa) which fixed in a cement pond (1.0 × 0.5 × 0.5 m each, replicates). The ponds were provided with water running regime and were in an outdoor system (12-h light: 12-h darkness). During the experimental period (5 months), fish were fed the respective test diets up to satiation two times daily (8:00 and 16:00). Every two weeks, fish weight was measured and the feed intake was recalculated accordingly. The water quality parameters (water temperature, dissolved oxygen and pH) were monitored regularly using a portable multiparameter meter (YSI, China). For measuring total ammonia and nitrite concentrations, commercial kits (La Chappelle, France) were used. The average water parameters were water temperature: 23.3 ± 0.51°C and dissolved oxygen: 6.78 ± 0.02 mg/L, while the other characteristics were 7.12 ± 0.11 for pH, 0.03 ± 0.003 and 0.017 ± 0.001 mg/L for ammonia and nitrite respectively.
2.4 Survival percentage and growth indices
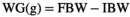
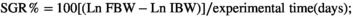
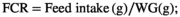
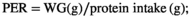
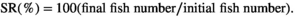
2.5 Sampling
After 5 months of trial, the fish were starved for 24 h before sampling. After that, the fish were anaesthetized using benzocaine solution (80 mg/L) according to Gontijo et al. (2003) to avoid stress during sampling. The blood samples without anticoagulant were collected (three fish / hapa) and left to clot for 2 h. Serum samples were separated by centrifugation for 15 min at 2146.56×g and stored at −20°C until use for biochemical analysis. The intestine was dissected out; then, the proximal part of intestine was taken and rinsed in Bouin's solution for histology sections. Moreover, three fish were taken from each hapa and kept at −20°C for the fish body composition analysis. Another three fish per hapa were collected, and the gastric tissues were taken and washed with ice-cold phosphate buffered saline and stored at −80°C until be used for determination of ghrelin gene expression. Also, intestinal tissues were collected and kept in liquid nitrogen for digestive enzymes analysis.
2.6 Proximate whole-body composition analysis
The chemical composition (moisture, ash, crude lipids and crude protein) of whole fish body was determined according to standard method (AOAC, 2007). The moisture content was estimated by complete drying of samples in a drying oven (GCA, model 18EM, Chicago, IL, USA) to attain the constant weight. The total ash was determined by burning samples at a muffle furnace (Thermolyne Corporation) for 6 h at 550°C, while the crude lipids were estimated by ether extraction using Soxhlet apparatus (FOSS, Hoganas). Finally, crude protein (N × 6.25) was determined using Kjeltec (FOSS 2300, Hoganas) by a Kjeldahl method.
2.7 Digestive enzymes assays
The whole intestine was carefully weighed and homogenized in a tissue homogenizer in an ice bath with addition of 10 volumes (v/w) of chilled saline. Then, the extract was centrifuged at 3500 ×g, 10 min, 4°C. The activity of protease, amylase and lipase was evaluated according methods of Jiang (1982), Worthington (1993) and Jin (1995) respectively.
2.8 Intestinal morphometric measures
The proximal part of intestine was fixed in 10% buffered neutral formalin and processed according to standard histological methods (Survarna et al., 2012). Tissue sections (5 μm thick) were prepared and stained with haematoxylin and eosin stain (H&E). Slides were examined by Image J software version 1.45 (National Institutes of Health) for morphometric analysis (villus height and width and Goblet cells number) according to Pirarat et al. (2015) and Abdel Rahman, Hassanin, et al. (2019).
2.9 Biochemical assays
Serum growth hormone evaluation was done using fish growth hormone ELISA kit (MyBiosource Co.) CAT. NO. MBS044656 according to a described method of Lugo et al. (2008). Levels of aspartate and alanine aminotransferases (AST and ALT), urea and creatinine were estimated in fish serum by using diagnostic test kits (Biodiagnostic Co.) CAT. NO. AS 10 61 (45), AL 10 31 (45), UR 21 10 and CR 12 50 according to previously described methods (Chaney & Marbach, 1962; Perakis & Wolff, 1984; Reitman & Frankel, 1957) respectively.
2.10 The total RNA extraction and appetite-related gene (ghrelin) expression determination
Total RNA was extracted from the gastric tissue (three samples/ replicate; nine samples/ group) of each groups using RNA Purification Kit CAT. NO. 74004 according to the manufacturer's instructions (Qiagen). The extracted RNA was used to produce complementary DNA (cDNA) using Quantitect® Reverse Transcription kit CAT. NO. 205311 following the manufacturer's protocol (Qiagen). Specific primers (Table 3) of β-actin as a reference gene and ghrelin gene (Qiang et al., 2014; Zou et al., 2017 respectively) were used to perform Quantitative real-time polymerase chain reaction (qPCR) analysis with SYBR green PCR master mix (StepOnePlus, Applied Biosystem). The amplification curves and CT values were done by using the stratagene MX3005P software. Gene expression results were analysed using the 2−ΔΔCt method (Yuan et al., 2006). Data are represented as the mean fold changes ± standard error, which was calculated by comparison of the gene expression level (normalized to the reference gene β-actin) between the control group (RPC0) and experimental groups.
| Target gene | Primers sequences | Reverse transcription | Primary denaturation | Amplification (40 cycles) | Dissociation curve (1 cycle) | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Secondary denaturation | Annealing (Optics on) | Extension | Secondary denaturation | Annealing | Final denaturation | |||||
| β-actin (EU887951) | CCACACAGTGCCCATCTACGA CCACGCTCTGTCAGGATCTTCA |
50˚C 30 min. |
94˚C 5 min. |
94˚C 15 sec. |
62˚C 30 sec. |
72˚C 30 sec. |
94˚C 1 min. |
62˚C 1 min. |
94˚C 1 min. |
Qiang et al. 2014 |
| Ghrelin (AB104859) | GCAGAAGACTTGGCGGACTACAT ATAAACCAGAAAGAAGGGACAACC |
50˚C 30 min. |
94˚C 5 min. |
94˚C 15 sec. |
59˚C 30 sec. |
72˚C 30 sec. |
94˚C 1 min. |
59˚C 1 min. |
94˚C 1 min. |
Zou et al. 2017 |
2.10.1 Statistical analysis
All data were checked for normality after transformation (ASIN). Based on polynomial orthogonal contrasts, the ANOVA (Analysis of Variance) test was utilized. Linear and quadratic regression equations were performed using SPSS (Statistical Package for Social Sciences) Version 16 for Windows (SPSS Inc.) on growth, body composition, digestive enzymes, intestinal morphometric and biochemical parameters as well as ghrelin gene expression to evaluate the relationship between the graded RPC levels and these parameters at a significance value of p < .05. Post hoc Tukey's test was employed to see whether there were any differences between the means and all statistical significance statements are based on p < .05. The results were expressed as mean ± standard error (SE).
3 RESULTS
3.1 Growth and whole-body composition
Fish in all groups accepted all diets with a 100% SR during the 5 months of feeding. The RPC25 diet linearly and quadratically elevated the FBW (p < .001), WG (p < .001), FI (p < .001), SGR (p < .001) and PER (p < .001) and decreased FCR (p < .001), respectively, of fish when comparing to that of the control group (RPC0) as was presented in Table 4. Replacement of FM with RPC50 did not affect (p > .05) these parameters when compared to RPC0; however, linearly and quadratically decreases in FBW, WG, FI, SGR and PER and increase in FCR were observed in RPC75.
| Parameters | Regression analysis1 | Experimental groups | ||||
|---|---|---|---|---|---|---|
| RPC0 | RPC25 | RPC50 | RPC75 | Linear | Quadratic | |
| Growth performance | ||||||
| IBW (g) | 27.84 ± 0.13 | 27.89 ± 0.23 | 27.77 ± 0.18 | 27.66 ± 0.44 | 0.59 | 0.79 |
| FBW (g) | 208.33 ± 0.45 b | 214.05 ± 0.43 a | 207.50 ± 0.83 b | 189.93 ± 2.49 c | <0.001 | <0.001 |
| WG (g) | 180.48 ± 0.48 b | 186.16 ± 0.64 a | 179.74 ± 0.84 b | 162.27 ± 2.09 c | <0.001 | <0.001 |
| SGR (%) | 1.34 ± 0.006 b | 1.36 ± 0.001 a | 1.34 ± 0.006 b | 1.28 ± 0.003 c | <0.001 | <0.001 |
| FI (g day–1 fish–1) | 2.33 ± 0.003 b | 2.39 ± 0.001 a | 2.32 ± 0.01 b | 2.15 ± 0.03 c | <0.001 | <0.001 |
| FCR (g feed/ g gain) | 1.94 ± 0.003 b | 1.92 ± 0.006 c | 1.94 ± 0.006 b | 1.99 ± 0.006 a | <0.001 | <0.001 |
| PER (gain/protein intake) | 5.72 ± 0.02 b | 5.89 ± 0.02 a | 5.71 ± 0.03 b | 5.17 ± 0.06 c | <0.001 | <0.001 |
- RPC0 (control group) = fish fed normal base diet. RPC25, RPC50 and RPC75= fish fed diet, with fishmeal replaced by 25, 50, and 75% RPC, respectively.
- IBW, initial body weight; FBW, final body weight; WG, weight gain; SGR, specific growth rate; FI, feed intake; FCR, feed conversion ratio; PER, protein efficiency ratio.
- 1 The regressions were considered significant at p < 0.05. Values are mean ± SE.
- a-c Means in the same row within each classification bearing different letters are significantly (p < 0.05) different.
Concerning the whole-body composition (Table 5), the results highlighted that the moisture, ash, dry matter, crude lipid and protein contents in the fish body in RPC25 to RPC75 did not significantly alter (p > .05) from RPC0.
| Parameters | Experimental groups | Regression Analysis1 | ||||
|---|---|---|---|---|---|---|
| RPC0 | RPC25 | RPC50 | RPC75 | Linear | Quadratic | |
| Whole body composition % (on the wet weight) | ||||||
| Moisture | 74.55 ± 0.51 | 75.15 ± 0.50 | 73.65 ± 1.22 | 74.38 ± 0.46 | 0.56 | 0.93 |
| Dry matter | 25.45 ± 0.51 | 24.85 ± 0.50 | 26.35 ± 1.22 | 25.61 ± 0.46 | 0.57 | 0.93 |
| Ash | 5.62 ± 0.07 | 5.65 ± 0.34 | 5.53 ± 0.32 | 5.29 ± 0.11 | 0.33 | 0.59 |
| Crude lipids | 3.68 ± 0.33 | 3.31 ± 0.22 | 3.42 ± 0.23 | 3.46 ± 0.52 | 0.73 | 0.58 |
| Crude protein | 11.55 ± 0.21 | 11.69 ± 2.46 | 12.08 ± 0.55 | 11.45 ± 0.81 | 0.98 | 0.77 |
- RPC0 (control group) = fish fed normal base diet. RPC25, RPC50 and RPC75= fish fed diet, with fishmeal replaced by 25, 50, and 75% RPC, respectively.
- 1 The regressions were considered significant at p < 0.05. Values are mean ± SE.
3.2 Intestinal enzymes activities
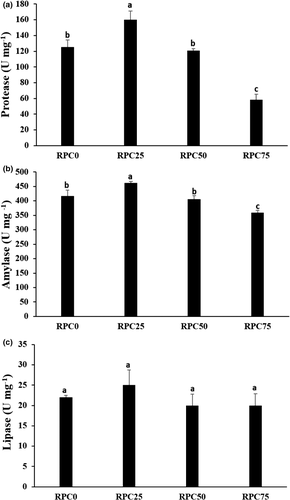
The activities of digestive enzymes of Nile tilapia fed on RPC diets for 5 months were presented in Figure 1. The fish fed RPC25 diet displayed linear and quadratic higher activity of protease (p < .001) and amylase enzymes (p = .004 and p = .007), respectively, than RPC0. There were no significant differences (p > .05) in these parameters in RPC50 group and RPC0. Compared with RPC0, the activity of these enzymes linearly and quadratically decreased in RPC75 group. Replacement of FM with RPC did not affect lipase enzyme (p > .05) neither linearly (p = .08) nor quadratically (p = .56), when compared to RPC0.
3.3 Intestinal morphometric measures and histological findings
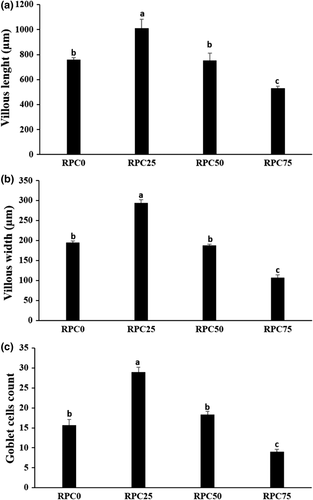
As investigated in Figure 2, linear and quadratic augmentations were detected in the intestinal villous length (p = .002 and p = .001) and width (p < .001) as well as goblet cells count (p < .001), respectively, of fish fed RPC25 diet compared with RPC0. However, replacement of FM with RPC75 induced linear and quadratic decline in these indices. There were no significant differences (p > .05) in these parameters in RPC50 group and RPC0.
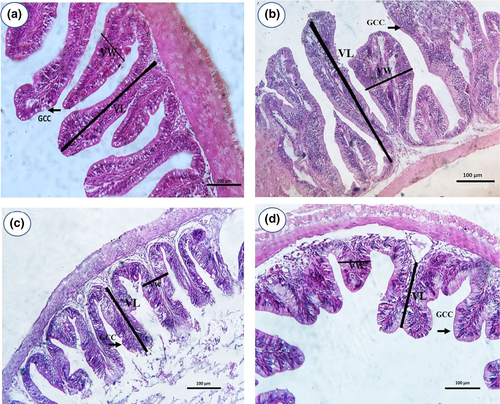
The RPC0 group had tall and isolated villi with intact enterocytes and a mild goblet cell metaplasia with intact crypts, as shown in Figure 3. Furthermore, the most branched and tallest villi were found to have abundant large tips and a pronounced metaplasia of goblet cells were observed in the RPC25 group; meanwhile, thin and tall villi with a narrow lumen with a moderate goblet cells metaplasia in the RPC50 were seen. On the contrary, short and thick villi with a villus fusion in lumen and low degree of goblet cells metaplasia were noticed in the RPC75 group. No pathological alterations were detected in all RPC groups.
3.4 Serum biochemical indices
As shown in Table 6, linear and quadratic elevations in the growth hormone (p = .003 and p = .02), respectively, of fish fed RPC25 diet compared with RPC0 were detected, but it declined in RPC75 group followed by RPC50. The results of ALT and AST did not differ (p > .05) in RPC25 and RPC50 groups compared to the control. A linear decrease (p = .01) in ALT activity and linear and quadratic decreases (p = .007 and p = .001), respectively, of RPC75 group relative to that of control (RPC0). Serum levels of urea and creatinine were not linearly or quadratically affected (p > .05) by RPC inclusion (Table 6).
| Parameters | Experimental groups | Regression Analysis1 | ||||
|---|---|---|---|---|---|---|
| RPC0 | RPC25 | RPC50 | RPC75 | Quadratic | Linear | |
| GH (ng/mL) | 442±7.21 ab | 467 ± 21.88 a | 421 ± 4.17 b | 382 ± 1.53 c | 0.02 | 0.003 |
| ALT (U/L) | 9.93±1.51 a | 10.63 ± 1.52 a | 8.92 ± 0.24 a | 4.98 ± 0.94 b | 0.08 | 0.010 |
| AST (U/L) | 10.97±0.63 a | 11.92 ± 1.05 a | 9.99 ± 0.09 a | 6.31 ±0.36 b | 0.007 | 0.001 |
| Urea (mg/dL) | 0.93±0.18 | 0.82 ± 0.02 | 0.79 ± 0.06 | 0.76 ± 0.08 | 0.58 | 0.28 |
| Creatinine (mg/dL) | 4.87±1.14 | 4.47 ± 0.46 | 4.42 ± 0.61 | 4.34 ± 0.07 | 0.71 | 0.67 |
- RPC0 (control group) = fish fed normal base diet. RPC25, RPC50 and RPC75= fish fed diet, with fishmeal replaced by 25, 50, and 75% RPC, respectively.
- GH, growth hormone; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
- 1 The regressions were considered significant at p < 0.05. Values are mean ± SE.
- a-c Means in the same row within each classification bearing different letters are significantly (p < 0.05) different.
3.5 Relative expression of ghrelin gene
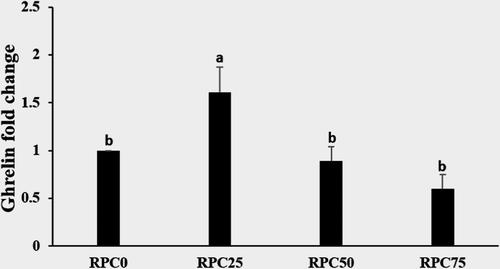
The quantitative expression of gastric ghrelin gene was presented in Figure 4, where the highest linear and quadratic expression value was observed in RPC25 group (1.61-fold, p = .004 and p = .003), respectively, among all groups. Compared with RPC0, there were no significant differences (p > .05) in the expression value of ghrelin of RPC50 (0.89-fold) and RPC75 groups (0.60-fold).
4 DISCUSSION
Selection of feed ingredients, taking into account the nutritional value and cost for dietary formulation, is a vital factor for fish growth and survival in aquaculture industry (Mahmoud et al., 2021; Sharif et al., 2021). In this concern, this work was planned to evaluate the growth rate, digestive and absorptive capacity Nile tilapia in response to different RPC diets as a partial replacement to FM.
In the present study, the fish fed a diet partially replacing up to 25% of FM with RPC revealed a marked elevation in growth parameters. This may be due to the role of RPC25 diet in augmenting the gut health by improving the intestinal digestive enzymes and absorptive surface that was noticed in our results. Also, RPC25 diet induced elevation in growth hormone, the latter is a one member of prolactin and somatostatin family that is produced by the somatotropic cells of pituitary gland and plays a vital role in growth of animals (Velloso, 2008).
However, a lowering trend occurred in growth indices including growth hormone at 75% RPC substitution level and this may return back to the decreased feed intake and digestibility of RPC than FM as reported by Oujifard et al. (2012). One important factor to determine feed utilization is feed digestibility because it reflects food absorption from intestinal tract of animal. The animal protein sources are more easily digested than that of plant sources (Akiyama et al., 1989). Furthermore, high inclusion level of RPC could inhibit the digestive enzymes; hence, fish did not utilize RPC (Gai et al., 2005) and this was confirmed in our study. In this concern, it was suggested that for obtaining better performance, a lower FM substitution by a mixture of plant protein should take in consideration (Sitjà-Bobadilla et al., 2005).
Similarly, it was reported that RPC could replace dietary total protein up to 20% in rainbow trout, 21% in blackspot seabream, Pagellus bogaraveo, and 50% in Pacific white shrimp without any adverse effects on growth and the latter decreased with increasing substitution level (Oujifard et al., 2012; Palmegiano et al., 2006, 2007) respectively. Khan et al. (2013) suggested that more than 20% of rice polish in Nile tilapia diet could replace FM with marked changes in growth.
The results of this study regarding the whole-body composition were relatively similar among all groups, elucidating that all substitution percentages of FM by RPC had no marked impact on body composition of Nile tilapia. It was compatible with the findings recorded in blunt snout bream (Abasubong et al., 2019) and Chinese soft-shelled turtle (Sun et al., 2018).
Investigation of digestive enzymes profile gives an index to the digestive capacity and metabolic functionality of the intestinal tract that influenced by feed components (Daprà et al., 2009). In this study, the digestive enzyme activity (protease and amylase) displayed a marked increase in 25% replacement level, highlighting enhanced secretion of digestive enzymes and significant increase in growth rate. This may be explained by activation of compensatory mechanism in response to anti-nutritional factors that found in plant sources (Haard et al., 1996). A similar stimulatory effect had been elucidated in blackspot seabream by 35% RPC concentrate diet (Caruso et al., 2005; Daprà et al., 2009).
On the other hand, a decreased level of these enzymes with 75% replacement level was obtained, indicating alterations in proteins and carbohydrate digestion. The unfavourable impact of RPC50 and RPC75 diets on these enzymes may be returned back to the increased amount of anti-nutritional factors at high RPC level as protease inhibitors that may decrease the secretion of digestive enzymes and consequently reduce digestibility and growth (Ghosh & Mandal, 2015). Significant reduction in protease and amylase activity in intestinal content of blunt snout bream (Abasubong et al., 2019) and turtle (Sun et al., 2018) fed a RPC concentrate diet had been reported. Unlikely, no marked difference was found in lipase activity in all treatments, proposing efficient utilization of lipids by fish without adverse impact.
Studying the intestinal morphometric indices highlights the vital role of fish intestine in feed utilization and absorption (Abdel Rahman, Hassanin, et al., 2019). Modification in fish intestinal morphology that are induced by plant proteins is a decisive in the evaluating the prospect value of dietary ingredient. In addition to that, villous length is worthy histological factor that can be evaluated in experiments concerning the fishmeal substitution to assess different commercial diets (Tran-Ngoc et al., 2019). Replacing 25% of FM with RPC revealed an increase in villus length and goblet cells count in our study without any pathological alterations recorded. These findings giving reason for enhanced integrity of intestinal brush border, feed absorption and utilization and consequently reflected on growth rate. In contrast, these parameters were diminished in 75% replacement level, suggesting declined intestinal function of Nile tilapia. This finding may be attributed to increasing anti-nutritional factors in RPC diets or decreased nutrient digestibility that led to a lowering in villi length and width. Cai et al. (2018) and Abasubong et al. (2019) assured our results that RPC concentrate at 100% replacement level induced reduction in the intestinal morphometric parameters of blunt snout bream.
Aminotransferases are enzymes that catalyse the transfer of nitrogen between amino acids and their corresponding oxoacids involving in both protein metabolism and gluconeogenesis (Lu et al., 2014; Vroon & Israili, 1990). Also, their high levels are used as diagnostic indicators of liver diseases (Abdel Rahman et al., 2020; Kew, 2000). Regarding ALT and AST activity, our results showed no significant difference in their levels in RPC25 and RPC50 groups and control indicating normal liver structure, but there was a non-significant increase in RPC25 group. The low activation level of ALT and AST may be in response to the boosted utilization of dietary protein and amino acids catabolism as reported by Lin and Li (2011) to support fast growth as observed in the results of this study. In this regard, Sun et al. (2018) reported a marked increase in ALT and AST levels of fish fed RPC18% and squid paste replacing FM in P. sinensis.
Our study on fish fed a diet partially replacing up to 75% of FM with RPC revealed no significant effects on serum levels of urea and creatinine, indicating RPC had no adverse impact of kidney functions owing to relatively low levels of anti-nutrients in RPC as comparable other plant proteins sources (Oujifard et al., 2012). Other researches on different FM replacers in Nile tilapia (Metwalli, 2013; Yones & Metwalli, 2015) confirmed these results.
The appetite stimulating factor, namely ghrelin, has a boosting impact on fish feed intake (Zou et al., 2017). Our results revealed that dietary RPC at 25% substitution level markedly augmented gastric ghrelin gene expression, highlighting accelerated expression level and enhanced fish palatability that led to marked increase in fed intake and growth, but the mechanism needs further investigation. These finding may be attributed to the relatively higher protein content of RPC25 diet than control diet that induced an increasing in ghrelin level. In this regard, Babaei et al. (2017) reported high expression of preproghrelin of sea bream fed high protein diets. Also, Sissener et al. (2013) noticed a minor effect on ghrelin expression of Atlantic salmon, Salmo salar, fed high levels of plant protein and vegetable oil.
5 CONCLUSION
The current study confirms that RPC can be integrated up to 25% in traditional tilapia realistic diets to replace FM with enhancing the growth performance, nutrients utilization, intestinal digestive and absorptive capabilities. This study also recommends the feasibility of using RPC as an alternative and cheaper protein source in the Nile tilapia feed formulations to substitute FM, but with the supplementation of synthetic amino acids.
ACKNOWLEDGEMENT
This work was financially supported by grant from Executive Program of the agreement on scientific cooperation between the Academy of Scientific Research and Technology (ASRT) and the National Research Council of Italy (CNR).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data are published in the cited literature and reported in the text of this manuscript.




