Dietary supplementation of betaine improves growth performance and reduces lipid peroxidation in Nile tilapia
Abstract
In this study, we evaluated the growth performance, body composition and feed efficiency, muscle and gut histology, blood haematology and plasma biochemical parameters and the activity of antioxidant enzymes in the liver of Nile tilapia fed diets supplemented with betaine. Increasing doses (0.00, 0.50, 1.00 and 2.00 g kg−1) were added to a basal diet and randomly assigned to three groups, composed of 12 fish (27.76 ± 1.31 g) in each experimental units. The fish were fed to satiation four times a day during 100 days. Dietary inclusion of 0.50 g kg−1 of betaine resulted in greater results in weight gain, daily weight gain, specific growth rate, as well as energy retention rate. Dietary inclusion of betaine resulted in less height, width and thickness of the tunic of the gut folds. Fish fed 0.50 and 1.00 g kg−1 of dietary betaine showed higher plasma glucose. The concentration of substances reactive to thiobarbituric acid was lower in fish fed with 0.50 g kg−1 of dietary betaine, while those fed with 1.00 g kg−1 dietary betaine show greater activity of the catalase enzyme. The dietary inclusion of 0.50 g kg−1 of betaine improves growth performance probably by reducing lipid peroxidation.
1 INTRODUCTION
World fish consumption has grown significantly in recent years (FAO, 2018), influencing the intensification of aquaculture production, as extractive fisheries are stagnant and expected to decline even more over the coming years. This scenario is currently established worldwide, especially in tropical regions, which, in turn, favours the production of one of the most reared species in the world, the Nile tilapia Oreochromis niloticus (Cacho et al., 2020; FAO, 2018).
Tilapia is currently produced in more than 140 countries (Fitzsimmons, 2015), and this scope is due to its outstanding characteristics, such as rapid growth, easy reproduction, resistance to high stocking densities and different temperatures (Castagnolli, 1992). Therefore, its increased production is related to advances in the fields of genetic improvement, nutrition, hygiene and management, with nutrition playing an important role in terms of reaching high productivity at low production costs, while maintaining the quality of marketed fish (Bostock, 2011; Henchion et al., 2017; Hua et al., 2019).
The efficiency of fish productions mostly relies in the feed that is offered to the animals and its daily management practices, as the animals should be able to express its highest potential when fed diets that meet their dietary requirements. In this sense, feed occupies the most expensive input in aquaculture farms, especially because of the elevated prices of protein ingredients (Ferreira et al., 2013). Thus, the establishment of strategies that allows an efficient use of this nutrient is imperative (Cho et al., 2005). One way to improve the use of dietary nutrients is through the use of feed additives, which can act as flavour enhancers, preservatives, chemotherapeutic agents, immunostimulants, antioxidants, probiotics and prebiotics (NRC, 2011; Rodrigues et al., 2015).
In this sense, betaine (trimethylglycine) is a quaternary amine derived from the amino acid glycine, with three groups of methyl linked to the nitrogen atom of this molecule (Holmstrom et al., 2000). Its main function is to provide methyl groups that are necessary for the synthesis of several substances, such as sulphur amino acids and carnitine. Thus, it acts indirectly in the lipid and protein metabolism (Eklund et al., 2005; Lever & Slow, 2010; Ratriyanto et al., 2009). Moreover, it has an effective action on the osmotic balance, because it is a polar molecule capable of retaining water inside cells and facilitating the function of ion pumps (Moeckel et al., 2002), besides improving the maintenance of gut structures, which, in turn, benefits nutrients digestibility (Eklund et al., 2005).
Thus, here, we evaluated the growth performance, body composition and feed efficiency, muscle and gut histology, blood haematology and plasma biochemical parameters and the activity of antioxidant enzymes in the liver of Nile tilapia juveniles fed diets supplemented with different levels of betaine.
2 MATERIAL AND METHODS
The experiment was conducted at the State University of West Paraná (Unioeste), in Toledo, Paraná, Brazil. The experimental procedures adopted in this study were approved by the Ethics Committee on Animal Use of the State University of West Paraná (CEUA/Unioeste), under protocol n° 45/17.
2.1 Experimental diets
A basal diet composed of practical ingredients was formulated to meet the dietary requirements for Nile tilapia. The betaine was included in a basal diet at three levels: 0.50, 1.00 and 2.00 g kg−1 (Table 1). Each level of betaine inclusion, in addition to the control diet, was tested in triplicate.
| Inclusion levels of betaine g kg−1 | ||||
|---|---|---|---|---|
| 0 | 0.50 | 1.00 | 2.00 | |
| Ingredients, g kg−1 | ||||
| Soybean meal | 447.40 | 447.20 | 447.00 | 446.50 |
| Ground corn | 219.30 | 219.20 | 219.10 | 218.90 |
| Wheat meal | 200.00 | 199.90 | 199.80 | 199.60 |
| Tilapia by-product | 50.00 | 50.00 | 50.00 | 49.90 |
| Poultry by-product meal | 50.00 | 50.00 | 50.00 | 49.90 |
| Soybean oil | 12.90 | 12.90 | 12.90 | 12.90 |
| Limestone | 12.10 | 12.10 | 12.10 | 12.10 |
| Mineral and vitamin Premixa | 5.00 | 5.00 | 5.00 | 5.00 |
| Sodium chloride (NaCl) | 3.00 | 0.30 | 0.30 | 0.30 |
| Butyl-hydroxyl-toluene | 0.20 | 0.20 | 0.20 | 0.20 |
| Calcium propionate | 0.10 | 0.10 | 0.10 | 0.10 |
| Betaine | 0.00 | 0.50 | 1.00 | 2.00 |
| Total | 1000 | 1000 | 1000 | 1000 |
| Nutrients, g kg−1 | ||||
| Digestible protein | 280.00 | 280.00 | 280.00 | 280.00 |
| Starch | 255.10 | 255.10 | 255.10 | 255.10 |
| Calcium | 12.00 | 12.00 | 12.00 | 12.00 |
| Digestible energy (kcal kg−1) | 3200 | 3200 | 3200 | 3200 |
| Total phosphorus | 7.60 | 7.60 | 7.60 | 7.60 |
| Lipids | 48.70 | 48.70 | 48.70 | 48.70 |
| Diet composition, g kg−1 | ||||
| Crude protein | 333.50 | 336.40 | 343.90 | 323.70 |
| Crude energy (kcal kg−1) | 4255 | 4239 | 4291 | 4236 |
| Crude fibre | 72.70 | 71.30 | 67.50 | 63.30 |
| Mineral matter | 98.80 | 89.00 | 89.20 | 85.90 |
- a Guarantee levels per kilogram of product. Vit. A – 500,000 UI; vit. D3 – 250,000 UI; vit. E – 5000 mg; vit. K3 – 500 mg; vit. B1 – 1500 mg; vit. B2 – 1500 mg; vit. B6 – 1500 mg; vit. B12 – 4000 mg; folic acid – 500 mg; calcium pantothenate – 4000 mg; vit. C – 10,000 mg; biotin – 10 mg; Inositol – 1000; nicotinamide – 7000; choline – 10,000 mg; Cobalt – 10 mg; Copper – 1000 mg; Iron – 5000 mg; Iodine – 200 mg; Manganese – 1500 mg; Selenium – 30 mg; Zinc – 9000 mg.
The ingredients used in the diets were ground individually in a hammer-type mill (0.5-mm screen mesh), manually sieved (0.5 mm), weighed and manually homogenized. Subsequently, the humidity of the diets was adjusted to 24% with water and extruded in an EX-LABORATORY extruder (EXTEEC, Ribeirão Preto, Brazil). The extrusion parameters were as follows: temperature, 100°C; thread speed, 200 rpm; flow rate, 40% of nominal capacity; thread diameter, 30 mm and cylinder length, 100 mm. After extrusion, the diets were dried in a forced air circulation oven at 55°C and, subsequently, packaged and stored in a freezer (−20°C) until the time of use.
2.2 Experimental design and procedures
One hundred and sixty masculinized Nile tilapia juveniles from the GIFT strain were used in the experiment (27.76 ± 1.31 g), of which 16 were used to the initial fillets composition, for further calculations of nutrients retention. The other 144 fish were randomly distributed in 12 experimental units (0.45 m3 useful volume), disposed in a water recirculation system (RAS) with controlled temperature and constant aeration, totalling four treatments with three replicates. The experimental design was completely randomized with four treatments, in triplicate, where the fish were fed four times a day (08:00, 11:00, 14:00 and 17:00) during 105 days, with the first 5 days being destined to the adaptation of fish to the experimental diets.
2.3 Water quality
Throughout the study, the following water quality parameters were evaluated: temperature (27.36 ± 0.92°C) with a digital thermometer, dissolved oxygen (7.94 ± 0.82 mg L−1), pH (7.5 ± 0.5) and electrical conductivity (80.22 ± 0.71 µS cm−1), with the aid of a portable multiparameter probe (YSI 556). The concentrations of ammonia and nitrite were assessed with colorimetric kits (LabconTest), but these did not exceed 0 ppm. According to Boyd (1990), all variables were found within the comfort ranges for the species.
2.4 Analytical procedures
At the end of feeding trial, the fish were anaesthetized with benzocaine solution (100 mg L−1) (Gomes et al., 2001) and subsequently weighed individually measured for further growth performance calculations. Three fish of each experimental unit had blood samples collected for further analysis of blood haematology and plasma biochemical parameters. Similarly, three fish per experimental unit were collected and anaesthetized and euthanized with benzocaine overdose (200 mg L−1) (Gomes et al., 2001) to collect samples for the following analyses: somatic indices, fillets composition, muscle and gut histology and the activity of antioxidant enzymes in the liver. The biological samples used in these analyses were collected according to the metabolic peak of blood concentration presented in Nile tilapia; thus, at the final day of experiment and previous to the analyses, the fish were schematically fed, respecting a 3-h metabolic time.
Fillet samples were stored at −20°C until processing (Weiler et al., 2019). For histological analysis, the muscle samples were fixed in ALFAC solution (Alcohol/Formaldehyde/Acetic Acid) for 16 h and samples were stored in 70% alcohol until processed, while the gut samples were placed on a polystyrene plate, opened longitudinally, washed in saline solution and fixed in 10% formaldehyde solution for 12 h and then preserved in 70% alcohol. Samples of liver tissues were stored in liquid nitrogen for further quantification of the concentration of oxidative stress-related enzymes.
2.5 Growth performance and somatic indexes
From the quantification of consumed feed and the animals' final weight, the following variables were calculated: weight gain WG (g) = final weight − initial weight; daily weight gain DWG (g) = weight gain/days of experiment; apparent feed conversion AFC = feed supplied/weight gain; specific growth rate SGR (%) = 100 × [(ln final weight − ln initial weight/days]); protein efficiency ratio PER (%) = 100 × (weight gain/crude protein consumed); fillet yield FY (%) = (weight of fillet/weight of the animal) × 100 and survival.
The somatic indexes were determined from the weight of the fish, the liver and fat tissues, according to the following calculations: hepatosomatic index HSI (%) = 100 × (weight of hepatic tissue/body weight) and viscerosomatic fat index VSFI (%) = 100 × (weight of visceral fat/body weight).
2.6 Fillets and diets composition, and feed utilization
The proximate composition of fillets was determined from the pre-dried, milled filet (55°C for 72 h). After milling the samples, the moisture content was determined by drying samples at 105°C for 8 h, followed by burning the material at 550°C in a muffle-oven to determine the ash content. The crude protein content was determined by the Kjeldahl method, and ether extract with the aid of a Soxhlet extractor as solvent (AOAC, 2005). The same procedures were used to assess the chemical composition of the experimental diets.
With the initial and final values of crude protein and gross energy of filets, the following calculations were made: protein retention rate PRR (%) = 100 × [(Fw × CPf) − (Iw × CPi)]/ingested CP; and the energy retention rate ERR (%) = 100 × [(Fw × CEf) − (CEi)]/ingested CE, in which Fw = final weight, Iw = initial weight, CPf = final crude protein, CPi = initial crude protein, CEi = initial crude energy and CEf = final crude energy content.
2.7 Histological analysis
For muscle histological analysis, the white muscle samples on the left side of the fish, above the lateral line, were sampled with the aid of a scalpel blade. Regarding the histological analysis of the intestines, small portions of approximately 5 µm length were collected from the medial gut of fish. Prior to analysis, all samples were dehydrated in increasing concentrations of alcohol, underwent diaphanization in xylol, then were embedded in histological paraffin, included and cut in a microtome (Thermo Scientific – Microm HM 340E). Seven-micrometre sections were stained using haematoxylin–eosin solution (HE) (Behmer et al., 1976). For analysis of muscle fibres, the smallest diameter of at least 200 fibres per fish were analysed, totalling 2000 measured fibres in each treatment, which were then classified according to its morphometry as smaller than 20 µm, from 20.01 to 50 µm, and larger than 50 µm. Regarding the histological analysis of the gut, the morphometry of intestinal mucosa was performed in 10 villi per animal, totalling 90 measurements per treatment, in which the following parameters were analysed: villus height, width and thickness of the tunic. The photo documentation of muscle samples was performed in light microscopy in an optical microscope (P1 Olympus BX 50 – Manila, Philippines) using 40× lens, while the photo documentation of gut samples was made in a microscope using 20× lens. Measurements were made using the software CellSens Standard 1.15®.
2.8 Haematological and biochemical analysis
Blood samples for haematological analysis were collected by puncturing the caudal vein of fish, using syringes containing EDTA 3%. The number of erythrocytes was determined in a Neubauer chamber, using Hayen's liquid in the proportion of 1:200. The haemoglobin rate was performed according to the methodology described by Collier (1944), and the percentage of haematocrit was determined according to the recommendations of Goldenfarb et al. (1971). Based on the obtained results, the following haematimetric indexes were calculated: mean corpuscular volume MCV = (haematocrit × 10)/erythrocytes; and concentration of mean corpuscular haemoglobin CMCH = (haemoglobin rate × haematocrit) × 100, according to Wintrobe (1934).
The analysis of biochemical blood parameters was performed in blood samples collected with the aid of syringes without anticoagulants. Those samples were centrifuged at 2500 rotations per minute (rpm) for 5 min, and from the serum, we determined the concentrations of glucose (mg dl−1), total cholesterol (mg dl−1), triglycerides (mg dl−1) and total plasmatic protein (g dl−1), with the aid of specific commercial analysis kits (Gold Analisa Diagnostica®), following the procedures recommended by the manufacturer and carrying out the readings in a spectrophotometer.
2.9 Enzymatic analysis
Regarding the analysis of substances reactive to thiobarbituric acid (TBARS) glutathione S-transferase (GST) and catalase (CAT), two fish from each tank were placed in ice and had their liver removed, which were immediately placed in liquid nitrogen and then in a freezer (−80°C). Before analysis, liver samples were thawed and homogenized in 9 ml of a phosphate buffer solution 0.3 M (KCl 140 mM, pH 7.4) per gram of tissue. Subsequently, a protease inhibitor was added to the solution, together with phenylmethylsulfonyl fluoride (PMSF) at a concentration of 10 nM, diluted in isopropanol (0.03484 g PMSF in 2 ml of isopropyl alcohol). For each ml of the buffer solution, 10 µl of PMSF was added.
TBARS determination was performed according to Buege and Aust (1978), and the results were expressed in nmol MDA mg−1 of protein. The evaluation of CAT activity was determined according to Nelson and Kiesow (1972), in a wavelength of 240 nm, and the results were expressed in µmol mg−1 protein min−1. The GST activity was analysed according to the methodology proposed by Ellman (1959), using trichloroacetic acid 20% (TCA 20% = 20 g of TCA in 100 ml of distilled water) in the proportion of 0.2 g of tissue for 1 ml of TCA. The reading was made in a spectrophotometer, in a wavelength of 412 nm, and the activity was expressed in µmoles mg−1 of protein.
2.10 Statistical analysis
The obtained data were submitted to normality (Shapiro–Wilk) and homoscedasticity (Levene) tests, and the means obtained for each micronutrient were investigated by means of a variance analysis (ANOVA), and in case of significant difference, the Tukey's test was applied at a 5% significance level, with the aid of the software Statistica 7.0® (STATSOFT & Inc., 2005). The results are presented as means ± standard deviation (SD) for each variable.
3 RESULTS
3.1 Growth performance and somatic indexes
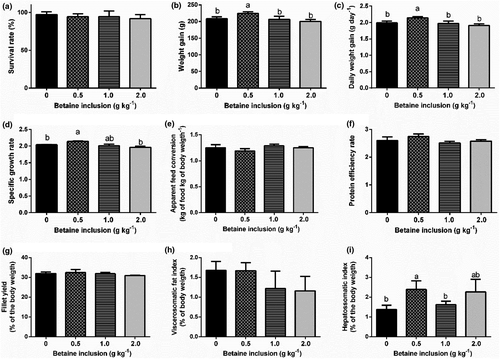
Dietary supplementation with 0.50 g kg−1 of betaine increases (p < .05) WG, DWG and SGR (Figure 1b–d respectively). Fish-fed diets containing 0.50 and 2.00 g kg−1 betaine had the highest HSI values compared to fish-fed diets without betaine (p < .05) (Figure 1i). Betaine inclusion levels did not affect the results of AFC, PER, FY, VSFI and survival (Figure 1e, f, g, h and a respectively).
3.2 Enzymatic analysis of liver
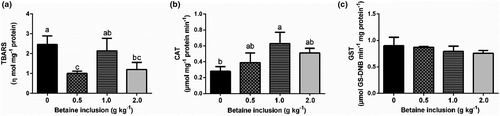
The concentration of TBARS was significantly reduced in the liver of fish fed 0.50 and 2.00 g kg−1 of betaine in diets (p < .05) (Figure 2a). For catalase activity, the highest result was shown in fish fed 1.00 g kg−1 of betaine, while the lowest result for this variable was shown in fish in the control group (p < .05) (Figure 2b). On the other hand, there were no significant differences for glutathione S-transferase activity between the treatments evaluated (p > .05) (Figure 2c).
3.3 Histological analysis
The inclusion of betaine in diets resulted in alteration in the gut histomorphometry of Nile tilapia (Table 2). There was a trend to decrease the height of the gut folds with the increase in the dose of betaine in the diet, while the fish in the control group showed the highest results for this variable (p < .05). Fish fed 0.50 and 1.00 g kg−1 of betaine in the diets showed the smallest gut folds width, while the largest gut fold width was observed in fish fed without betaine (p < .05). The tunic thickness was smaller in fish fed 2.00 g kg−1 of betaine in the diet and fish fed with control diet showed the highest result for this variable (p < .05). On the other hand, the frequency of the smallest and largest diameters of muscle fibres of juveniles of tilapia was not affected by the inclusion of betaine in the diets (p > .05).
| Betaine inclusion g kg−1 | p-Value | ||||
|---|---|---|---|---|---|
| 0 | 0.50 | 1.00 | 2.00 | ||
| Gut histomorphometry | |||||
| Villus height (µm) | 358.68 ± 71.17a | 223.80 ± 28.69b | 225.26 ± 51.52b | 144.75 ± 32.94c | <.01 |
| Villus width (µm) | 214.16 ± 61.15a | 68.79 ± 09.03c | 84.97 ± 28.42c | 134.39 ± 25.62b | <.01 |
| Tunic thickness (µm) | 70.14 ± 12.20a | 49.02 ± 07.02b | 45.92 ± 08.55b | 23.20 ± 04.41c | <.01 |
| Frequency of muscle fibres | |||||
| <20 µm (%) | 0.07 ± 0.24 | 0.08 ± 0.14 | 0.17 ± 0.22 | 0.17 ± 0.32 | NS |
| 20–50 µm (%) | 10.43 ± 2.49 | 7.58 ± 2.06 | 12.33 ± 3.61 | 15.17 ± 5.78 | NS |
| >50 µm (%) | 89.50 ± 2.57 | 92.33 ± 1.94 | 87.50 ± 3.67 | 84.67 ± 5.89 | NS |
Note
- Values are presented as mean ± SD of three replicates. Different letters in the same line indicate significant differences among treatments.
3.4 Blood haematology and plasma biochemical parameters
Fish fed with 0.50 or 2.00 g kg−1 of betaine in diets showed higher levels of plasma glucose compared to fish from the other treatments (p < .05). Other serum elements, such as cholesterol and total proteins, were not altered with the dietary supplementation (Table 3). The haematological parameters evaluated, such as number of erythrocytes, percentage of haematocrit, concentration of haemoglobin, mean corpuscular volume and concentration of mean corpuscular haemoglobin, did not present significant (p > .05) (Table 3).
| Betaine inclusion g kg−1 | p Value | ||||
|---|---|---|---|---|---|
| 0 | 0.50 | 1.00 | 2.00 | ||
| Haematological variables | |||||
| Erythrocyte (×106 μl−¹) | 2.17 ± 0.13 | 1.98 ± 0.07 | 2.15 ± 0.16 | 2.01 ± 0.09 | NS |
| Haematocrit (%) | 40.89 ± 5.46 | 40.78 ± 4.02 | 40.29 ± 5.10 | 36.25 ± 5.06 | NS |
| Haemoglobin (g dl−1) | 8.44 ± 0.72 | 8.86 ± 0.50 | 9.19 ± 1.03 | 8.33 ± 0.87 | NS |
| MCV (fl) | 18.84 ± 1.95 | 20.70 ± 2.37 | 18.81 ± 2.03 | 17.89 ± 1.95 | NS |
| CMCH (%) | 4.87 ± 5.46 | 4.63 ± 4.02 | 4.56 ± 5.10 | 4.42 ± 5.06 | NS |
| Biochemical variables | |||||
| Glucose (mg dl−1) | 49.82 ± 3.20a | 69.37 ± 8.86b | 51.48 ± 2.58a | 71.96 ± 3.10b | <.01 |
| Cholesterol (mg dl−1) | 80.01 ± 18.06 | 83.62 ± 10.44 | 72.88 ± 7.62 | 88.92 ± 12.03 | NS |
| Triglycerides (mg dl−1) | 94.60 ± 17.86 | 126.65 ± 31.12 | 118.25 ± 16.41 | 149.43 ± 22.55 | NS |
| Total proteins (g dl−1) | 3.16 ± 0.48 | 2.96 ± 0.14 | 2.62 ± 0.18 | 2.77 ± 0.16 | NS |
Note
- Values are presented as mean ± SD of three replicates.
- Abbreviations: CMCH, concentration of mean corpuscular haemoglobin; MCV, mean corpuscular volume; NS, not significant.
3.5 Filets composition and feed utilization
The addition of 0.50 g kg−1 of betaine in diets provided a higher energy retention rate in tilapia filets, while fish fed the control diet showed the lowest result for this variable (p < .05). Dry matter, protein, lipids, ash, as well as the protein retention rate did not differ significantly (p > .05) as shown in Table 4.
| Variables | Betaine inclusion g kg−1 | p Value | |||
|---|---|---|---|---|---|
| 0 | 0.50 | 1.00 | 2.00 | ||
| Moisture | 79.21 ± 0.52 | 78.86 ± 0.46 | 79.52 ± 0.74 | 79.48 ± 0.32 | NS |
| Protein | 19.29 ± 0.83 | 19.68 ± 1.41 | 18.91 ± 0.65 | 18.11 ± 0.58 | NS |
| Lipids | 1.68 ± 0.28 | 1.65 ± 0.19 | 1.55 ± 0.28 | 1.41 ± 0.11 | NS |
| Ahs | 1.28 ± 0.57 | 1.64 ± 0.33 | 1.64 ± 0.33 | 1.46 ± 0.58 | NS |
| PRR | 30.67 ± 0.45 | 32.39 ± 11.89 | 27.80 ± 6.12 | 16.83 ± 0.00 | NS |
| ERR | 7.45 ± 0.01b | 8.39 ± 0.01a | 6.36 ± 0.01bc | 5.76 ± 0.02c | <.01 |
Note
- Values are presented as mean ± SD of three replicates. Different letters in the same line indicate significant differences among treatments.
- Abbreviations: ERR, energy retention rate; NS, not significant; PRR, protein retention rate.
4 DISCUSSION
Here, we show that the inclusion of 0.50 g kg−1 of betaine in the juveniles of Nile tilapia (O. niloticus) food improves growth performance and reduces lipid peroxidation. We also show that higher betaine inclusion percentual did not alter the growth performance of fish. In fact, fish-fed diets with 0.50 g kg−1 of betaine presented increased weight gain (WG), daily weight gain (DWG) and specific growth rate (SGR) when compared to control fish and to those fed diets with 1.0 and 2.0 g kg−1 of betaine inclusion (Figure 1).
Since all betaine inclusion levels changed similarly the gut histology, reducing the length and the width of the intestinal microvilli as well as reducing the tunic thickness (Table 2) and since no relevant haematological changes were found (Table 3), our main hypothesis is that the mechanism underlying this positive effect of 0.5 g kg−1 of betaine inclusion is the reduced lipid peroxidation (Figure 2a). In fact, the lower betaine supplementation significantly reduced the concentration of TBARS. This effect might be related to the capacity of betaine to reduce the amounts of free fatty acids, causing those to be well utilized in the metabolism of β-oxidation. Besides, these can protect other cells from oxidative damage, inhibiting the propagation of free radicals, thus helping to repair phospholipids of oxidized membranes (Rebouche, 1992).
In this line, it is well known that the lipid peroxidation stimulates the formation of free radicals as a final product and may cause alterations both in the cell membranes and in the DNA (Panzetta et al., 1995), due to the presence of reactive oxygen species (ROS) and nitrogen (RNS). The most susceptible products to oxidation are polyunsaturated fatty acids, seen that these are more abundant in cells (Nelson & Cox, 2018). In this sense, betaine acts because of its functional and antioxidant attributes that secure the organism against factors related to stress (Pinedo-Gil et al., 2018), in addition to being involved in the synthesis of methionine, which acts as a provider of cellular cysteine via trans-sulphuring, thus promoting the synthesis of reduced glutathione and protecting the cell from metabolites and ROS (Craig, 2004).
The enzymatic defence system includes several antioxidant enzymes, such as catalase (CAT) and glutathione S-transferase (GST) among others, which act in preventing and controlling the formation of free radicals and non-radical species (Yonar & Sakin, 2011). The betaine supplementation did not alter GST activity of fish, which confirms that the inclusion levels designed for this study were non-toxic. According to Salbego et al. (2017), GST is usually utilized to identify the oxidative stress of cells from endogenous or exogenous intoxication. Although GST remained unaltered, the enzyme CAT was found to have a higher activity in fish-fed diets supplemented with betaine (1.00 g kg−1), which might, in turn, assist in the processes against cellular oxidative stress (Pinedo-Gil et al., 2018). This enzyme acts in the removal of hydrogen peroxide, which is transformed in water and molecular oxygen. Its reduction is commonly related to factors that provoke increases in superoxide radicals (Toni et al., 2010).
A relevant question is how the inclusion of betaine improves growth performance. The answer seems to be related to the fact that betaine provides methyl groups to the organism, which are needed for the synthesis of several substances, such as L-carnitine and creatine (Kidd et al., 1997); therefore, it acts on the lipid metabolism (Eklund et al., 2005; Lever & Slow, 2010; Ratriyanto et al., 2009). It comes from the oxidation of choline that derives from the loss of a methyl group of adenosyl-methionine to cysteine. Therefore, it can be understood that betaine supplementation acted synergistically in the oxidative metabolism of the animals, providing a higher availability of L-carnitine, and consequently a greater use of the dietary fatty acids (Eklund et al., 2005). It is possible that the availability of carnitine supported the use of fatty acids as energy source, and caused a sparing effect for proteins (Nelson & Cox, 2018) to be used in other tissues related to growth performance when 0.50 g kg−1 of betaine was used, presenting the highest growth-related results in this study.
Due to the chemical structure of betaine and its methyl radicals, it may have participated of catalytic reactions as a source of methyl groups, reducing the supply of methionine and choline for this end, and increasing the availability of these compounds for growth (Eklund et al., 2005). Thus, choline was redirected to the process of re-methylation, whilst methionine was directed to the protein synthesis, sparing the participation of this amino acid in the donation of methyl groups (Metzler-Zebeli et al., 2009).
This clear relationship of betaine with lipid metabolism (Eklund et al., 2005; Lever & Slow, 2010; Ratriyanto et al., 2009) reinforces the hypothesis that the reduced lipid peroxidation verified in fish fed with betaine supplementation of 0.50 g kg−1. This lower betaine inclusion level seems to prevent the oxidative stress, increasing the availability of lipids for energy production by increasing fatty acids transport to the β-oxidation metabolism since they were not peroxidate (Rani & Panneerselvam, 2002). It is noteworthy that the highest inclusion levels of betaine provided the highest concentration of visceral fat; thus, it is possible that this higher supplementation increased the quantity of carnitine, favouring the lipolysis process (Nelson & Cox, 2018).
In addition, betaine stimulates the expression of genes related to growth, acting as a modulator as observed in studies with Labeo rohita (Dar et al., 2019). In juveniles of tilapia GIFT lineage, supplementation of 0.50 g kg−1 of feed provided better weight gain, feed conversion and specific growth rate (Luo et al., 2011). Betaine supplementation at levels of 2.0 g kg−1 ratio for ingredients of plant origin, such as corn gluten and soybean, stimulates the consumption of fish feed, improving their growth, intestinal development and blood biochemistry for Nile tilapia fingerlings (Ismail et al., 2020).
Betaine supplementation influenced on the increase of the HSI of fish, and the inclusion level of 0.50 g kg−1 resulted in a considerable increase of the ERR, in comparison with other treatments. Presumably, the process of homeostasis in these animals, triggered by the metabolic activity of betaine supplementation, possibly provided a higher accumulation of glycogen in their liver (Enes et al., 2008).
Regarding the gut, histological analysis, we found that betaine supplementation resulted in reduced intestinal microvilli; however, performance was not reduced in all experimental groups. Silva and Nörnberg (2003) pointed out that the use of additives in fish diets is common, and most of the supplements lead to some kind of anatomical change in the gastrointestinal tract of the animals, promoting increased fold size and consequently, an increased absorption surface that leads to higher performance.
According to Ferreira et al. (2014), the integrity of the gut mucosa can be evaluated from morphological measurements of gut fold, in order to determine an animal's gut digestive and absorptive capacity. However, in poultry, chicken, betaine supplementation was beneficial for the gut epithelium (Kettunen et al., 2001), considering its capacity of acting as an osmolyte, maintaining the integrity and structure of villi and promoting an improved digestibility and absorption of nutrients (Niang, 2005).
Again, this similar effect of betaine on gut histology and the absence of reduction in growth performance of 1.0 and 2.0 g kg−1 treatments reinforce the hypothesis that the better balance of the redox state in fish-fed diets with 0.50 g kg−1 of betaine (decreased TBARS levels, Figure 2) increases the availability of lipids for energy production by increasing fatty acids transport to the β-oxidation metabolism (Rani & Panneerselvam, 2002).
Regarding haematological and biochemical parameters, no relevant effects were found, except the increased levels of plasma glucose in fish-fed diets supplemented with 0.50 and 2.00 g kg−1 of betaine. Allied to that, according to Anderson and Kolozlovsky (1985), glucose is converted into energy that is used as fuel for protein synthesis, supporting the growth of muscle fibres, as observed in fish fed 0.50 g kg−1. Both the glucose and total protein levels observed in this study are close to those obtained by Bittencourt et al. (2003), who evaluated tilapia juveniles at the growth stage in a semi-intensive system and reported a mean glucose concentration of 60 mg dl−1 and 3 g dl−1 of total proteins. The plasmatic proteins are represented by albumins, globulins and fibrinogen, and reductions in its concentrations may either indicate hepatic alterations or that the supplied diet is not balanced. These compounds may have varying concentrations in the blood in case the feed have low concentrations of amino acids (Guyton & Hall, 2011). The total protein concentrations observed in this study are considered adequate for healthy tilapia individuals (Chen et al., 2009), indicating that a lack of amino acids probably did not occur due to the inclusion of betaine in the experimental diets.
It is important to mention that the erythrocyte variables of fish-fed diets containing betaine revealed no alterations in the number of erythrocytes, haematocrit, haemoglobin, MCV and CMCH, showing that there was no lack of any essential nutrient in the diets, and that none of the inclusion levels caused intoxication of the fish. In addition, the fish haemogram revealed that the observed values remained within the reference range for the species (Hrubec & Smith, 2010).
Finally, the betaine inclusion did not alter the fillets composition and protein retention rate. In fact, the proximate composition of the fillets obtained in this study is in accordance with the expected for the species, according to Caula et al. (2008), which suggests that betaine supplementation did not influence fillet composition.
5 CONCLUSION
Betaine supplementation for tilapia juveniles at the level of 0.50 g kg−1 improves growth performance probably through a reduction in lipid peroxidation.
ACKNOWLEDGEMENTS
This study was financed, in part, by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001. LJGB is supported by grants from the Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) 19/2551-0001-873-8 and holds a research fellowship of the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) 303263/2018-0.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




