Effects of dietary Bacillus subtilis DSM 32315 supplementation on the growth, immunity and intestinal morphology, microbiota and inflammatory response of juvenile largemouth bass Micropterus salmoides
Rui-Yu Du and Hai-Qi Zhang contributed equally to this work.
Abstract
This study aimed at evaluating the effects of dietary inclusion of Bacillus subtilis DSM 32315 on the growth, immunity, intestinal architecture and gut microbiota of juvenile largemouth bass Micropterus salmoides (LMB). B. subtilis DSM 32315 was supplemented in the diets at the levels of 0 (control, CON), 1.5 (BS1.5, 3 × 106 CFU/g feed) and 3 g/kg (BS3, 6 × 106 CFU/g feed). Each diet was assigned to four replicate tanks of LMB juveniles (initial mean body weight, 8.32 ± 0.06 g) with 20 fish per tank for 9 weeks. The results showed that the growth and feed utilization of LMB were not sensitive to dietary modifications (p > .05). Compared with the CON fish, dietary BS supplementation did not affect the haematological parameters (white and red blood cell counts and haematocrit value) of LMB (p > .05), but the BS1.5 fish performed better than the BS3 fish. Serum innate immunity indicators (lysozyme activity and complement C3 and C4 levels) were improved with dietary BS supplementation at both doses, while higher serum IgM level was only recorded at 1.5 g/kg feed (p < .05). All the sampled fish exhibited intact intestines, but the intestinal architecture (villus height and muscular layer thickness) still benefited from dietary BS inclusion at both doses (p < .05). Although intestinal anti-inflammatory response (mRNA levels of IL-10 and TGF-β1) did not differ between the BS supplemented fish (p > .05), the BS1.5 fish obtained superior pro-inflammatory response (mRNA levels of IL-1β, IL-8, and TNF-α) over the BS3 fish (p < .05). Compared with the CON fish, the abundance of the harmful Plesiomonas shigelloides decreased in the gut microbiota of the BS1.5 fish (p < .05). Despite the BS3 fish obtained higher diversity of gut microbiota, the abundances of potential beneficial (Cetobacterium somerae and Shewanella xiamenensis) and harmful bacteria (Acinetobacter johnsonii and P. shigelloides) increased or decreased simultaneously (p < .05). Taken together, it was concluded that dietary BS DSM 32315 inclusion presented some positive effects on LMB. Although the BS1.5 fish performed better than the BS3 fish, the best supplemental dose need to be further studied.
1 INTRODUCTION
Aquaculture is the fastest growing food-producing sector to provide animal protein for human consumption (FAO, 2018). The current tendency in China is towards a banning on the use of antibiotics as growth promoters in animal production, due to the fact that excessive application of antibiotics has led to the development of resistant bacteria, elimination of animal immune system, environmental hazards and food safety problems (Ai et al., 2011; Cabello et al., 2013; Done et al., 2015). In this context, significant efforts have been devoted to find functional feed additives as alternatives for antibiotics in aquaculture industry. Probiotics are one of the feed additives under investigation for this purpose to improve disease resistance and growth of aquatic species (Wang et al., 2019). Bacillus species are one of the most widely used probiotics in aquaculture (Merrifield et al., 2010), as the spore-forming ability endows them greater viability after pelleting and high resistance to gastric conditions (He et al., 2013; Liu et al., 2017). In the last two decades, it has been well documented that dietary Bacillus spp. supplementation benefits the digestive function, immune response, gut microbiota and disease resistance as well as the quality of rearing water thus improving production performance and feed utilization of fish (Ai et al., 2011; Cha et al., 2013; Zaineldin et al., 2018). The beneficial effects of dietary Bacillus spp. administration are markedly dose and strain dependent in fish. For instance, Sun et al. (2016) found that dietary inclusion of B. subtilis B115 at 2 × 107 and 2 × 108 CFU/g feed improved growth and feed utilization of juvenile blunt snout bream Megalobrama amblycephala, but excessive supply of this strain (2 × 109 CFU/g feed) resulted in negative effects. Addo et al. (2017a) reported that dietary inclusion of B. subtilis SB3086 and SB3615 rather than SB3295 improved serum bactericidal activity of Nile tilapia Oreohromis niloticus.
Largemouth bass Micropterus salmoides (LMB) is one of the most commercially important freshwater fish in China. Since its introduction from the United States to China in the early 1980s, the production of LMB increased tremendously and reached 477,808 tonnes in 2019 with the help of the development of formulated feed (China Fishery Statistics Yearbook, 2020). However, the application of formulated feed (especially when dietary starch was >100 g/kg) is frequently associated with nutrition metabolism disturbances in the intestine and liver of LMB, subsequently leading to anorexia, poor disease resistance, and growth retardation (Li & Chen, 2011; Lin et al., 2018; Yu et al., 2019; Zhang et al., 2020). Therefore, optimizing feed formula with functional feed additives is highly needed to improve the nutrient utilization and health status for the sustainable culture of LMB. To our knowledge, there is scarce information regarding the application of probiotics in the diets of LMB except the study of Yang et al. (2020), in which B. subtilis was supplemented together with yeast powder and mannan oligosaccharides as the form of synbiotics. However, the probiotic role of B. subtilis alone in LMB is still unknown. As a unique probiotic Bacillus strain (patent number: US2017340683-A1), B. subtilis DSM 32315 was derived from a multi-parameter selection process (Ma et al., 2018; Petri et al., 2017). Recently, it was reported that dietary administration of this strain exhibited promising values on the intestinal architecture and gut microbiota, disease resistance and production performance of poultry and livestock animals (Bortoluzzi et al., 2019; Ma et al., 2018; Sokale et al., 2019; Tang et al., 2019). It was possible that dietary B. subtilis DSM 32315 supplementation could exert similar probiotic roles in fish. Therefore, this study was performed to evaluate dietary inclusion of this strain on the growth, feed utilization, immunity and intestinal health of juvenile LMB.
2 MATERIALS AND METHODS
2.1 Experimental design and diets
Formula and proximate composition of the experimental diets is shown in Table 1. Fish meal, soybean meal and chicken meal were used as the sources of protein, wheat flour was used as the carbohydrate source, while fish oil, soybean oil and soybean lecithin were used as the sources of lipid. A basal diet, containing ca 490 g/kg protein and 160 g/kg lipid, was prepared as the control (CON). Another two test diets were supplemented with 1.5 and 3 g/kg B. subtilis DSM 323152 (spore content, 2 × 109 CFU/g; Evonik Degussa) in the diet of CON, being designated as diets BS1.5 and BS3, respectively. Portions of wheat flour were replaced as needed to accommodate the test probiotics. Feed samples were sent to headquarters of Evonik Industries AG for determination of the actual BS counts by the dilution plate series culture method. Feed samples were suspended in phosphate-buffered saline, heat-treated at 80°C for 10 min, spread plated on tryptone soya agar and incubated at 37°C for 16–24 h aerobically (VDLUFA, 2012). The test results showed that the spore counts of BS in diets CON, BS1.5 and BS3 were 66, 2.8 × 106 and 5.5 × 106 CFU/g feed, respectively.
| Ingredients (g/kg) | CON | BS1.5 | BS3 |
|---|---|---|---|
| Fish meala | 450 | 450 | 450 |
| Soybean mealb | 150 | 150 | 150 |
| Chicken mealb | 100 | 100 | 100 |
| Wheat flourb | 183 | 181.5 | 180 |
| Bacillus subtilis DSM 32315c | 0 | 1.5 | 3 |
| Fish oilb | 30 | 30 | 30 |
| Soybean oilb | 40 | 40 | 40 |
| Soybean lecithinb | 10 | 10 | 10 |
| Monocalcium phosphateb | 10 | 10 | 10 |
| Vitamin & mineral premixb | 20 | 20 | 20 |
| Ascorbic phosphate esterb | 2 | 2 | 2 |
| Choline chloride (50%)b | 5 | 5 | 5 |
| Analysed nutrient composition (g/kg, dry matter basis) | |||
| Moisture | 90.5 | 98.9 | 99.8 |
| Crude protein | 487 | 490 | 492 |
| Crude lipid | 158 | 155 | 157 |
| Ash | 123 | 125 | 126 |
Note
- Vitamin and mineral premix (IU or mg/kg diet): vitamin B1, 800; vitamin B2, 1600; vitamin B6, 1200; nicotinic acid, 3800; D-Ca pantothenate, 2500; myo-inositol, 4000; d-biotin, 40; folic acid, 320; vitamin A, 400,000 IU; vitamin E, 16,000; vitamin K3, 600; vitamin D3, 80,000 IU; vitamin B12, 4; vitamin C, 35,000; magnesium, 3000; iron, 1800; manganese, 800; iodine, 250; copper, 350; zinc, 6500; selenium, 15; cobalt, 60. CON, BS1.5 and BS3 indicated that diets were supplemented with 0, 1.5 and 3 g/kg of B. subtilis DSM 32315, respectively.
- a Imported from Peru.
- b Purchased from Guangzhou Jieda Feed Co., LTD.
- c Supplied by Evonik Degussa (China) Co., Ltd.
All dry feed ingredients were ground through a 60-mesh screen, weighed using a digital scale, and mixed for 20 min in a Hobart mixer (A-200T Mixer Bench Model unit; Russell Food Equipment). Then, oil and distilled water (about 350 ml/kg dry ingredients) were slowly added to the dry ingredients for another 15 min mixing to form a dough. The dough was passed through a pelletizer (Institute of Chemical Engineering, South China University of Technology, Guangdong, China) with a 2.5-mm diameter die. The diet pellets were dried under forced air at room temperature until the moisture was reduced to <100 g/kg. The dry pellets were placed in covered plastic bags and stored at −20°C until fed.
2.2 Growth trial
Five hundred feed-trained LMB juveniles (mean body weight, about 5 g/fish) were purchased from a commercial producer (Three Gorges Ecological Fishery Co. Ltd). Upon arrival, the fish were transferred into a holding recirculating system located in Fish Nutrition Research Laboratory, Southwest University. The system comprised of two 2500-L circular plastic tanks, and the water capacity of each tank was maintained at 1500 L. In this system, fish were hand-fed twice daily at 9:00 and 17:00 to near satiation for two weeks with a commercial diet. The diet was provided by Guangdong Haid Group and was analysed to contain 70.1, 537, 142 and 138 g/kg moisture, protein, lipid and ash, respectively.
After the acclimatization, groups of healthy LMB juveniles (20 fish per tank) were bulk-weighed, graded to a similar size (initial mean body weight, 8.32 ± 0.06 g/fish) and randomly distributed into 12 rectangular glass tanks (0.7 m × 0.45 m × 0.8 m, 250 L) operating as a recirculating aquaculture system. Water quality was maintained through sponges and gravel absorption, and the water exchange volume of each tank was maintained at approximately 6 L/min. All tanks were connected with continuous aeration through air stones to maintain sufficient dissolved oxygen. Each experimental diet was assigned to four replicate tanks of LMB. During the next 9 weeks, fish were fed the diets manually to apparent satiation twice daily at 9:00 and 17:00. Fish were fed three rounds each meal and were considered satiated when four to five diet pellets were not eaten within 5 min at the bottom of the tank during the last round of feeding. Uneaten diet pellets were collected immediately after feeding, dried, and weighed to accurately estimate feed consumption. Fish were kept under a natural photoperiod regime (12 h light: 12 h dark). Faeces wastes were siphoned off the tanks after 2 h of the last feeding. About one-third of the water in each tank was exchanged with tap water after aeration daily. Water quality parameters were monitored twice a week. During the growth trial, water temperature, pH, dissolved oxygen and ammonia were maintained at 25.6 ± 1.8°C, 7.60 ± 0.06, 8.52 ± 0.44 mg/L and 0.10 ± 0.01 mg/L, respectively.
2.3 Fish sampling
Prior to the start of the feeding trial, 12 LMB juveniles (pooled) were sampled from stock for an estimate of initial whole-body composition. During the feeding trial, fish were bulk-weighed and counted tank by tank to evaluate the production performance and survival at the end of the 3rd, 6th and 9th week.
At the end of the trial, 12 fish per tank were randomly selected, anaesthetized with MS-222 (100 mg/L, Sigma) and sampled after 24 h of feed deprivation. Three fish (pooled) were used for analysis of whole-body composition. Three fish were used for blood collection from the caudal vein with sterile syringes. Blood samples were kept at room temperature for 4 h and then centrifuged at 3000 g for 15 min at 4°C. Serum was separated and stored at −20°C pending analysis of immune parameters. Whole-blood samples were collected from three another fish with heparinized sterile syringes and used for the analysis of haematological parameters. Before blood collection, individual body weight and length of these fish were measured to calculate condition factor. After blood collection, these fish were dissected, and viscera (including the gonads) were collected and weighed to calculate viscerosomatic index. Then, the spleen and entire intestine were separated from the viscera and weighed to calculate spleen and intestine somatic indices, respectively. The remaining three fish were dissected, and foregut (1 cm after the stomach), midgut (1 cm after the first bend of the intestine) and hindgut (2 cm before the anus) samples were collected for histological analysis. About 50 mg of the jejunum samples was immediately frozen in liquid nitrogen and then transferred to −80°C pending analysis of the expression of representative genes involved with inflammatory response.
The remaining fish in each tank were fed for three more days. Approximately 6 h after the last feeding, 3 fish per tank were randomly selected, anaesthetized as described above and dissected aseptically to obtain the entire intestine. Once collected, the intestine samples were stored on ice. The digesta samples were separated sterilely, pooled by tank and frozen at −80°C pending microbiota analysis through 16S rRNA sequencing. All the experiments were conducted under the standard code of protocol for the Care and Use of Laboratory Animals in China. This research was approved by the Animal Ethics Committee of Southwest University (no. IACUC-20181015-12, 15 October 2018).
2.4 Proximate composition analysis
The proximate composition (moisture, protein, lipid and ash levels) of the diets and whole-body samples was analysed following the standard methods (AOAC, 1995). Moisture was determined by oven-drying at 105°C for 24 h. Crude protein (N × 6.25) was determined by the Kjeldahl method after an acid digestion. Crude lipid was determined by the ether-extraction method. Ash was determined using a muffle furnace at 550°C for 24 h.
2.5 Haematological and serum immunity analysis
White blood cell, red blood cell and platelet counts, haematocrit value and haemoglobin level of the whole blood were measured by automatic biochemical analyser (BC-3000, Mindray). Lysozyme activity of the serum was measured through spectrophotometric assay, while serum complement 3 (C3) and C4 as well as IgM levels were determined through enzyme-linked immunosorbent assays. All the aforementioned immune parameters were measured using commercial available kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions. Lysozyme activity was expressed as U/L, while the other serum immune parameters were presented as g/L.
2.6 Histological analysis
The intestine samples were fixed in 4% paraformaldehyde solution (Servicebio) for at least 24 h, dehydrated in graded ethanol concentrations and embedded in paraffin wax. Serial sections were cut at 5 µm of thickness and mounted on glass slides. Sections were deparaffinized in xylene, hydrated in ethanol and stained with haematoxylin and eosin (HE). The images were examined under a light microscope with a computerized image system (Olympus, DP73). Villus height, villus width and muscular layer thickness of each slice were measured by an image analysis software (ImageJ v.1.8.0, National Institute of Health).
2.7 Intestinal microbiota identification
The intestinal digesta samples were transported in dry ice to Biomarker Technologies Co. Ltd for microbiota identification through 16S rRNA sequencing. Briefly, total DNA of intestinal microbes was extracted using PowerSoil® DNA Isolation kit (MO BIO Laboratories) according to the manufacturer's instructions. DNA quality was assessed with 1% agarose gel electrophoresis, while the quantity was assessed with NanoDrop-2000. Full-length of 16S rRNA was amplified by PCR with the barcoded fusion primers 27F (AGRGTTT-GATYNTGGCTCAG) and 1492R (TASGGHTACCTTGTTASGACTT). Then, the PCR products were purified, quantified, homogenized and sequenced on the Illumina HiSeq platform.
The quality of the sequencing data was evaluated using lima v1.7.0 and UCHIME v4.2. Sequences with ≥97% similarity were defined as the same operational taxonomic unit (OTU). Alpha diversity indexes (Chao1, ACE, Shannon, Simpson and Faith's phylogenetic diversity whole tree) were assessed with Mothur v.1.30. Beta diversity was evaluated by principal component analysis (PCoA) based on unweighted UniFrac distance using QIIME. The abundance of relative species at the levels of phylum, class, order, family, genus and species was statistically analysed using QIIME.
2.8 RNA extraction, cDNA synthesis and real-time PCR
Total RNA from the jejunal samples was extracted using RNAiso Plus reagent (TaKaRa) according to the manufacturer's instructions. The quality and concentration of total RNA were assessed with NanoDrop-2000. Then, cDNA was synthesized from RNA with PrimeScript RT Master Mix Perfect Real Time Kit (TaKaRa) and stored at −20°C pending real-time PCR analysis.
The primer sequences of inflammatory cytokines encoding genes were obtained according to Yu et al. (2018), while the primer sequence of β-actin (Table 2) was designed by Primer Premier 6 (Premier Biosoft Int.). The construction of linear standard curve and real-time PCR analysis was performed in the ABI-7500 fast real-time PCR system (Applied Biosystems) according to our previous study (Chen et al., 2017). The relative abundance of gene expression versus the β-actin was calculated according to the formula: 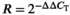 as described by Livak and Schmittgen (2001).
as described by Livak and Schmittgen (2001).
| Genes | Forward primer (5′–3′) | Reverse primer (5′–3′) | Product length (bp) | Tm (°C) | Reference/accession no. |
|---|---|---|---|---|---|
| IL-1β | CGTGACTGACAGCAAAAAGAGG | GATGCCCAGAGCCACAGTTC | 166 | 59.4 | Yu et al. (2018) |
| IL-8 | CGTTGAACAGACTGGGAGAGATG | AGTGGGATGGCTTCATTATCTTGT | 101 | 64.9 | Yu et al. (2018) |
| IL-10 | CGGCACAGAAATCCCAGAGC | CAGCAGGCTCACAAAATAAACATCT | 113 | 62.1 | Yu et al. (2018) |
| TNF-α | CTTCGTCTACAGCCAGGCATCG | TTTGGCACACCGACCTCACC | 161 | 63.0 | Yu et al. (2018) |
| TGF-β1 | GCTCAAAGAGAGCGAGGATG | TCCTCTACCATTCGCAATCC | 118 | 59.0 | Yu et al. (2018) |
| β-actin | TTCACCACCACAGCCGAAAG | TCTGGGCAACGGAACCTCT | 179 | 57.0 | KJ669298.1 |
- Abbreviations: IL-10, interleukin-10; IL-1β, interleukin-1β; IL-8, interleukin-8; TGF-β1, transforming growth factor-β1; TNF-α, tumour necrosis factor-α.
2.9 Statistical analysis
Data are presented as mean ± SD (standard deviation) in the present study. The statistical analyses were carried out with SPSS22 software for Windows (SPSS Inc), and the minimal statistical significance level was set at 0.05 (p < .05). After validation of the normality of distribution (Kolmogorov–Smirnov test) and homogeneity of variances (Levene's test), all data were subjected to one-way ANOVA to detect treatment effects. When a treatment effect was detected, Tukey's multiple comparison tests were used to identify the differences.
3 RESULTS
3.1 Growth, feed utilization and body composition
At the end of the feeding trial, survival rates of different treatments varied from 92.5% to 95%. Data for production performance of LMB during the feeding trial are shown in Table 3. At the end of week 6 and week 9, mean body weight of the BS1.5 fish increased by 3.74% and 2.95%, respectively, as compared with the CON fish, but the differences were not significant (p > .05). During the whole trial, mean body weight of the BS3 fish did not differ from that of the CON fish (p > .05). Specific growth rate followed a similar pattern to that of mean body weight. Data for feed utilization and biometric indices of LMB at the end of week 9 are presented in Table 4. Feeding ratio (2.22–2.27%) and feed utilization (feed and protein efficiency ratios and protein productive value) were not sensitive to dietary modifications (p > .05). All the tested morphometric parameters including condition factor, viscerosomatic index and spleen and intestine somatic indices were not significantly different among treatments (p > .05). As shown in Table 5, whole-body moisture (690–693 g/kg), crude protein (177–186 g/kg), crude lipid (90.2–91.4 g/kg) and ash (51.1–53.0 g/kg) levels were not significantly different among treatments (p > .05).
| Diets | CON | BS1.5 | BS3 |
|---|---|---|---|
| Initial mean body weight | 8.33 ± 0.09 | 8.31 ± 0.05 | 8.31 ± 0.03 |
| Mean body weight (week 3) | 19.2 ± 1.0 | 18.9 ± 0.7 | 19.2 ± 1.0 |
| Specific growth rate (week 3) | 3.97 ± 0.25 | 3.90 ± 0.20 | 3.99 ± 0.23 |
| Mean body weight (week 6) | 37.4 ± 2.9 | 38.8 ± 2.0 | 37.6 ± 1.3 |
| Specific growth rate (week 6) | 3.49 ± 0.16 | 3.58 ± 0.13 | 3.51 ± 0.08 |
| Mean body weight (week 9) | 54.2 ± 4.3 | 55.8 ± 3.9 | 54.1 ± 1.7 |
| Specific growth rate (week 9) | 3.12 ± 0.13 | 3.17 ± 0.12 | 3.12 ± 0.05 |
Note
- Data are presented as mean ± SD (n = 4). CON, BS1.5 and BS3 indicated that diets were supplemented with 0, 1.5 and 3 g/kg of B. subtilis DSM 32315, respectively.
- Specific growth rate = 100 × [ln (final mean body weight/initial mean body weight)]/days.
| Diets | CON | BS1.5 | BS3 |
|---|---|---|---|
| Feeding ratio (%)a | 2.24 ± 0.07 | 2.27 ± 0.04 | 2.22 ± 0.04 |
| Feed efficiency ratiob | 1.12 ± 0.03 | 1.10 ± 0.02 | 1.11 ± 0.02 |
| Protein efficiency ratioc | 2.08 ± 0.06 | 2.06 ± 0.03 | 2.07 ± 0.04 |
| Protein productive value (%)d | 42.4 ± 1.99 | 42.5 ± 4.35 | 41.8 ± 3.06 |
| Condition factore | 2.30 ± 0.17 | 2.36 ± 0.07 | 2.43 ± 0.02 |
| Viscerosomatic index (%)f | 10.0 ± 0.39 | 9.94 ± 0.30 | 10.0 ± 0.48 |
| Spleen somatic index (%)g | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.01 |
| Intestine somatic index (%)h | 0.83 ± 0.08 | 0.83 ± 0.04 | 0.84 ± 0.02 |
Note
- Data are presented as mean ± SD (n = 4). CON, BS1.5 and BS3 indicated that diets were supplemented with 0, 1.5 and 3 g/kg of B. subtilis DSM 32315, respectively.
- a Feeding ratio = 100 × feed intake/[(initial body weight + final body weight)/2]/day.
- b Feed efficiency ratio = total weight gain/feed intake.
- c Protein efficiency ratio = total weight gain/protein intake.
- d Protein productive value = 100 × (final body protein – initial body protein)/protein intake.
- e Condition factor = 100 × body weight (g)/body length3 (cm).
- f Viscerosomatic index = 100 × viscera weight/body weight.
- g Spleen somatic index = 100 × spleen weight/body weight.
- h Intestine somatic index = 100 × intestine weight/body weight.
| Diets | CON | BS1.5 | BS3 |
|---|---|---|---|
| Moisture | 693 ± 5 | 690 ± 5 | 693 ± 3 |
| Crude protein | 186 ± 4 | 178 ± 4 | 177 ± 5 |
| Crude lipid | 90.8 ± 2.0 | 91.4 ± 7.8 | 90.2 ± 5.8 |
| Crude Ash | 51.6 ± 0.9 | 53.0 ± 4.6 | 51.1 ± 2.7 |
Note
- Data are presented as mean ± SD (n = 4). CON, BS1.5 and BS3 indicated that diets were supplemented with 0, 1.5 and 3 g/kg of B. subtilis DSM 32315, respectively.
3.2 Haematological parameters and serum immune indicators
Data for haematological parameters of LMB are shown in Table 6. The counts of white and red blood cell, haematocrit value and haemoglobin level were highest in the whole blood of the BS1.5 fish, which were significantly higher than those of the BS3 fish (p < .05). However, the above-mentioned parameters were not significantly different between the CON and BS1.5 fish (p > .05). Platelet count of the whole-blood trended to decrease with elevated doses of dietary BS supplementation, but the differences were not significant (p > .05).
| Diets | CON | BS1.5 | BS3 |
|---|---|---|---|
| White blood cell (1011/L) | 1.24 ± 0.13ab | 1.35 ± 0.12b | 1.02 ± 0.13a |
| Red blood cell (1012/L) | 1.69 ± 0.12ab | 1.83 ± 0.23b | 1.46 ± 0.20a |
| Haematocrit (%) | 25.8 ± 1.5ab | 27.3 ± 2.3b | 22.1 ± 3.2a |
| Haemoglobin (g/L) | 81.5 ± 4.2b | 86.7 ± 3.5b | 70.5 ± 4.6a |
| Platelet (1010/L) | 2.24 ± 0.20 | 2.08 ± 0.22 | 1.86 ± 0.18 |
Note
- Data are presented as mean ± SD (n = 4). Different letters at the same row indicate significant differences among treatments (p < .05). CON, BS1.5 and BS3 indicated that diets were supplemented with 0, 1.5 and 3 g/kg of B. subtilis DSM 32315, respectively.
Data for serum immune indicators of LMB are presented in Figure 1. Compared with the CON fish (2.38 U/L), serum lysozyme activity did not significantly increase until the dose of BS reached 3 g/kg feed (2.97 U/L; p > .05). Serum complement C3 level of the BS supplemented fish was markedly higher than that of the CON fish (p < .05). Compared with the CON fish (1.14 g/L), serum complement C4 level significantly increased when BS was supplemented at the dose of 1.5 g/kg feed (1.77 g/L; p < .05). Serum IgM level was significantly higher in the BS1.5 fish (32.0 g/L) than in the CON (26.1 g/L) and BS3 fish (27.5 g/L; p < .05).
3.3 Intestinal morphology
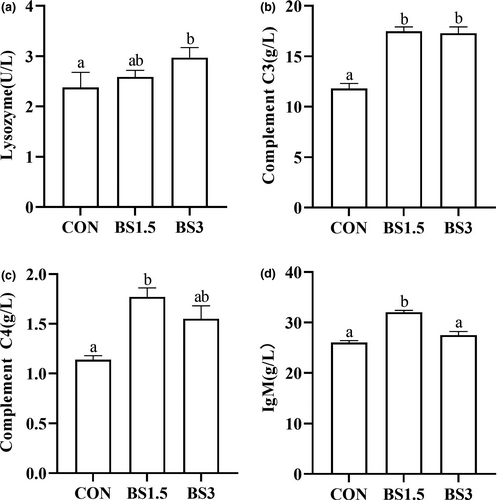
Data for foregut, midgut and hindgut architectures of LMB are shown in Figure 2. Intestinal histological appearance showed that all the sampled fish had intact epithelial barriers, extensive mucosal folds and abundant villi. Villus height of the foregut (679–697 μm) was not affected by dietary modifications (p > .05). The BS1.5 fish obtained markedly higher midgut villus height (752 μm) than the CON (691 μm) and BS3 fish (710 μm; p < .05). Villus height in the hindgut of the CON fish (545 μm) was significantly lower than that of the other treatments (639–655 μm; p < .05). Villus width of the foregut and midgut was significantly lower in the BS supplemented fish than in the CON fish (p < .05). Compared with the CON fish (155 μm), muscular layer thickness of the foregut was significantly higher in the BS3 fish (168 μm; p < .05). The BS supplemented fish obtained markedly higher midgut muscular layer thickness (167–169 μm) than the CON fish (152 μm; p < .05). Compared with the CON fish, muscular layer thickness of the hindgut was not affected by dietary BS supplementation (p > .05).
3.4 Diversity and composition of gut microbiota
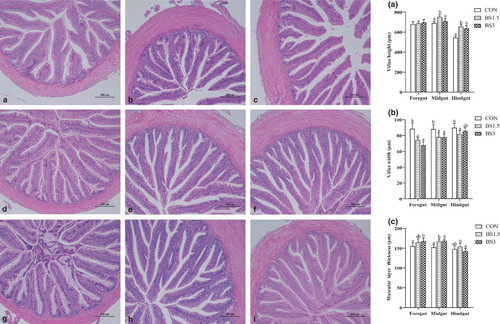
Data for alpha diversity indices of gut microbiota are shown in Table 7. The sequencing coverage was above 99.8% in this study, which could effectively reflect the diversity of gut microbiota in LMB. Compared with the CON fish, the OTUs, ACE, Chao1 and Faith's phylogenetic diversity (PD) whole tree indices of gut microbiota did not significantly increase until the supplemental dose of BS reached 3 g/kg feed (p > .05). Shannon index of gut microbiota was significantly lower in the CON fish than in the BS1.5 and BS3 fish, while the opposite was true for Simpson index (p < .05). The above-mentioned results suggested that the diversity of gut microbiota in LMB was improved with BS inclusion especially at the dose of 3 g/kg feed. Principal coordinate analysis (PCoA) plot defined treatments where the samples from the CON, BS1.5 and BS3 fish occupied distinct positions (Figure 3). The first axis of the PCoA explained 68.0% of the variation in microbial diversity, while the second axis explained 14.5% of it.
| Diets | OTUs | ACE | Chao1 | Shannon | Simpson | PD whole tree | Good's-coverage |
|---|---|---|---|---|---|---|---|
| CON | 54.7 ± 12.9a | 56.2 ± 12.0a | 55.8 ± 12.1a | 1.37 ± 0.34a | 0.49 ± 0.03b | 5.90 ± 1.66 a | 0.9996 |
| BS1.5 | 70.7 ± 9.1ab | 70.4 ± 9.4ab | 69.6 ± 10.0ab | 2.17 ± 0.29b | 0.28 ± 0.06a | 7.79 ± 0.95ab | 0.9991 |
| BS3 | 96.3 ± 19.1b | 99.2 ± 19.6b | 99.8 ± 18.7b | 2.76 ± 0.27b | 0.18 ± 0.06a | 9.23 ± 1.39b | 0.9989 |
Note
- Data are presented as mean ± SD (n = 3). Different letters at the same column indicate significant differences among treatments (p < .05). CON, BS1.5 and BS3 indicated that diets were supplemented with 0, 1.5 and 3 g/kg of B. subtilis DSM 32315, respectively.
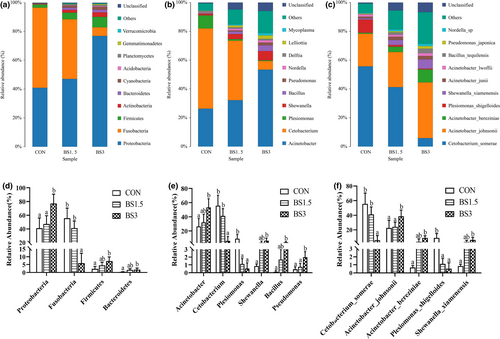
Data for the composition of gut microbiota are shown in Figure 4. Irrespective of dietary modifications, Proteobacteria, Fusobacteria and Firmicutes were the dominated phyla, which in total accounted for 98.8%, 93.0% and 90.1% of the gut microbiota in the CON, BS1.5 and BS3 fish, respectively (Figure 4a). The abundance of Proteobacteria was significantly lower in the gut microbiota of the CON (40.9%) and BS1.5 fish (47.2%) than that of the BS3 fish (77.0%), while the opposite was true for Fusobacteria abundance (Figure 4d; p < .05). Compared with the CON fish, the abundances of Firmicutes and Bacteroidetes did not markedly increase until the supplemental dose of BS reached 3 g/kg feed (p > .05). At the genus level, Cetobacterium and Acinetobacter dominated the gut microbiota of the CON and BS1.5 fish, while Acinetobacter was the top microbial community in the gut microbiota of the BS3 fish (Figure 4b). Compared with the CON fish, the abundances of Acinetobacter, Shewanella and Bacillus did not significantly increase until the supplemental dose of BS reached 3 g/kg feed (Figure 4e; p > .05). The abundance of Cetobacterium was markedly higher in the gut microbiota of the CON (55.6%) and BS1.5 fish (41.3%) than that of the BS3 fish (5.90%), while the opposite was true for Pseudomonas abundance (p < .05). Plesiomonas abundance was markedly lower in the gut microbiota of the BS supplemented fish as compared with the CON fish (p < .05). At the species level, C. somerae and A. johnsonii dominated the gut microbiota of the CON and BS1.5 fish, while A. johnsonii was the top microbial community in the gut microbiota of the BS3 fish (Figure 4c). The abundance of A. johnsonii was significantly lower in the gut microbiota of the CON (22.7%) and BS1.5 fish (24.3%) than that of the BS3 fish (38.6%), while the opposite was true for C. somerae abundance (Figure 4f; p < .05). Compared with the CON fish, the abundances of A. bereziniae and S. xiamenensis did not markedly increase until the supplemental dose of BS reached 3 g/kg feed (p > .05). P. shigelloides abundance was markedly lower in the gut microbiota of the BS supplemented fish as compared with the CON fish (p < .05).
3.5 The expression of inflammatory cytokines
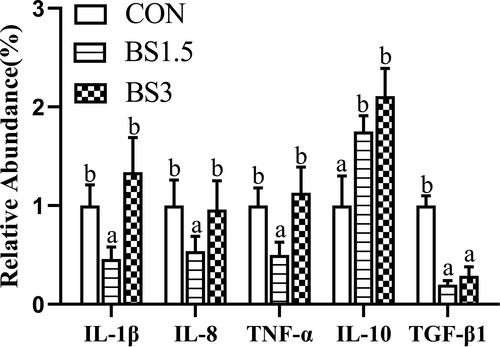
Data for the mRNA levels of intestinal pro-inflammatory (IL-1β, IL-8 and TNF-α) and anti-inflammatory cytokines (IL-10 and TGF-β1) are shown in Figure 5. The mRNA abundances of intestinal IL-1β, IL-8 and TNF-α were markedly lower in the BS1.5 fish than in the CON and BS3 fish (p < .05). Compared with the CON fish, the mRNA levels of IL-10 were 1.75- and 2.11-fold higher in the intestine of the BS1.5 and BS3 fish, while the opposite was true for TGF-β1 expression (p < .05).
4 DISCUSSION
It was recently reported that dietary BS DSM 32315 supplementation benefited the production performance of poultry animals under necrotic enteritis challenge (Bortoluzzi et al., 2019; Sokale et al., 2019) and livestock animals with suboptimal dietary protein provision (Tang, Qian, et al., 2019). In this study, however, the growth and feed utilization of LMB juveniles were not affected by the inclusion of this strain at 1.5 and 3 g/kg (corresponding to 3 × 106 and 6 × 106 CFU/g feed, respectively) during the 9-week feeding trial. This phenomenon might be attributed to the fact that the amounts of macronutrients (especially protein) in the feed had met the standard of requirements by LMB (Li & Chen, 2011). As this was the first study regarding the probiotic roles of BS DSM 32315 in aquaculture species, direct comparisons could not be made between LMB and other fish species. However, the result of this study was in accordance with the absence of growth promotion in the application of other BS strains such as Aqua NZ, SB3086, SB3295, SB3615 or AP193 in Nile tilapia Oreochromis niloticus (Addo et al., 2017a, 2017b) and C-3102 in hybrid tilapia O. niloticus × O. aureus (He et al., 2013). Previous investigations showed that the nutrient composition of whole body or white muscle was not sensitive to dietary Bacillus spp. supplementation in Nile tilapia (Telli et al., 2014) and parrot fish Oplegnathus fasciatus (Liu et al., 2018), and a similar situation was observed with respect to whole-body composition of LMB in this study.
Haematological parameters are useful monitoring tools for physiological and health status of fish (Fazio, 2019). In this study, BS additive did not affect all the tested haematological parameters except the haemoglobin level, which was lower in the whole blood of the BS3 fish than that of the CON fish, suggesting that dietary BS DSM 32315 supplementation at 3 g/kg might decrease the high oxygen-carrying capacity (De Pedro et al., 2005; Firouzbakhsh et al., 2011). Lysozyme and complement C3 and C4 are important components of the innate defence mechanism (Magnadottir et al., 2009; Saurabh & Sahoo, 2008), while IgM performs antigen-specific conditioning and phagocytosis to pathogenic bacteria in fish (Magnadottir et al., 2009). In this study, serum lysozyme activity and complement C3 and C4 levels of LMB were improved with BS DSM 32315 inclusion at either 1.5 or 3 g/kg feed, which were in accordance with the results from many other fish species fed diets containing endospores of other BS strains (Ai et al., 2011; Galagarza et al., 2018; Lee et al., 2017; Liu et al., 2017, 2018; Ramesh & Souissi, 2018; Zaineldin et al., 2018). However, serum IgM level was superior in the BS1.5 fish over the BS3 fish. Integrating the results of haematological parameters and serum immune indicators, it seemed that the immune regulatory role of BS DSM 32315 might be dose-dependent in LMB juveniles, and the inclusion of this strain at 1.5 g/kg feed contributed to better innate and adaptive immunities.
Nowadays, the gastrointestinal tract is not only seen as a physical and immunological barrier but also seen as an immune organ (Van Loo, 2007). Cytokines play an important role in adaptive and innate immune responses by binding to specific receptors on cell membranes (Secombes et al., 2001). It was previously reported that dietary BS (Ch9, GC-21 and GC22 strain) administration exerted anti-inflammatory ability in the intestine of grass carp Ctenopharyngodon idellus (Guo et al., 2016; Tang, Qian, et al., 2019). Although intestinal anti-inflammatory response (mRNA expression of IL-10 and TGF-β1) did not differ between the BS1.5 and BS3 fish, the pro-inflammatory response (mRNA levels of IL-1β, IL-8 and TNF-α) was superior in the intestine of the BS1.5 fish over the BS3 fish. The reason might be attributed to the increase of Proteobacteria abundance from 47.2% to 77.0% with further increment of dietary BS inclusion, as chronic enrichment of this phylum was associated with an unstable gut microbial community structure, subsequently leading to the occurrence of inflammatory response (Shin et al., 2015). Surprisingly, the expression of intestinal TGF-β1 in LMB was downregulated with dietary BS inclusion, and the reason was unknown. The modulatory role of TGF-β1 in inflammation response deserves further investigations in the intestine of LMB.
Intestine is the main place for digestion and absorption of nutrients; thus, intestinal health is an important guarantee for the normal growth and development of fish (Galagarza et al., 2018). Villus height, villus width and muscular layer thickness are important indicators for intestinal health. Though all the sampled LMB juveniles had intact intestines, the inclusion of BS DSM 32315 at either 1.5 or 3 g/kg feed still improved intestinal digestion and absorption capability to some extent, as higher villus height and/or muscular layer thickness were recorded in the foregut, midgut or hindgut of the BS supplemented fish as compared with the CON fish. However, villus width of the intestine in LMB decreased with dietary BS DSM 32315 inclusion in this study, and the reason may be due to the limited space in gut (Poolsawat et al., 2021). Similarly, villus height increased with dietary inclusion of this strain in the midgut of broiler chickens (Sokale et al., 2019) and in the ileum of weaned piglets (Tang, Qian, et al., 2019). It was also well documented that dietary inclusion of other strains of Bacillus spp. benefited the intestinal architecture and digestive function of many fish species (Fan et al., 2018; Lee et al., 2017; Liu et al., 2017; Zaineldin et al., 2018).
As an important inhabitant of the host, gut microbiota has profound impacts on the bioavailability of dietary components; thus, it plays crucial roles in host nutritional and physiological processes (Power et al., 2014). In accordance with the results of Zhou et al. (2018), Proteobacteria, Fusobacteria and Firmicutes were the dominant phyla in the gut microbiota of LMB. In this study, the abundance of Proteobacteria increased remarkably in the gut microbiota of the BS3 fish as compared with the other treatments. Consistently, the elevated abundances of Acinetobacter (specifically A. johnsonii and A. bereziniae), Shewanella (specifically S. xiamenensis) and Pseudomons accounted for such a change. Inconsistently, the abundance of Plesiomonas (specifically P. shigelloides) decreased with dietary BS inclusion. It was previously reported that the inclusion of S. xiamenensis in the feed enhanced the innate immunity and disease resistance of grass carp (Wu et al., 2015), but A. johnsonii and P. shigelloides were identified as opportunistic pathogens in fish (Behera et al., 2018; Hu et al., 2014; Kozińska et al., 2014; Liu et al., 2015; Pakingking et al., 2015), while the probiotic role of A. bereziniae is still unknown in fish. Opposite to Proteobacteria, the abundance of Fusobacteria decreased remarkably in the gut microbiota of the BS3 fish as compared with the CON and BS1.5 fish. Consistently, the reduced abundance of Cetobacterium (specifically C. somerae) contributed mostly to such a change. C. somerae is known to be positively associated with the production of acetic acid, propionic acid and vitamin B12 (Tsuchiya et al., 2008). As described above, although the BS3 fish obtained the highest diversity of gut microbiota, the abundances of potential beneficial and harmful bacteria increased or decreased simultaneously. Compared with the CON fish, the abundance of only one type of bacteria (the harmful Plesiomonas shigelloides) decreased in the gut microbiota of the BS1.5 fish. The regulatory role of BS DSM 32315 additive in the composition of gut microbiota in LMB is still ambiguous, which deserve further studies with more replications at each supplemental doses.
5 CONCLUSION
Taken together, it was concluded that dietary BS DSM 32315 inclusion presented some positive effects on LMB. Although the BS1.5 fish performed better than the BS3 fish, the best supplemental dose need to be further studied.
ACKNOWLEDGEMENTS
This study was mainly funded by Key Laboratory of Healthy Freshwater Aquaculture (Ministry of Agriculture and Rural Affairs), Key Laboratory of Fish Health and Nutrition of Zhejiang Province and Zhejiang Institute of Freshwater Fisheries (ZJK202006). Chongqing Ecological Fishery Technology System (2016-2021) and Research Project of Education and Teaching Reform of Southwest University (2019JY019) partially supported this study.
CONFLICT OF INTEREST
The authors declare that the research was conducted with no commercial or financial relationships that could be construed as a potential conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data supporting the results of this study can be obtained from corresponding author upon reasonable request.




