Effects of β-Conglycinin on intestinal structure and intestinal permeability in Rhynchocypris lagowski Dybowski
Abstract
The purpose of this study was to evaluate the effects of β-conglycinin on intestinal permeability, intestinal morphology and the expression of tight junction protein in Rhynchocypris lagowski Dybowski. 750 Rhynchocypris lagowski Dybowski (4.61 ± 0.01 g) were divided into 5 groups (CK, β-20, β-40, β-60 and β-80 groups) and fed with 5 diets contained, respectively (0, 20, 40, 60 and 80 g/kg), β-conglycinin for 8 weeks. These results showed dietary β-conglycinin could destroy the structural integrity of intestine, cause villus to break and shrink, and the epithelium and lamina propria to separate. Simultaneously, compared with CK group, the levels of Diamine oxidase (DAO), 5-Hydroxytryptamine (5-HT), D-lactic acid (D-LA) and Endothelin-1 (ET-1) in serum were significantly increased in β-20, β-40, β-60 and β-80 groups (P < .05). In proximal intestines (PI), the expression of Zonula occludens-1 (ZO-1) and Occludin-b was significantly reduced, and lamina propria width was significantly increased in β-60 and β-80 groups (P < .05), and fold height, muscular layer thickness and the expression of Claudin were significantly decreased in β-40, β-60 and β-80 groups (P < .05), and the expression of Occludin-a was significantly decreased in β-20, β-40, β-60 and β-80 groups (P < .05); in addition, the expression of ZO-1 in PI was significantly increased in β-20 and β-40 groups (P < .05). In mid intestines (MI), fold height and the expression of ZO-1 and Claudin were significantly reduced, and lamina propria width was significantly increased in β-40, β-60 and β-80 groups (P < .05), and the expression of ZO-1 was significantly increased in β-20 groups (P < .05), and muscular layer thickness and the expression of Occludin-a and Occludin-b were significantly decreased in β-20, β-40, β-60 and β-80 groups (P < .05). In distal intestines (DI), fold height and the expression of ZO-1, Claudin, Occludin-a and Occludin-b were significantly reduced, and lamina propria width was significantly increased in β-20, β-40, β-60 and β-80 groups (P < .05), and muscular layer thickness was significantly reduced in β-40, β-60 and β-80 groups (P < .05). In summary, dietary β-conglycinin had negative effects on tight junctions, intestinal permeability and morphology. Moreover, the negative effects of β-conglycinin on intestines from high to low were DI, MI and PI in our study.
1 INTRODUCTION
In recent decades, a growth number of soybean meal has been used in the feed industry (Ding et al., 2015; Hancock et al., 2000; Li et al., 2017). However, β-conglycinin, as one of soybean antigen protein, is strongly immunogenic and cannot be completely inactivated and considered to be the main factor restricting the widespread use of soybean products in the feed industry (Meinlschmidt et al., 2016; Wang et al., 2014). Simultaneously, previous studies suggested that β-conglycinin had obvious anti-nutritional effects on animals. These anti-nutritional effects are mainly manifested in reducing growth, causing intestinal inflammation and changes in intestinal morphology and reducing immune function and antioxidant capacity in pigs (Hao et al., 2010; Zhao et al., 2010), rats (Guo et al., 2008; Han et al., 2010) and mice (Liu et al., 2008). Compared with terrestrial animals, β-conglycinin has similar effects on aquatic animals. In turbot (Scophthalmus maximus L.) and Chinese mitten crabs (Eriocheir sinensis) studies, it was found that the growth performance, immune function and antioxidant capacity of turbot and Chinese mitten crabs were decreased by dietary β-conglycinin; in addition, intestinal morphology of turbot and Chinese mitten crabs was also destroyed (Han et al., 2018; Li et al., 2017). Besides these effects, β-conglycinin can also affect the expression of insulin-like growth factors-1 (IGF-I) or target of rapamycin (TOR) in Jian carp (Cyprinus carpio var. Jian) and golden crucian carp (Carassius auratus) studies, which found that the expression of IGF-I and TOR was decreased by dietary β-conglycinin (Li et al., 2019; Zhang et al., 2013).
Intestine is not only an important organ for the digestion and absorption of animal, but also the structural basis for the immune barrier and maintaining homeostasis (Guo et al., 2014). The tight junctions between intestinal epithelial cells play a vital role in maintaining the integrity of the intestinal structure (Walle et al., 2010). Tight junctions, which are composed of Claudins, Zonula occludens (ZO), Occludin and Junctional adhesion molecule (JAM), can effectively close the intercellular space and prevent the invasion of pathogens, endotoxins and germs (Groschwitz and Hogan, 2009; Jaladanki and Wang, 2010; Takuya, 2013). In generally, intestinal tight junction allow only nutrients, water and electrolyte ions to pass through. However, under pathological conditions, intestinal tight junction is destroyed by harmful substances and pathogens, causing bacterial endotoxins and toxic macromolecules to pass, which in turn causes a series of intestinal damage (Gao and Qin, 2005). Some studies have found that high concentration of β-conglycinin can reduce the tight junction protein Occludin and ZO-1 of small intestinal epithelial cells of piglet and the tight junction protein Claudin-1 of piglet intestinal tract, thereby causing intestinal barrier dysfunction (Zhao et al., 2014; Zhao et al., 2013). However, there is no study focused on the effects of β-conglycinin on intestinal tight junction in fish, and further investigation is needed.
On the other hand, due to different digestion and absorption capacities of different intestinal segments for nutrients in animals, when β-conglycinin enters the digestive tract, different amounts of complete β-conglycinin and its fragments or small peptides, which cannot be digested, are remained in different intestinal segments. This may be the reason that the difference in immune activity of β-conglycinin and the difference in degrees of damage for intestines among different intestinal segments. In piglet (Zhao et al., 2008) and rat (Perez et al., 2000) studies, it was found that β-conglycinin had different immune activities among different intestinal segments. In grass carp (Ctenopharyngodon idella) study, dietary β-conglycinin caused different degrees of intestinal inflammation and oxidative damage between proximal, mid and distal intestines (Duan et al., 2019). These above results indicated that β-conglycinin had different effects on different intestinal segments in animals. Therefore, dietary β-conglycinin might affect tight junctions varying among different intestinal segments in fish, which requires further investigation.
Rhynchocypris lagowski Dybowski is an omnivorous economic fish, which has the characteristic of less disease, no intermuscular spines, high nutritional value and is well received by farmers and consumers. In the present study, the growth trial had been used in our previous study, which determined effects of β-conglycinin on growth performance, antioxidant capacity and immunity (Li et al., 2020). In addition, the purpose of this study was to further investigate the effects of β-conglycinin on digestive enzymes, protein metabolism enzymes, intestinal tight junctions and morphology varying among different intestinal segments in Rhynchocypris lagowski Dybowski.
2 MATERIALS AND METHODS
2.1 Experimental diets and fish
These experimental diets and fish were consistent with our previous study (Li et al., 2020), and β-conglycinin was obtained according to the method described by Wu et al. (Wu et al., 1999). The purity of β-conglycinin was approximately 80% (Li et al., 2020). The trial was divided into 5 groups: CK, β-20 (containing 20 g/kg β-Conglycinin), β-40 (containing 40 g/kg β-Conglycinin), β-60 (containing 60 g/kg β-Conglycinin) and β-80 (containing 80 g/kg β-Conglycinin). Dietary formula were showed in Table 1. 750 juvenile Rhynchocypris lagowski Dybowski (4.61 ± 0.01 g) were obtained from Nian Nian You Yu aquaculture limited company in Jilin province, China and were randomly distributed into 15 barrels (200 L) with 50 fish per barrel. The daily management of 8-week feeding trial was exactly the same as the previous study (Li et al., 2020). All experimental and animal-handling protocols were performed following the guidelines approved by the Institutional Animal Care and Use Committee, Jilin Agricultural University.
| Ingredients (g/kg) | CK | β-20 | β-40 | β-60 | β-80 |
|---|---|---|---|---|---|
| Fish meal | 300.00 | 300.00 | 300.00 | 300.00 | 300.00 |
| Flour | 200.00 | 200.00 | 200.00 | 200.00 | 200.00 |
| Chromium sesquioxide | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Corn oil | 26.90 | 27.10 | 27.30 | 27.40 | 27.60 |
| Fish oil | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 |
| β-conglycinin | 0 | 20.00 | 40.00 | 60.00 | 80.00 |
| Casein | 107.60 | 90.40 | 73.20 | 56.00 | 38.80 |
| Gelatin | 26.90 | 22.60 | 18.30 | 14.00 | 9.70 |
| Chorine chloride (50%) | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Mineral and vitamin premixa | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Microcrystaline cellulose | 44.30 | 44.30 | 44.30 | 44.30 | 44.30 |
| DL-methionine | 5.70 | 6.00 | 6.30 | 6.70 | 7.00 |
| Lysine | 10.70 | 11.00 | 11.30 | 11.50 | 11.90 |
| Dextrin | 217.90 | 218.60 | 219.30 | 220.10 | 220.70 |
| Calcium dihydrogen phosphate | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 |
| Nutrition level (g/kg)b | |||||
| Crude protein | 357.40 | 358.10 | 360.80 | 359.00 | 358.70 |
| Crude lipid | 79.10 | 78.60 | 79.10 | 78.20 | 80.40 |
| Moisture | 78.20 | 77.40 | 77.80 | 78.80 | 78.10 |
| Ash | 66.80 | 67.10 | 67.70 | 66.90 | 66.20 |
| β-conglycininc | 0 | 16.40 | 33.00 | 50.10 | 67.10 |
- a Mineral and vitamin premix(per 100 g premix): vitamin A, 84~104 KIU; vitamin D, 56~59 KUI; vitamin E, ≥2400 mg; vitamin K, ≥162 mg; nicotinamide, ≥458 mg; pantothenic acid, ≥702 mg; folic acid, ≥106 mg; biotin,≥4 mg; inositol, ≥3020 mg; copper, 96~110 mg; iron, 4060~5500 mg; manganese, 552~633 mg; cobalt, 6~8 mg; iodine, 13~20 mg; zinc, 2320~2660 mg; selenium, 8~12 mg.
- b Measured value.
- c Determined by the method of (Perez et al., 2000).
2.2 Sample collection
Sample collection was consistent with our previous study (Li et al., 2020). After the feeding trial, all fish were fasted for 24 h and then anaesthetized with 3-Aminobenzoic acid ethyl ester methanesulfonate (MS-222; Sigma). The hepatopancreas and intestines which divided into proximal intestine (PI), mid intestine (MI) and distal intestine (DI) of all fish were collected. Hepatopancreas and intestines of 15 fish were used to determine the activities of digestive enzymes and protein metabolism enzymes. Hepatopancreas and intestines frozen in liquid nitrogen were used to determine the expression of relative genes. The intestines of 5 fish were fixed in 10% neutral formaldehyde solution for histological evaluation. The blood of all fish was collected from the tail veins of all fishes with a syringe, stored at 4°C for 24 h and centrifuged at 1400 g for 10 minutes to separate the serum. All samples were stored at −80°C until analysis.
2.3 Histological assay
Same as the operation of Li et al. (2019), 10% Neutral buffered formalin was used to fix PI, MI and DI, and then, these intestinal segments, which were cut into about 5 μm thick, were dehydrated by different concentrations of alcohol, xylene and embedded in paraffin. Subsequently, samples were stained with haematoxylin and eosin (H&E). Finally, sample slices were observed and relevant data were measured by the Axioskop microscope (IX71.Olympus).
2.4 Biochemical analysis
As described by Didelot et al. (2006), the activities of protease were measured by the Folin-phenol reagent method. The activities of lipase, amylase, glutamic-pyruvic transaminase (GPT) and glutamic-oxaloacetic transaminase (GOT), and the levels of Diamine oxidase (DAO), 5-Hydroxytryptamine (5-HT), D-lactic acid (D-LA) and Endothelin-1 (ET-1) were measured by commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.5 Analysis of genes expression
According to the description of previous study (Li et al., 2020), TRIzol kit, PrimeScripTM RT kit and Premix TaqTM kit (Takara) were used to extract total RNA from hepatopancreas and intestines (PI, MI and DI), synthesize cDNA and perform fluorescence quantitative PCR. Primers for insulin-like growth factors-1 (IGF-I), target of rapamycin (TOR), Zonula occludens-1 (ZO-1), Occludin-a, Occludin-b, Claudin and β-actin genes were synthesized by Sangon. The specific primers were shown in Table 2. The gene expression levels were analysed by the 2−ΔΔCT method and using the relative genes expression level of the control as a calibrator.
| Genes | Sequences (5'-3') | GenBank number |
|---|---|---|
| β-actin | ||
| Forward | CGGTATCCATGAGACCACCT | AAB97964.1 |
| Reverse | CTTCTGCATCCTGTCAGCAA | |
| IGF-I | ||
| Forward | GGACATCACCAGGACAG | AY533140.1 |
| Reverse | CTCGGCTTGAGTTCTTC | |
| TOR | ||
| Forward | AAGCCACCACTTTCTACGATG | AB290031.1 |
| Reverse | TCACTATGGCAAGGATACCG | |
| Occludin-a | ||
| Forward | TCCAGCATTTCTACCG | NM_212832.2 |
| Reverse | ACAGACAAAGATTCCCAC | |
| Occludin-b | ||
| Forward | CGATGAGTTCCAGCAT | NM_001008618.1 |
| Reverse | CAGACAAAGATTCCCAC | |
| Claudin | ||
| Forward | CGACCCGTTTACTCCA | NM_131762.2 |
| Reverse | CACTCCCACAGATACAGC | |
| ZO-1 | ||
| Forward | GAAGACAAGCCATACAGA | XM_009303250.3 |
| Reverse | GTGGGTCAAAGTTACGAG | |
- Abbreviations: IGF-I, Insulin-like growth factors-1; TOR, target of rapamycin; ZO-1, zonulaoccludens-1.
2.6 Statistic analysis
All data were analysed by SPSS statistic 22.0 (IBM, USA) and presented as mean ± standard errors (means ± SE). The data were analysed with one-way analysis of variance (ANOVA) to determine the significant differences. To determine significant differences among groups (P < .05), Duncan's multiple range test was used to rank all treatments.
3 RESULT
3.1 The morphology of intestinal tissue
The effects of β-conglycinin on the intestinal indexes were showed in Figure 1. β-conglycinin had no significant effect on intestinal somatic index and intestinal length index (P > .05) (Figure 1a,b). In PI and MI, fold height was significantly lower than the CK group in β-40, β-60 and β-80 groups (P < .05), and in DI, fold height was significantly lower than the CK group in β-20, β-40, β-60 and β-80 groups (P < .05) (Figure 1c).
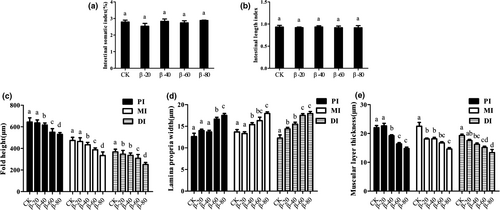
In PI, lamina propria width was significantly higher than the CK group in β-60 and β-80 groups (P < .05); in MI, lamina propria width was significantly higher than the CK group in β-40, β-60 and β-80 groups (P < .05); in DI, lamina propria width was significantly higher than the CK group in β-20, β-40, β-60 and β-80 groups (P < .05) (Figure 1d).
Compared with the CK group, in PI and DI, muscular layer thickness was significantly decreased in β-40, β-60 and β-80 groups (P < .05), and in MI, muscular layer thickness was significantly reduced in β-20, β-40, β-60 and β-80 groups (P < .05; Figure 1e).
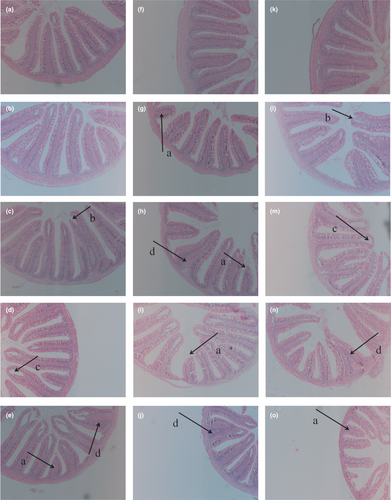
The morphology of intestinal tissue was shown in Figure 2. There were breaks in the villus by dietary β-conglycinin (Figure 2E,L). There was a separation between the epithelium and the lamina propria by dietary β-conglycinin (Figure 2D). In addition, the lamina propria widening and villi atrophy occurred respectively (Figure 2N,O).
3.2 The activities of digestive enzymes
The effects of β-conglycinin on the activities of digestive enzymes in hepatopancreas, PI, MI and DI were showed in Figures 3-5. The activities of protease in hepatopancreas and PI in β-40, β-60 and β-80 groups were significantly lower than the CK group (P < .05; Figure 3a,b). Compared with the CK group, the activities of protease in MI and DI in β-20, β-40, β-60 and β-80 groups were significantly decreased (P < .05; Figure 3c,d). In addition, β-conglycinin has no significant effects on the activities of lipase and amylase in hepatopancreas, PI, MI and DI (P > .05; Figures 4 and 5).

3.3 The activities of protein-metabolizing enzymes
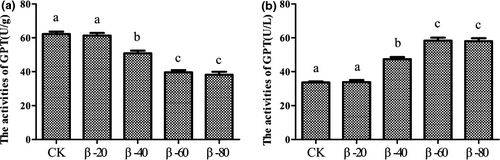
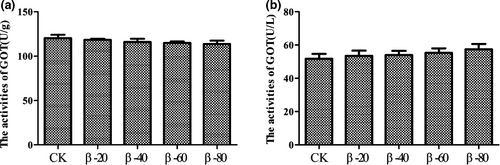
The results of activities of protein metabolizing enzymes were listed in Figures 6 and 7. Compared with the CK group, the activities of GPT in β-40, β-60 and β-80 groups were significantly reduced in hepatopancreas and were significantly increased in serum (P < .05; Figure 6a,b). However, there was no significant difference among each group found in the activities of GOT (P > .05; Figure 7a,b).
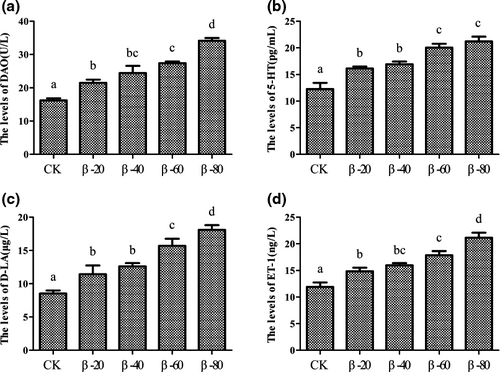
3.4 The levels of DAO, 5-HT, D-LA and ET-1 in serum
The effects on the levels of DAO, 5-HT, D-LA and ET-1 in serum were showed in Figure 8. Compared with the CK group, the levels of DAO, 5-HT, D-LA and ET-1 in serum were significantly increased in β-20, β-40, β-60 and β-80 groups (P < .05; Figure 8a–d).
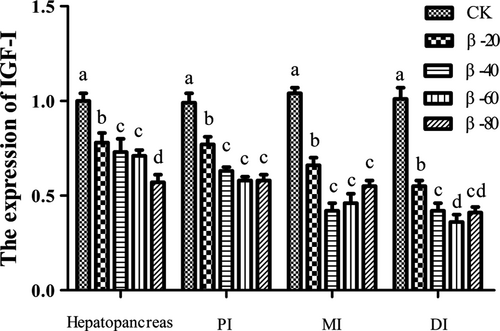
3.5 The expression of IGF-I and TOR
The results of the expression of IGF-I and TOR were listed in Figures 9 and 10. The expression of IGF-I and TOR in hepatopancreas, PI, MI and DI in β-20, β-40, β-60 and β-80 groups were significantly lower than the CK group (P < .05).
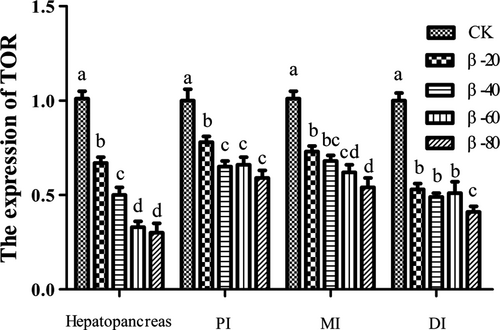
3.6 The expression of tight junction protein
The expression of tight junction protein was presented in Figures 11-14. In PI, the expression of ZO-1 in β-20 and β-40 groups was significantly higher than the CK group (P < .05), and the expression of ZO-1 in β-60 and β-80 groups was significantly lower than the CK group (P < .05). In MI, compared with the CK group, the expression of ZO-1 in β-20 was significantly increased (P < .05), and the expression of ZO-1 in β-40, β-60 and β-80 groups was significantly reduced (P < .05). In DI, compared with the CK group, the expression of ZO-1 in β-20, β-40, β-60 and β-80 groups was significantly decreased (P < .05; Figure 11).

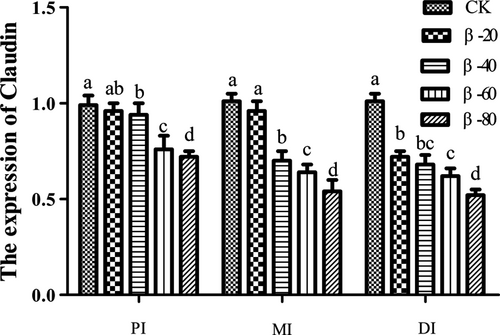
Compared with the CK group, the expression of Claudin in PI and MI was significantly reduced in β-40, β-60 and β-80 groups (P < .05), the expression of Claudin in DI in β-20, β-40, β-60 and β-80 groups was significantly reduced (P < .05; Figure 12).
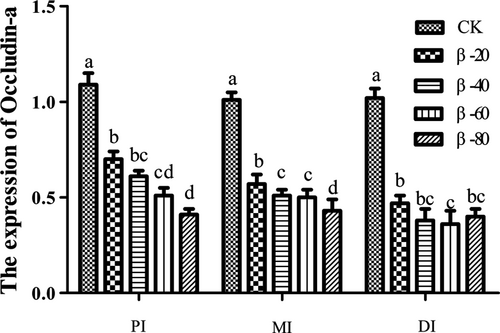
Compared with the CK group, the expression of Occludin-a in PI, MI and DI was significantly reduced in β-20, β-40, β-60 and β-80 groups (P < .05; Figure 13).
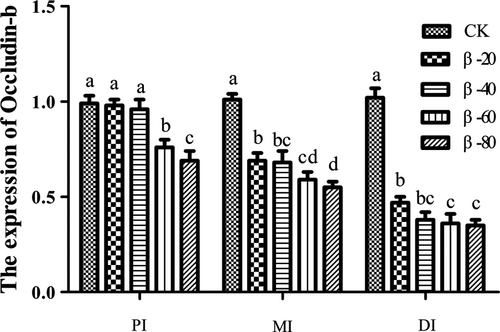
In PI, the expression of Occludin-b in β-60 and β-80 groups was significantly lower than the CK group (P < .05). In MI and DI, the expression of Occludin-b in β-20, β-40, β-60 and β-80 groups was significantly lower than the CK group (P < .05; Figure 14).
4 DISCUSSION
In fish, intestine is not only an important organ for the body’s digestion and absorption of nutrients, but also an immune barrier and the structural basis for maintaining homeostasis (Bairagi et al., 2002; Dong et al., 2017). The integrity of intestinal tissue structure plays a decisive role in intestinal health of fish (Li et al., 2019). The vigorous and regular swing of intestinal villi can effectively prevent pathogenic microorganisms from invading the host intestine and can also promote the digestion and absorption of nutrients in intestines (Sang and Fotedar, 2010; Singh et al., 2015). In our study, dietary β-conglycinin decreased fold height and muscular layer thickness, increased lamina propria width in intestines, simultaneously, the atrophy and break of mucosal fold and the separation of epithelial mucosa from the lamina propria were found. These results indicated dietary β-conglycinin could destroy the structure of intestine. This reason may be that β-conglycinin caused intestinal inflammation, leading to changes in intestinal structure. In our previous study, it had been found that dietary β-conglycinin caused changes in intestinal inflammatory factors (Li et al., 2020). In golden crucian carp and turbot studies, these results similar to results of our study were found that dietary β-conglycinin destroy the structure of intestine (Li et al., 2019; Li et al., 2017). Furthermore, our results suggested that the effects of dietary β-conglycinin on fold height, muscular layer thickness and lamina propria width among PI, MI and DI were inconsistent. The inconsistency in the effect of intestinal morphology is similar to the inconsistency in the effect of the activities of protease, due to the different functions of different intestinal segments.
The activities of digestive enzymes are important indicator reflecting the physiological digestive function of aquatic animals and determining the digestion and absorption capacity of aquatic animals for nutrients (Gisbert et al., 2009; Natalia et al., 2004). In our study, more than 40 g/kg dietary β-conglycinin could decrease the activities of protease in hepatopancreas and PI; however, exceeding 20 g/kg would cause a significant decrease in the activities of protease in MI and DI. In addition, there was no significant effect on the activities of lipase and amylase by dietary β-conglycinin. These results suggested that dietary β-conglycinin could decrease the activities of protease in hepatopancreas and intestine rather than the activities of lipase and amylase. This is because that the indigestibility of β-conglycinin could destroy the intestinal structure and affect the secretion of protease (Li et al., 2019). On the other hand, the digestive organs and system of Rhynchocypris lagowski Dybowski have not yet been fully developed, resulting in low tolerance to β-conglycinin, which in turn leads to a decline in the activities of protease. Similar results have been also reported that dietary β-conglycinin could reduce the activities of protease in Jian carp, golden crucian carp and Chinese mitten crabs studies (Han et al., 2018; Li et al., 2019; Zhang et al., 2013). Simultaneously, our study results also found an interesting phenomenon that 20 g/kg β-conglycinin could significantly reduce the activities of protease in MI and DI rather than PI whose the activities of protease need higher levels dietary β-conglycinin to significantly decrease the activities of protease. These results also proved that the effects of β-conglycinin on the activities of protease in PI, MI and DI are inconsistent. When β-conglycinin entered the intestine, β-conglycinin was digested and degraded in PI, resulting in more epitopes of β-conglycinin appearing, and then absorbed in MI and DI (Tengjaroenkul et al., 2000). This also caused β-conglycinin to damage MI and DI more than PI. Besides the activities of digestive enzymes, β-conglycinin could destroy hepatopancreas in fish and then affect the activities of protein-metabolizing enzymes in hepatopancreas and serum. Glutamic-pyruvic transaminase (GPT) and glutamic-oxaloacetic transaminase (GOT) are important aminotransferases in hepatopancreas (Helmut and Johan, 1994). Under normal conditions, the activities of GPT and GOT in serum are low. Moreover, when hepatopancreas is destroyed, GPT and GOT will be released into serum in large quantities, and the degree of increasing the activities of GPT and GOT is consistent with the degree of hepatopancreas damage (Barcellos et al., 2004; Nyblom et al., 2004). In our study, the activities of GPT in hepatopancreas were significantly decreased by 40–80 g/kg β-conglycinin, and the activities of GPT in serum, which was the opposite of hepatopancreas, were significantly enhanced by 40–80 g/kg β-conglycinin. However, the activities of GOT had a downward trend, and the difference was not significant in hepatopancreas; the activities of GOT had an upward trend, and the difference was not significant in serum. In golden crucian carp study, it was found that dietary β-conglycinin could decrease the activities of GPT in hepatopancreas (Li et al., 2019). The above results indicated that β-conglycinin could destroy the hepatopancreas of fish, resulting in a decrease in the activities of GPT in hepatopancreas and an increase in the activities of GPT in serum. IGF-I mRNA exists in all tissues of fish and has the highest content in hepatopancreas (Rutanen, 2000; Moorman et al., 2016). It has physiological functions such as regulating cell metabolism, promoting cell proliferation and differentiation (Husmann et al., 1996). Previous study showed that dietary nutritional levels can affect the expression of IGF-I (Thissen et al., 1994). The TOR mRNA is mainly regulated by amino acids and has the function of controlling protein synthesis and gene transcription (Destefano and Jacinto, 2013; Kim, 2009). In addition, IGF-I mRNA has an indirect effect on the activation of TOR signalling pathway (Glass, 2010). In our study, the expression of IGF-I and TOR was significantly decreased by dietary β-conglycinin in hepatopancreas and intestines. The results of golden crucian carp and Jian crap are consistent with our results, which suggested that dietary β-conglycinin could reduce the expression of IGF-I and TOR in golden crucian carp and Jian crap (Li et al., 2019; Zhang et al., 2013). However, the mechanism of β-conglycinin on the expression of IGF-I and TOR, as well as the mechanism of their downstream target proteins, is still unclear, and further research is needed.
In addition to destroying the morphological structure of fish intestines, soy antigen protein also affects intestinal permeability (Zhang et al., 2020). The levels of diamine oxidase (DAO), 5-Hydroxytryptamine (5-HT), D-lactic acid (D-LA) and endothelin-1 (ET-1) are important indicators of intestinal permeability (Ian et al., 2014). In our study, the levels of DAO, 5-HT, D-LA and ET-1 were significantly increased by dietary β-conglycinin in serum. Previous studies showed that an increase in level of DAO in serum indicated the destruction of the intestinal barrier, which can reflect the integrity of the intestinal mechanical barrier and the degree of damage (Liu et al., 2013; Rossaint et al., 2010). 5-HT has the effect of improving intestinal permeability (Wu et al., 2016). The level of D-LA can reflect intestinal permeability (Murdock et al., 2014). ET-1 is an endogenous pathogenic factor released during intestinal ischaemia, hypoxia and injury (Subrina et al., 2011). Thus, our results suggested that dietary β-conglycinin could cause an increase in intestinal permeability, destroy intestinal mucosal barrier and lead to digestion and absorption disorders. In grass carp study, glycinin, as one of soybean antigen protein, enhanced the levels of DAO and D-LA in serum, impaired the intestinal health and structural integrity (Zhang et al., 2020). In piglet studies, these results showed that dietary β-conglycinin could destroy intestinal mucosa, increase intestinal permeability and increase the levels of 5-HT in serum (Wu et al., 2016). An increase in intestinal permeability increases the possibility of harmful substances invading the body, seriously endangering intestinal health in animals (Tilg et al., 2019). The reason for the increase in intestinal permeability may be that dietary β-conglycinin destroys the tight junction structure (TJs) of intestine (Zhang et al., 2020). TJs are dynamic multi-protein complex, which can control the permeability of the intestinal barrier as a selective and semi-permeable intercellular barrier. TJs are composed of Claudin, Zonula occludens (ZO) and Occludin (Visser et al., 2009). These tight junction complex structures can effectively seal the intercellular space and prevent the invasion of pathogens, endotoxins and germs (Dokladny et al., 2016). In our study, dietary β-conglycinin could decrease the expressions of ZO-1, Claudin, Occludin-a and Occludin-b, except the expression of ZO-1 which were increased in PI (β-20 and β-40 groups) and in MI (β-20 group). These results indicated that dietary β-conglycinin could destroy the tight junction structure of intestine. However, the low level of β-conglycinin led to an increase in the expression of ZO-1 in PI and MI. It was speculated that the low level of β-conglycinin was equivalent to a stimulant, which stimulated the body to reduce intestinal permeability to improve intestinal health. Our results also found a phenomenon that the negative effects of β-conglycinin on PI were lower than MI, MI were lower than DI. As far as the expression of ZO-1 was concerned, 40 g/kg β-conglycinin could increase the expression of ZO-1 in PI, but 40 g/kg β-conglycinin could reduce the expression of ZO-1 in MI and DI. These results of the expression of ZO-1 and Claudin indicated that β-conglycinin had different effects on MI and DI. Previous study was consistent with our results that the soybean antigen protein glycinin had different effects in different intestinal segments and had the most serious impact on DI (Zhang et al., 2020). The reason for different effects of soybean antigen protein on intestinal tight junctions among three intestinal segments may be that soybean antigen protein has different oxidative damage to different intestinal segments. Glycinin increased the content of ROS in the PI, MI and DI of grass carp, from high to low, DI, MI and PI respectively. Moreover, the unique epitope of β-conglycinin is also the main reason for the different effects of β-conglycinin on the tight junctions of different intestinal segments in Rhynchocypris lagowski Dybowski (Zhao et al., 2008). However, the effects of β-conglycinin on intestinal tight junctions among three segments are not clear and need further study.
5 CONCLUSION
In our study, these results suggested that dietary β-conglycinin could reduce the activities of protease in intestines and hepatopancreas, decrease the activities of protein metabolizing enzymes(GPT) in hepatopancreas, increase the activities of GPT in serum, increase intestinal permeability, reduce the expression of intestinal tight junctions-related genes and reduce the expression of IGF-I and TOR in Rhynchocypris lagowski Dybowski. Moreover, the negative effects of β-conglycinin on intestines from high to low were DI, MI and PI in Rhynchocypris lagowski Dybowski.
ACKNOWLEDGEMENT
The work was supported by Natural science fund project of science and technology department of Jilin province (20170101026JC).
CONFLICT OF INTEREST
No conflict of interest exists in the submission of this manuscript, and the manuscript is approved by all authors for publication.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available.




