Liver immune parameters, complement pathway, inflammatory factor and TOR genes expression of silver sillago, Sillago sihama, fed with diets replacing fish meal with low-gossypol cottonseed meal
Funding information
National Key R&D Program of China (2019YFD0900200); National Natural Science Foundation of China (31972808); China Agriculture Research System (CARS-47); ‘Chong yi liu (231419011)’ project of Guangdong Ocean University
Abstract
A 56-day feeding trial was conducted to evaluate the effects of replacing fish meal (FM) with low-gossypol cottonseed meal (LCSM) on liver immune of silver sillago Sillago sihama Forsskál (1775). Five isonitrogenous and isolipidic diets were formulated with 0%, 16%, 32%, 48% and 64% LCSM replacing FM level (supplemented with methionine and lysine, named LCSM0, LCSM160, LCSM320, LCSM480 and LCSM640). Growth indices were significantly higher in the LCSM0 and LCSM160 groups than in the LCSM480 and LCSM640 groups. Malondialdehyde (MDA) content, alkaline phosphatase (AKP) activity and reactive oxygen species (ROS) concentrations in the liver of the control group were higher than those of the treatment group. Liver catalase (CAT), glutathione peroxidase (GSH-Px), total superoxide dismutase (T-SOD), lysozyme (LYZ) activity, immunoglobulin M (IgM) levels and total antioxidative capacity (T-AOC) levels were highest in LCSM320 group. Dietary LCSM substitution up-regulated the expression of liver interleukin 10 (IL-10) and downregulated tumour necrosis factor-α (TNF-α), target of rapamycin (TOR), insulin-like growth factor 1 (IGF-1) and transforming growth factor beta-3 (TGF-β3) expression. The relative mRNA expression level in liver nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and interleukin one beta (IL-1β) was not affected by dietary LCSM levels. The fish meal replacement level reached 32% and some genes in the complement pathway were significantly up-regulated (p < .05) including complement component 3 (C3), complement factor H (CFH), complement factor B (CFB) and MBL-associated serine protease 1 (MASP1). These results show that 16% of the fish meal replaced by LCSM with amino acids (methionine and lysine) supplementation did not significantly reduce immunity or antioxidant parameter compared with the FM-based control.
1 INTRODUCTION
Fish meal is a vital source of essential amino acids, essential fatty acids and energy in aquatic animal diets (Miles & Chapman, 2006). However, steadily rising costs in fish meal have become the main limiting factor in aquaculture expansion; hence, fish meal is being replaced with cheaper plant proteins (Bian et al., 2017). Cottonseed meal (CSM) is much cheaper per unit of protein than fish meal (FM) and other FM alternative protein sources due to global mass production of cotton and cottonseed by-products (Anderson et al., 2016). CSM is a high-quality plant protein source for aquaculture fish diets (Anderson et al., 2016; Hu et al., 2007). However, free gossypol (FG) within CSM has anti-nutritional and anti-fertility effects on warm-blooded animals and fish (Anderson et al., 2016; Romano & Scheffler, 2008). On one hand, FG can bind with the amino group of lysine, reducing the utilization rate of lysine and emphasizing the characteristics of lysine as the first limiting amino acid of CSM (Wilson et al., 2018); on the other hand, FG affects the growth of aquatic animals (Bu et al., 2017; Cai et al., 2011; Gui et al., 2010; Wang, Li, et al., 2014; Zhou et al., 2017). Due to the use of low-temperature primary leaching and two solvent step-by-step extraction processes in processing, low-gossypol cottonseed meal (LCSM) has dramatically reduced protein denaturation and removed gossypol, qualitatively improving nutrient level (Liu et al., 2000). The FG content of LCSM is ≤0.06 g/kg, the crude protein content is above 500.0 g/kg and the ratio of total amino acid in the crude protein is 95% (He et al., 2015). The concentration of CSM added to animal and fish diets is increased by reducing or eliminating gossypol (Alam et al., 2018; He et al., 2015; Li et al., 2008). LCSM can replace some plant and animal protein sources such as soybean meal and fish meal and is widely used in livestock, poultry (Lindsey et al., 1980) and aquatic diets (Anderson et al., 2016), and is a potential substitute for FM in the fish diet.
Sillago sihama Forsskál (S. sihama) is mainly distributed in the shallow seas of the India-West Pacific Ocean and coastal areas of China. S. sihama is economically important in coastal areas of China due to the excellent meat quality and extremely high nutritional value, and plays a vital role in fishing activities in Chinese coastal waters. Recent overfishing has depleted natural sources of S. sihama, and the market price has continued to rise (Huang et al., 2020). To meet market demand, Guangdong Ocean University has successfully cultivated the first batch of artificially breeding S. sihama. Current research on S. sihama mainly focuses on morphology (Cao et al., 2010; Wan, 1996), reproductive biology and artificial breeding (Huang et al., 2013; Lee, 1981), genetics (Guo et al., 2014; Zhang et al., 2018), tissue physiology (Cao et al., 2008; Cao et al., 2010) and ecology (Lu et al., 2008).
Few studies have addressed nutrition in S. sihama, aside from our laboratory studies that investigated vitamin A, vitamin C and vitamin E requirement in the species (Huang et al., 2018, 2020, 2020). In addition, the lack of exclusive forage production is due to the paucity of nutritional physiology research, leading to the use of shrimps or marine fish diets to cultivate the S. sihama. Previously, our laboratory has demonstrated that high levels of LCSM in diet can have a negative impact on the intestinal health of S. sihama (Liu et al., 2020); however, the effect of LCSM on the liver of S. sihama has not been reported. In this study, the effect of dietary LCSM on the antioxidant and non-specific immune parameters of the liver of S. sihama was investigated to provide basic data for the use of LCSM in the diet of S. sihama.
2 MATERIAL AND METHODS
2.1 Feed ingredients
Low-gossypol cottonseed meal (free gossypol content is 0.0079 g/kg, tested by Société Générale de Surveillance [SGS]) was supplied by the Hunan Xinrui Biological Technology Co. Ltd, and FM was supplied by the China National Township Enterprises Corporation (68% crude protein and 7.3% total lipid on dry matter basis). Chemical compositions of the test ingredients are shown in Table 1.
| Components | White fish meal (FM) | Low-gossypol cottonseed meal (LCSM) |
|---|---|---|
| Dry matter | 951.0 | 929.0 |
| Crude protein | 680.0 | 649.0 |
| Crude lipid | 73.0 | 5.0 |
| Free gossypol | 0.0079 | |
| NEAAa | ||
| Ala | 40.3 | 22.9 |
| Asp | 62.4 | 54.2 |
| Tyr | 24.0 | 17.4 |
| Ser | 30.4 | 24.8 |
| Glu | 91.9 | 123.3 |
| Gly | 46.3 | 24.9 |
| Cys | 6.2 | 11.0 |
| Pro | 29.4 | 21.3 |
| EAAb | ||
| Val | 31.8 | 25.4 |
| Met | 19.9 | 7.4 |
| Ile | 27.7 | 18.3 |
| Leu | 49.3 | 34.3 |
| Thr | 29.2 | 18.7 |
| Phe | 26.0 | 33.1 |
| His | 13.5 | 16.8 |
| Lys | 50.7 | 24.7 |
| Arg | 43.0 | 72.2 |
- a NEAA: nonessential amino acid.
- b EAA: essential amino acid.
2.2 Fish, diets and experimental design
The present study used the same growth trial as our previous study (Liu et al., 2020). Composition and nutrients contents of the experimental diets are presented in Table 2. Five isonitrogenous, isolipidic diets were formulated to replace 0%, 16%, 32%, 48% or 64% of fish meal with a corresponding quantity of protein with LCSM (LCSM0, LCSM160, LCSM320, LCSM480 and LCSM640). Methionine and lysine were added to experimental diets to compensate for the imbalance. All raw materials were crushed and passed through 60 mesh screens. Feedstuffs were weighed accurately according to the formula and thoroughly mixed in a Hobart-type mixer. After addition of oils, choline chloride and water, the compound was made into pellets through F-26 double-screw extruder. The diets were air-dried, sealed in plastic Ziploc bags and stored in a −20°C refrigerator with a moisture content of approximately 10%.
| Ingredients | Experimental diets | ||||
|---|---|---|---|---|---|
| LCSM0 | LCSM160 | LCSM320 | LCSM480 | LCSM640 | |
| White fish meal | 550.0 | 461.5 | 373.0 | 284.5 | 196.0 |
| Low-gossypol cottonseed meal | 0.0 | 88.5 | 177.0 | 265.5 | 354.0 |
| Soybean protein concentrated | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 |
| Vital wheat gluten | 70.0 | 70.0 | 70.0 | 70.0 | 70.0 |
| High-protein flour | 189.5 | 189.5 | 189.5 | 189.5 | 189.5 |
| Fish oila | 21.9 | 27.6 | 33.3 | 39.1 | 44.8 |
| Phospholipid | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 |
| Mineral mixtureb | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Vitamin mixturec | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Ca(H2PO4)2 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Antioxidant | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Choline chloride | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Microcrystalline cellulose | 50.3 | 41.2 | 32.1 | 23.0 | 13.9 |
| Methionined | 0.00 | 2.3 | 4.6 | 6.9 | 9.2 |
| Lysined | 0.00 | 1.1 | 2.2 | 3.3 | 4.3 |
| Carboxymethyl cellulose sodium | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Attractante | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Proximate compositionf | |||||
| Dry matter | 883.7 | 880.9 | 879.8 | 880.9 | 877.1 |
| Crude protein | 495.6 | 495.3 | 492.9 | 499.4 | 499.2 |
| Crude lipid | 89.7 | 92.5 | 94.7 | 96.1 | 92.3 |
| Ash | 147.9 | 135.0 | 120.1 | 108.3 | 94.6 |
| Free gossypol content (mg/kg) | 0 | 6.99 | 13.98 | 20.97 | 27.96 |
- a Semirefined fish oil, Oleaginosa Victoria S.A.
- b Mineral mixture (mg/kg diet): KIO4 0.15, CoCl2 ·6H2O 20.27, CuSO4·5H2O 99.2, FeC6H5O7 68.55, ZnSO4 · 7H2O 141.4, MgSO4·7H2O 0.6, CaH2PO4 400, KCl 76.65, Na2SeO3 10, elite power 4183.1. (Obtained from Zhanjiang Yuehai Feed Co. Ltd).
- c Vitamin mixture (mg/kg diet): vitamin B1, 51; vitamin B2, 50; vitamin B6, 100; vitamin B12, 0.2; vitamin K3, 10; vitamin E, 198; Vitamin A, 20; vitamin D3, 100; nicotinic acid, 202; D-calcium pantothenate, 122; biotin, 50; folic acid, 12.5; inositol, 306.12; cellulose,778.18. (Obtained from Zhanjiang Yuehai Feed Co. Ltd).
- d Methionine and lysine were added to balance amino acid with control group.
- e Attractant composition: taurine: glycine: betaine = 1:3:3; they are obtained from Hangzhou King Techina Technology.
- f Moisture, crude protein, crude lipid and ash contents were measured value.
The proximate composition of the experimental diets contained an average of 49.64% and 9.30% of crude protein and lipid, respectively, without any significant difference. The proximate composition of the test diets and essential amino acid content are shown in Tables 2 and 3, respectively.
| Amino acid | Experimental diets | ||||
|---|---|---|---|---|---|
| LCSM0 | LCSM160 | LCSM320 | LCSM480 | LCSM640 | |
| NEAAa | |||||
| Ala | 25.56 | 24.02 | 22.48 | 20.91 | 19.40 |
| Asp | 40.65 | 39.92 | 39.20 | 38.41 | 37.75 |
| Tyr | 16.90 | 16.31 | 15.73 | 15.12 | 14.56 |
| Ser | 21.26 | 20.77 | 20.27 | 19.75 | 19.28 |
| Glu | 82.09 | 84.87 | 87.65 | 90.32 | 93.21 |
| Gly | 29.29 | 27.40 | 25.50 | 23.57 | 21.71 |
| Cys | 5.60 | 6.02 | 6.45 | 6.86 | 7.30 |
| Pro | 26.16 | 25.44 | 24.73 | 23.98 | 23.29 |
| EAAb | |||||
| Val | 21.88 | 21.31 | 20.74 | 20.15 | 19.61 |
| Met | 12.25 | 13.44 | 14.63 | 15.81 | 17.02 |
| Ile | 19.58 | 18.74 | 17.91 | 17.06 | 16.25 |
| Leu | 33.61 | 32.28 | 30.95 | 29.58 | 28.30 |
| Thr | 19.05 | 18.12 | 17.19 | 16.24 | 15.33 |
| Phe | 19.63 | 20.26 | 20.89 | 21.49 | 22.15 |
| His | 9.90 | 10.19 | 10.48 | 10.76 | 11.07 |
| Lys | 31.30 | 30.10 | 28.90 | 27.66 | 26.39 |
| Arg | 28.89 | 31.47 | 34.06 | 36.58 | 39.23 |
- a NEAA: nonessential amino acid.
- b EAA: essential amino acid.
2.3 Experimental animals and breeding management
Juvenile S. sihama were obtained from Guangdong Ocean University. Before the trial, juvenile fish were temporarily cultured in a cement pond measuring 4.5 × 3.45 × 1.8 m and fed commercial diets. A total of 450 healthy juvenile S. sihama (5.8 ± 0.58 g) were randomly distributed into 15 fibreglass tanks. Each diet was randomly assigned to three groups of 30 fish per tank. The experiment was carried out in an indoor marine culture system of the Marine Biology Research Base of Guangdong Ocean University. Salinity of the seawater was adjusted to 6–8 during 56 days experimental period. Seawater was disinfected with chlorine dioxide and aerated for 24 h before changing. The daily water temperature ranged 28.4–31.5°C and the pH ranged 7.5–8.0. The dissolved oxygen content was ≥6 mg/L, and the ammonia nitrogen and nitrite concentrations were ≤0. 5 mg/L. Fish were fed twice daily (8:00 and 16:00) to apparent saturation (at the beginning of feeding, fish fed at the surface of the water and feeding intensity subsequently decreased; when the fish swam towards the bottom of the tank and no longer came to feed, this was considered apparent saturation). Procedures involving animals were conducted in conformity with NIH guidelines and were approved by the Animal Care and Use Committee of the Guangdong Ocean University.
2.4 Sample collection and analysis
At the end of the experiment, fasting for 24 h, three fish were randomly taken from each repeating tank, dissected the liver and quickly placed in liquid nitrogen for the subsequent determination of enzyme activity and real-time fluorescence quantitative determination of related genes. The raw materials and dietary proximate analysis of dry matter (dried at 105°C), crude protein(by Kjeldahl apparatus, nitrogen ×6.25), crude lipid (extraction with petroleum ether by Soxhlet apparatus) and ash content(incineration at 550°C) were determined according to the established methods of Association of Official Analytical Chemists (AOAC, 1995). The raw materials and dietary amino acid profile were measured by an automatic amino acid analyzer 433D (SYKAM) after acid hydrolysis in 6 m HCl for 24 h at 110°C (Tryptophan in diet was alkaline hydrolysed in 4 m LiOH for 24 h at 110°C). Liver immune parameters (lysozyme [LYZ], alkaline phosphatase [AKP], immunoglobulin M [IgM]) and liver antioxidant parameters (total antioxidative capacity [T-AOC], total superoxide dismutase [T-SOD], catalase [CAT], glutathione peroxidase [GSH-Px], reactive oxygen species (ROS) and malondialdehyde [MDA]) were determined using the ELISA kit (Shanghai Enzyme-linked Biotechnology Co., Ltd). The liver was weighed, thawed and homogenized in a 9-volume frozen buffer and centrifuged at 4°C for 20 min. Supernatants were carefully collected where aliquots of samples were used in the testing process. The final tissue concentration was obtained by dividing the result by total protein (TP) in liver.
2.5 Real-time quantitative RT-PCR analysis of gene expression
Total RNA was extracted from the liver using a General RNA Extraction Kit, and integrity was detected by electrophoresis on 1.2% denatured agarose gel. RNA integrity and quality were assessed using a NanoDrop 1000 spectrophotometer. A PrimeScript™ RT-PCR Kit was used to perform first-strand cDNA synthesis in RT according to the manufacturer's instructions (Wang et al., 2008). The RNA was treated with gDNA Eraser, and 1.0 μg was used for reverse transcription with a PrimeScript RT Reagent Kit. Real-time PCR assays were carried out in a quantitative thermal cycler in a 10 μl reaction volume containing 5 μl SYBR@ Green Real-time PCR Master Mix, 1 μl of cDNA, 0.8 μm of each primer and 3.2 μl of sterilized double-distilled water. Each sample in the reaction was treated in triplicate. Date acquisition occurred every 6 s; hence, the thermal programmer included 30 s at 95°C, 40 cycles at 95°C for 5 s, 60°C for 34 s and a melt curve step from 60°C gradually increasing 0.5°C/s–95℃. According to the results of our preliminary experiment concerning the evaluation of internal control genes, 60S ribosomal protein L38 was used as a reference gene to normalize cDNA loading. Gene expression results were analysed using the 2−ΔΔCT method according to Livak and Schmittgen (2001). Normalized gene expression for the control group was set at 1. All primers were designed using PrimerQuest Tool, and primer sequences are listed in Table 4.
| Name | Primer type | Sequence | Genbank no. | Function | Amplicon |
|---|---|---|---|---|---|
| 60s | Forward Reverse |
GACAGCCAGGAGGAAGGATG TGTCTGTGATGACCAGGGTG |
ACN10033.1 | Housekeeping gene | 219 |
| C1RA |
Forward Reverse |
CAAACCTCATCTCTGGCTATCA CTCCTGCTCTGGCTCTATTTAC |
AIZ96979.1 | Classical pathway | 105 |
| C3 |
Forward Reverse |
GGAGGTCCTCTACAGGTTTAGT CCAGGGCAAGAAGACCATAAG |
ADU33222.1 | Central components | 104 |
| C4 |
Forward Reverse |
GTGAGCATGCCAAAGGAAAC GGCTTCAGTTGTCCAGAGATAG |
AIN76766.1 | Classical pathway | 102 |
| C5 |
Forward Reverse |
TGTGATCCTTCACGTCCAATC GGTTTGTCGGTCTGGATGAA |
XP_020505432.1 | Central components | 96 |
| CFH |
Forward Reverse |
GAACTTCCGGTGATCCCTAATG CTGTCATTCTGAGCCTGGTATC |
XP_018526364.1 | Alternative pathway | 98 |
| CFB |
Forward Reverse |
CAGACCTGAACCTCGTCTTTAC GCAGGTGAACCACCCATATTA |
XP_018547243.1 | Alternative pathway | 142 |
| MASP1 |
Forward Reverse |
CAACCTCAATGCCTCCTTCT CTCACACTCGTCCTGAGAAAC |
XM_027280128.1 | MB-Lectin pathway | 118 |
| TGF-β3 |
Forward Reverse |
TTCAGGTTCTGGCCCTTTAC TACTCAGTCTCCGTGTTATCCT |
XP_010767476.1 | Inflammation related genes | 100 |
| NF-κB |
Forward Reverse |
GGGATGGTTCAGGTTCTACTG CTGAGAGAGGACAGCTGATTG |
XP_027136364.1 | 119 | |
| IL-1β |
Forward Reverse |
GACAGCGACATGGTGAGATT CCCTTGCTGTGCTGATGTA |
AQR55700.1 | 97 | |
| TNF-α |
Forward Reverse |
CTGGTCCACCACATATGGAAA ACCAGCTACCCTCATCATCTA |
AAZ20770.1 | 119 | |
| IL-10 |
Forward Reverse |
GCAGATCTTCGACCAGATCAA GTACGTGGAGTTCAGGGTATTT |
AIC33826.1 | 97 | |
| TOR | Forward | GCTGTACCAGGCACTTATGA | KY985002.1 |
TOR pathway genes |
88 |
| Reverse | GCTGCTTGGAGGTGATGA | ||||
| IGF-I | Forward | GCCATAGCCTGGTTTACTGA | JQ794830.1 | 118 | |
| Reverse | CCTGACTCCGACGGCAACA |
- Abbreviations: 60S, 60S ribosomal protein L38; C1RA, complement component 1r; C3, complement component 3; C4, complement component 4; C5, complement component 5; CFB, complement factor B; CFH, complement factor H; IGF-1, insulin-like growth factor 1; IL-10, interleukin 10; IL-1β, interleukin one beta; MASP1, MBL-associated serine protease 1; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; TGF-β3, transforming growth factor beta-3; TNF-α, tumour necrosis factor-α; TOR, target of rapamycin.
2.6 Statistical analysis
Normality and homoscedasticity analyses were adopted before applying a one-way ANOVA test using SPSS statistics 16 (IBM Corp) for Windows software. Tukey's HSD post hoc test was subsequently used to determine differences among treatments with significance set at p < .05 (Dossou et al., 2018; Wu et al., 2018). Polynomial contrasts were executed to determine whether a linear or quadratic effect existed (Huang et al., 2020; Wu et al., 2018).
3 RESULTS
3.1 Liver antioxidant parameters
Results of liver antioxidant parameters are shown in Table 5. T-SOD activity initially increased and subsequently decreased significantly linearly and quadratically (p < .05) with the highest value occurring in the LCSM320 group. CAT and GSH-Px increased significantly in the LCSM320 group and subsequently decreased significantly quadratically (p < .05) in the LCSM640 group. MDA and ROS concentration in the LCSM0 group were significantly higher than those in other groups and were all reduced in the LCSM320 group. The T-AOC concentration was not affected by the dietary LCSM level (p > .05).
| Items | LCSM0 | LCSM160 | LCSM320 | LCSM480 | LCSM640 | SEM | p value | Polynomial contrasts | |
|---|---|---|---|---|---|---|---|---|---|
| Linear | Quadratic | ||||||||
| T-SOD (U/g prot) | 10.40a | 13.65b | 18.60c | 14.68b | 13.98b | 0.605 | .000 | 0.001 | 0.000 |
| T-AOC (U/mg prot) | 1.92 | 1.86 | 1.95 | 1.93 | 1.64 | 0.042 | .099 | 0.078 | 0.082 |
| CAT (U/mg prot) | 52.56a | 58.37ab | 64.90b | 55.41a | 49.73a | 1.448 | .001 | 0.202 | 0.000 |
| GSH-Px (U/g prot) | 12.21a | 13.80bc | 14.67c | 13.49abc | 13.14ab | 0.247 | .019 | 0.251 | 0.002 |
| ROS (U/mg prot) | 40.53c | 25.33b | 20.47a | 25.12b | 24.85b | 1.655 | .000 | 0.000 | 0.000 |
| MDA (nmol/mg prot) | 1.27b | 0.97a | 0.96a | 0.82a | 0.78a | 0.049 | .002 | 0.000 | 0.149 |
Note
- Values in the same row not sharing the same letter are significantly different (a,b,c, ANOVA, p < .05); SEM, Pooled standard error of the mean.
- Abbreviations: CAT, catalase; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; ROS, reactive oxygen species; T-AOC, total antioxidative capacity; T-SOD, total superoxide dismutase.
3.2 Liver immunological parameters
Liver immunological parameters are presented in Table 6. LYZ activity changed quadratically (p < .05) with significantly higher values in LCSM160, LCSM320 and LCSM480 than in other diets. IgM content initially increased and subsequently decreased linearly and quadratically (p < .05) with the maximum occurring in LCSM320 (p < .05). Higher AKP activity was detected in control group compared with all treated groups, with linear and quadratic models (p < .05).
| Items | LCSM0 | LCSM160 | LCSM320 | LCSM480 | LCSM640 | SEM | p value | Polynomial contrasts | |
|---|---|---|---|---|---|---|---|---|---|
| Linear | Quadratic | ||||||||
| LYZ (U/g prot) | 3.05a | 4.17b | 4.63b | 4.11b | 3.22a | 0.164 | .001 | 0.701 | 0.000 |
| IgM (μg/g prot) | 24.45a | 32.77bc | 35.69c | 29.66b | 31.20b | 0.981 | .000 | 0.016 | 0.000 |
| AKP (U/g prot) | 22.07b | 12.34a | 11.43a | 12.27a | 12.00a | 0.701 | .000 | 0.000 | 0.000 |
Note
- Values in the same row not sharing the same letter are significantly different (a,b,c, ANOVA, p < .05); SEM, Pooled standard error of the mean.
- Abbreviations: AKP, alkaline phosphatase; IgM, immunoglobulin M; LYZ, lysozyme.
3.3 Liver gene expression
3.3.1 Expression levels of complement-related genes
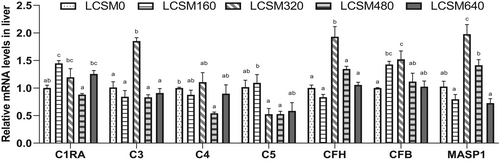
Expression levels of complement component 3 (C3), complement component 4 (C4), complement component H (CFH), complement component B (CFB) and MBL-associated serine protease 1 (MASP1) were highest in the LCSM320 group, and C3 and CFH were significantly higher in the LCSM320 group than those in other groups (Figure 1). The expression level of C4 in the LCSM320 group was significantly higher than that in the LCSM480 group, but there was no significant difference with other groups. CFB expression in the LCSM320 group was significantly higher than that in the LCSM0, LCSM480 and LCSM640 groups. Maximum expression of liver complement component 1r (C1RA) and complement component 5 (C5) occurred in the LCSM160 group and was significantly higher than those in the LCSM480 group.
3.3.2 Expression levels of inflammation-related genes
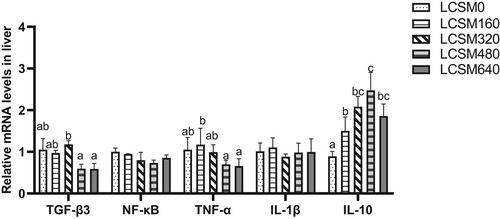
Expression levels of transforming growth factor beta-3 (TGF-β3) genes in LCSM480 and LCSM640 groups were significantly lower than that in LCSM320 group (Figure 2). The expression of the tumours necrosis factor-α (TNF-α) gene was significantly higher in LCSM160 group than that in LCSM480 and LCSM640 groups but not significantly different from the control group. There was no significant difference in the expression levels of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and interleukin one beta (IL-1β) among treatments. The expression of interleukin 10 (IL-10) was higher in LCSM480 group than that in LCSM0, LCSM160 and LCSM640 groups.
3.3.3 Expression levels of TOR and IGF-1genes
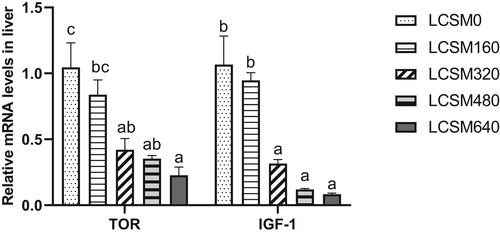
Expression levels of target of rapamycin (TOR) and insulin-like growth factor I (IGF-1) in the liver decreased significantly with the increase of LCSM replacement FM level (Figure 3). Expression levels of TOR and IGF-1 were not significantly different between the control group and the LCSM160 group, but these levels were significantly higher in the control group than other treatments.
4 DISCUSSION
This study used the same growth trial as in our previous study, which observed that 88.5 g/kg (16%) of FM replaced by LCSM with amino acids (methionine and lysine) supplementation did not significantly reduce growth or feed utilization compared with FM-based control, and excess LCSM in the diet can affect the intestinal health and thus the digestive and absorption function of the S. sihama (Liu et al., 2020). The liver, as the centre of metabolic function, plays an important role in both fish growth and immunity (Carambia & Herkel, 2018). Many previous studies showed that the metabolic and immunological function mainly rely on the liver health in aquatic animals (Caballero-Solares et al., 2018; Naderi et al., 2017; Wang, Liu, et al., 2014). Therefore, the present study aimed to investigate the effects of LCSM on liver antioxidant and immunity of S. sihama and their possible mechanisms, and to provide basic data for the utilization of LCSM in the marine fish diet.
Antioxidant enzymes are the first line of defence against free radicals in the complex immune system of fish (Deng et al., 2015). During healthy metabolism in organisms, the production and elimination of reactive oxygen species such as superoxide anion radical, hydroxyl radical and hydrogen peroxide maintain the dynamic balance (Bu et al., 2017). MDA is the end product of lipid peroxidation by free radicals that can cause the cross-linking polymerization of proteins, nucleic acids and other living macromolecules, and has cytotoxicity as an indicator of the antioxidant reactions of body (Buege & Aust, 1978). During the transformation of oxygen (O2) into water (H2O) in the cells of the body, many ROS will be produced. A few ROS will leak into the reaction process and become redundant oxygen radicals, and this will lead to tissue damage, induce various diseases and promote the ageing of the body (Johnson, 2002). The substitution of CSM for fish meal in the Ussuri catfish (Pseudobagrus ussuriensis) would lead to an increase in MDA and decrease in total antioxidant parameters (Bu et al., 2017), as found similarly in grass carp (Zheng et al., 2012). However, in this study, we found that liver MDA content and ROS activity were significantly lower in the LCSM320, LCSM480 and LCSM640 groups than those in the LCSM0 group. CAT, T-AOC and T-SOD activities in liver initially increased and subsequently decreased with increasing LCSM replacement levels and reached the maximum value in the LCSM320 group. This difference could be due to LCSM having a much lower gossypol content than CSM, thereby reducing liver damage and improving S. sihama tolerance of plant protein. In addition, a number of studies have concluded that the level of arginine in the diet affects liver MDA content. Studies in hybrid sturgeon (Acipenser schrenckii♀ × Acipenser baeri♂) (Wu, 2016) found that liver MDA content decreased with increasing levels of dietary arginine, and similar conclusions were reached in the current study. However, it is not known whether there are other unknown active factors that affect liver MDA content, which requires further study.
The non-specific immune system plays a vital role in disease resistance and health status of fish (Anderson, 1992). In this study, we found that LYZ activity was significantly higher than that of the LCSM0 group in all treatment groups except the LCSM640 group; liver IgM concentrations were significantly lower in the control group than in the other treatment groups. Results indicated that replacement of an appropriate quantity of fish meal with LCSM could enhance the non-specific immunity of S. sihama, whereas excessively high replacement of fish meal would inhibit non-specific immunity. Study of blunt snout bream (Abasubong et al., 2018) has also shown that replacing fish meal with rice protein powder could improve the non-specific immunity of blunt snout bream to a certain extent. Also, studies have shown that gossypol in CSM can increase chemotaxis of macrophages in juvenile channel catfish (Ictalurus punctatus) (Yildirim et al., 2003), thereby improving the immune response of the juvenile fish. However, LCSM replacement of fish meal reduced intestinal immunity and disease resistance in hybrid grouper (Yin et al., 2018). Excessive CSM replacement of fish meal reduced serum lysozyme and alkaline phosphatase activity, resulting in adverse effects on non-specific immunity of juvenile Ussuri catfish (Bu et al., 2017). These results differ from those in this study, but these differences are justified in that plant protein may have different effects on immune function depending on species, size, nutritional status and dietary composition of the fish (Azeredo et al., 2017).
Replacing fish meal with high concentrations of plant proteins can cause inflammatory reactions in the intestinal tract (Urán et al., 2008; Yuan et al., 2019). Most research on the inflammatory response focuses on the intestines rather than the liver. In this study, the high level of substitution did not significantly up-regulate the liver pro-inflammatory genes (NF-κB and IL-1β) compared with control groups and the anti-inflammatory gene TGF-β3 was found to be inhibited at a high level of substitution. We also found that the anti-inflammatory gene IL-10 was significantly up-regulated with the increase in LCSM concentration and the relative mRNA expression level of IL-10 reached the maximum in group LCSM480. Results showed that the high substitution diet might inhibit the occurrence of inflammation by up-regulating the expression of IL-10. Replacing more than 36% of fishmeal in the diet with cottonseed protein concentrate resulted in up-regulation of pro-inflammatory genes and down-regulation of anti-inflammatory genes in the gut of hybrid grouper, which is inconsistent with the present study (Yin et al., 2018). This difference in results may differ with the species of fish and the tissues measured. There is currently less research on the effects of LCSM on the fish liver, and further research is needed in this area in the future.
The complement system plays a vital role in alerting the host to the presence of potential pathogens and thereby leads to removal of the pathogens (Boshra et al., 2006). Although extrahepatic synthesis of complement proteins is well documented and different cell types are involved in the production of complements, most complement proteins are synthesized as inactive precursors in the liver (Laufer, 2001). There are three activation pathways in the complement namely: classical complement pathway (CCP), the MB-Lectin complement pathway (LCP) and alternative complement pathway (ACP) (Yuan et al., 2017). This study showed that a moderate amount of low-gossypol cottonseed protein, as added to the LCSM320 diet, can activate CCP, LCP and ACP to significantly improve the relative expression levels of C3, C4, CFH, CFB and MASP1. In contrast to the relative expression level of C5 in the LCSM320 group, the relative expression level of C3 in the LCSM320 group was significantly higher than that in the fish meal group. The reasons for this discrepancy may be linked to the CFH gene. This gene has been reported to be activated by ACP in the central regulatory factors formed in the C3b proteolytic inactivation iC3b process as the cofactor of CFI (Rodríguez De Córdoba et al., 2004). CFH also accelerated the attenuation of C3 invertase C3bBb and competed with B factor to bind C3b that could lead to the decrease of C42b3b and finally affect the expression of C5 (Rodriguez et al., 2014). This result was similar to that for Jian carp (Cyprinus carpio var. Jian) (Yuan et al., 2017), in which the replacement of fish meal by yeast hydrolysate was able to activate the complement system using both CCP and ACP. Also, some studies have shown that complement factor H could bind malondialdehyde epitopes to prevent oxidative stress. Weismann et al. (2011) found that CFH was a major MDA binding protein that could block the uptake of MDA-modified protein by macrophages and the MDA inducing the pro-inflammatory effect in mice. In this study, we found that the expression of CFH was initially up-regulated and subsequently down-regulated with an increasing substitution level. This indicated that replacing the appropriate level of fish meal with LCSM could play a role in preventing oxidative stress, thereby being consistent with MDA content measured in the liver.
Protein synthesis is a vital component of the processes involved in the growth response (Anthony et al., 2001). The limiting step in protein synthesis is translation initiation that is regulated by the TOR-signalling pathway (Zhou et al., 2017). However, the TOR pathway is in turn regulated by insulin-like growth factors (IGFs) through activation of phosphatidylinositide 3-kinase. And IGFs modulated TOR-signalling pathway via activating phosphoinositide 3-kinase. In this study, IGF-1 mRNA levels decreased significantly with increasing LCSM substitution levels, similarly to a study on cobia (Luo et al., 2013), which found corn gluten meal reduced IGF-1 gene expression in the liver. As the level of LCSM substitution in fishmeal increased, the expression level of TOR in the liver and whole-body crude protein content decreased (whole-body crude protein data were published in a previous article (Liu et al., 2020)), suggesting that an excessively high concentration of LCSM in the diet may inhibit protein synthesis by inhibiting the expression of genes related to the TOR pathway, thereby inhibiting growth.
The expression level of TOR in the liver decreased as the substitution level of LCSM in fish meal increased, suggesting that an excessively high concentration of LCSM in the diet may inhibit protein synthesis by inhibiting the expression of genes related to the TOR pathway, thereby inhibiting growth. Dietary amino acids such as threonine, isoleucine and leucine regulated IGF-1 and TOR gene expression in the liver of blunt snout bream (Qian et al., 2014; Zhou et al., 2017), Jian carp (Cai et al., 2012) and rainbow trout (Wacyk et al., 2012). As the substitution level increased, dietary threonine, isoleucine and leucine showed a downward trend, which may be the reason why the expression of TOR and IGF-1 was inhibited. In addition, many studies have found that gossyphenols in diet also inhibit the expression of TOR and IGF-1(Cai et al., 2011; Wang, Li, et al., 2014). Studies of blunt snout bream found that dietary gossypol did not exceed 108 mg/kg without affecting growth performance and without significantly inhibiting TOR expression levels. But in this study, when the free dietary gossypol reached 13.98 mg/kg, growth performance reduced (Liu et al., 2020) and liver TOR expression inhibited. This was less than juvenile common carp (323.5 mg/kg, Wang, Li, et al. (2014)) and allogynogenetic silver crucian carp (642 mg/kg, Cai et al. (2012)). Yang et al. (2015) demonstrated that gossypol mediated the down-regulation of thymidylate synthase, the cyclin D1 protein and the inhibition of the mTOR/p70S6K1 signalling pathway. This may also occur because S. sihama is more sensitive to dietary gossypol, which affects the growth of S. sihama by inhibiting tor and IGF-1 in the liver. However, the underlying mechanism by which LCSM influences the expression of these genes in S. sihama remains to be elucidated.
5 CONCLUSIONS
Results of the current study demonstrated that 16% of FM replaced by LCSM with amino acid (methionine and lysine) supplementation did not negatively affect the non-specific immunity and antioxidant parameter of S. sihama liver compared with the FM-based control. Immune and antioxidant parameters both reached a maximum in the LCSM320 group. Yet, an excessively high concentration of LCSM in the S. sihama diet inhibited the expression of TOR and IGF-1 in the liver, which may affect growth.
ACKNOWLEDGEMENTS
National Key R&D Program of China (2019YFD0900200); National Natural Science Foundation of China (31972808); China Agriculture Research System (CARS-47); ‘Chong yi liu (231419011)’ project of Guangdong Ocean University. We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work. There is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled, ‘Liver immune parameters, complement pathway, inflammatory factor, and TOR genes expression of silver sillago, Sillago sihama, fed with diets replacing fish meal with low-gossypol cottonseed meal’.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethics.




