Evaluation of yeast hydrolysate as a substitute to dietary fish meal of| juvenile Jian carp (Cyprinus carpio var. Jian): Protein synthesis via TOR pathway
Abstract
This study was performed to evaluate effects of fish meal replacement by yeast hydrolysate (YH) on crude protein via TOR pathway of Jian carp, Cyprinus carpio var. Jian (initial average weight 19.44 ± 0.06 g). Six hundred fish were assigned into five groups and fed with five isonitrogenous and isocaloric diets replacing fish meal by 0g/kg (G1), 10g/kg (G2), 30g/kg (G3), 50g/kg (G4) and 70g/kg YH (G5). The results showed crude protein of fish in G2 and G3 were significantly higher (p < .05) than in G1. YH significantly increased AST and ALT activities of hepatocytes (p < .05) compared with the control group, but had no effects (p > .05) on AST and ALT contents in conditioned medium of hepatocytes. Furthermore, MyoD, TOR and S6K1 mRNA levels in muscle of G2 and G3 were significantly higher than of G1 (p < .05). Meanwhile, YH significantly increased MyoD, TOR and S6K1 mRNA levels in hepatocytes compared with the control group. In conclusion, 10g/kg and 30g/kg YH increased crude protein content of muscle in fish and increased TOR pathway genes expression levels in muscle and hepatocytes.
1 INTRODUCTION
Jian carp, Cyprinus carpio var. Jian, is one of the most important farmed species that lives in river and reservoir in China. It is reported by the China Fishery Statistics Yearbook (China, 2019) that yield of carp in 2018 is about 2.96 million tons. Fish meal (FM) has been regarded as a kind of major protein source for aquaculture (Murray et al., 2010). Tremendous market demand and volatile price urge (Burr et al., 2012) scholars and researchers to reduce the application of fish meal in aquafeed by substituting it with cheaper protein sources that have no negative effects on the environment. Yeast has been recognized as a potential alternative to fish meal among single-cell proteins due to its high protein, vitamins, free nucleotide and some special functional components, such as mannan oligosaccharide (MOS). Research regarding yeast as a substitute to fish meal in aquafeed has been studied in several species, such as sea bass (Dicentrarchus labrax) (Santacroce et al., 2012), Jian carp (Yuan et al., 2017) and Thai Panga (Pangasianodon hypophthalmus × Pangasius bocourti) (Pongpet et al., 2016). Study regarding mechanism of fish protein metabolism influenced by replacing dietary fishmeal with different protein sources, however, is still lacking.
Muscle of animal generally grows by two possible mechanisms: hyperplasia and hypertrophy of muscle fibres. These two mechanisms are regulated by myogenic regulatory factors, structural gene and other growth factors (Rescan, 2005). Hyperplasia of muscle fibres is correlated with the density of muscle fibre, as the increase in new fibres leading to a greater potential for hyperplastic growth (Periago et al., 2005). Hypertrophy of muscle fibres usually is decided by growth rate of individual muscle fibre (Egginton & Johnston, 1982). Early study reported that the rates of hypertrophy and hyperplasia of muscle fibre change between different strains of the same species (Weatherley et al., 1979) and are influenced by farmed conditions such as diet (Kiessling et al., 1991; Lo´pez-Albors et al., 2010). More research evaluated the effects of feed additives on flesh quality of fish in wild and farmed in different species, such as Turbot (Psetta maxima) (Regost et al., 2003), blunt snout bream (Megalobrama amblycephala) (Cai et al., 2018) and golden pompano (Trachinotus ovatus) (Zhang et al., 2019). Research on regulation of muscle fibres growth by myogenic regulatory factors, however, is very few.
Fish growth is mainly the result of cell proliferation and protein growth, which is regulated by target of rapamycin (TOR) signal pathway (Laplante & Sabatini, 2012; Roux & Topisirovic, 2012). TOR pathway in fish activates cell growth in response to dietary nutrients such as amino acids, growth factors (such as insulin and insulin-like growth factor) and cellular energy status (ATP) (Wullschleger et al., 2006). Nutrient composition of food could also respond TOR pathway. Previous studies found that dietary arginine or leucine level regulated TOR expression levels in the muscle of carp (Ren et al., 2015). Wacyk et al. (2012) reported that isolated protein from diets decreased the relative expression of TOR gene in rainbow trout (Salmo gairdneri). Thus, in view of apparent relation between change in protein source and TOR pathway, we speculated that protein can affect protein synthesis via TOR signal pathway in fish, a theory that requires investigation.
Molecular biology technique is a crucial way to find signal pathway involved in fish protein metabolism when dietary fishmeal is replaced by different protein sources. However, the underlying mechanism that limits fishmeal replacement remains largely unknown. It is known in multiple species that activation of TOR signal pathway is required for protein synthesis and postprandial anabolism (Laplante & Sabatini, 2012). Previous study in our laboratory found that YH could increase the growth of Jian carp (). Therefore, it is important to understand whether replacement level of fishmeal is associated with changes in transcription of genes known to participate in protein synthesis. This study used yeast hydrolysate as a protein source to replacing fish meal in diets and study protein synthesis via TOR signalling pathway.
2 MATERIAL AND METHODS
2.1 Ethics statement
Experimental fish in this study was carried based on the guidelines for the Care and Use of Laboratory Animals in Jiangsu Province.
2.2 Experimental diets
Ingredients and proximate composition of the basal diet were presented in Table 1. Five isonitrogenous and isocaloric diets (G1, G2, G3, G4 and G5) were formulated. Fish meal in diets was replaced by 0g/kg, 10g/kg, 30g/kg, 50g/kg and 70g/kg YH respectively. YH was obtained from Guangdong Hinabiotech CO., Ltd (Guangdong, China). Carbohydrate is obtained from wheat flour. Protein was provided by soybean meal, fish meal, rapeseed meal and cottonseed meal. All ingredients were ground into powders and weighed, then mixed with oil. Finally, water was added to mixture. These diets were made using a pellet mill (Guangyuan Engineering Co., Ltd.) and dried at room temperature. The diets were kept in −20℃ fridge after drying.
| Ingredients | g/kg | Proximate composition (g/kg air-dry basis) | |
|---|---|---|---|
| Fish meal | 80.0 | Crude Protein | 346.3 |
| Yeast hydrolyate | 0.0 | Crude lipid | 52.1 |
| Wheat bran | 80.0 | Moisture | 90.6 |
| Fish oil | 12.8 | Ash | 77.2 |
| Soybean oil | 12.8 | Energy (MJ kg−1) | 181.4 |
| Premixa | 10.0 | ||
| Soybean meal | 268.0 | ||
| Rapeseed meal | 120.0 | ||
| Cottonseed meal | 130.0 | ||
| Wheat flour | 265.4 | ||
| Ca(H2PO4)2 | 18.0 | ||
| Premix | 10.0 | ||
| Salt | 3.0 | ||
- a Premix supplied the following minerals (g kg−1) and vitamins (IU or mg kg−1): CuSO4·5H2O, 2.0 g; FeSO4·7H2O, 25 g; ZnSO4·7H2O, 22 g; MnSO4·4H2O, 7 g; Na2SeO3, 0.04 g; KI, 0.026 g; CoCl2·6H2O, 0.1 g; Vitamin A, 900,000 IU; Vitamin D, 200,000 IU; Vitamin E, 4500 mg; Vitamin K3, 220 mg; Vitamin B1, 320 mg; Vitamin B2, 1090 mg; Vitamin B5, 2000 mg; Vitamin B6, 500 mg; Vitamin B12, 1.6 mg; Vitamin C, 5000 mg; Pantothenate, 1000 mg; Folic acid, 165 mg; Choline, 60,000 mg.
2.3 Experimental fish and design
Six hundred healthy juvenile fish (average weight 19.44 ± 0.06 g) were distributed into five groups (2.0 × 1.0 × 1.7 m), and each group has four cages. Juveniles were acclimated for 14 days, and during acclimation, fish fed with a commercial diet. Fish were fed with one of five diets with four replicates after acclimation. All fish were hand-fed to apparent satiation three times daily for 10 weeks. During trial period, water temperature was 23–29℃, pH was 6.5 and dissolved oxygen was 6.5 mg L−1.
2.4 Sample
Fish were fast for 24 h before sampling. Three fish from each cage were anaesthetized using MS-222 (Sigma, USA). Body weight and length of these selected fish were measured. Blood was collected using caudal vessel with heparinized syringes and then centrifuged at 1000 g, 4℃ for 10 minutes (min). Liver was removed and collected for enzymes activity. Muscle was collected for proximate composition and real-time PCR analysis.
2.5 Hepatocytes culture and experimental design
Firstly, fish were disinfected with 0.1% KMnO₄ solution for 10 min. Liver sample was carefully removed and transferred into a sterile petri dish. Then, the sample was washed with PBS supplemented with penicillin. The sample was aseptically cut into small pieces by medical scissors. Secondly, the tissue was digested with sterile trypsin (Gibco, USA) at 37℃ for 40 min. The digested solution was filtered through a cell sieve and collected in a sterilized centrifuge tube and centrifuged at 1000 rpm for 5 min. Then isolated hepatocytes were obtained and washed using the fresh DMEM/F12. Finally, hepatocytes were diluted with DMEM/F12 medium to 105 cells ml−1.
Isolated hepatocytes were transferred into each well of plates and cultured in CO2 incubator for 48 h. Then, hepatocytes were divided into control group and YH group, and each group has four replicates. Hepatocytes of control group were incubated with cell medium, and hepatocytes of YH group were incubated with 0.1 g L−1 YH (Lee et al., 2011). After 24 h, cultured medium and primary hepatocytes were collected for subsequent analyses.
2.6 Proximate composition analysis
Proximate composition of muscle was determined based on AOAC protocols (AOAC, 2003). Crude protein was analysed using Kjeldahl method; lipid content was estimated using ether extraction; moisture was determined using oven; and ash content was determined using combustion at 550℃ for 4 h.
2.7 Enzymes activity assay
Aspartate aminotransferase (AST) and alanineaminotransferase (ALT) activities were measured by enzymatic colorimetric methods according to the method of Rietman and Frankel (1957). Lactate dehydrogenase (LDH) level was determined based on the modified way stated by Lygren et al. (1999).
2.8 RNA extract and cDNA synthesis
RNA was extracted from 50 mg muscle of fish using TRIzol (Sigma, United States). The purity of total RNA was analysed using OD260/OD280 ratio. Then, the concentration of the extracted RNA was measured using ultra micro spectrophotometer (Thermo Scientific, United States). The first-strand cDNA was generated from RNA using reverse transcription kit (Takara, Japan).
The PCR primers (Table 2) were designed using premier 5.0 program and synthesized by Shanghai Generay Biotech Co., Ltd. (Shanghai, China). The PCR reaction condition was 95℃ for 5 s, 60℃ for 30 s, followed by a melt curve analysis of 15 s from 95 to 60℃, 1 min for 60℃ and then up to 95℃ for 15 s. Finally, gene expression levels were analysed by the 2−ΔΔct method.
| Gene | Primer type | Sequence | Genbank no. |
|---|---|---|---|
| 40S | Sense | GGAAGTGGCAAGGAGAAG | AB012087 |
| Anti-sense | GGAGAGGTGGACAGACAT | ||
| TOR | Sense | TTTACACGAGCAAGTCTACGGA | 109094977 |
| Anti-sense | CTTCATCTTGGCTCAGCTCTCT | ||
| S6K1 | Sense | GGTGCATGTCACCTTATGGG | EF373664.1 |
| Anti-sense | AGCTGGCAGCACTTCTAGTC | ||
| 4E-BP2 | Sense | ATGTCGTCCAGTCGTCAGTTT | HQ010440.1 |
| Anti-sense | AGGAGTGGTGCAATAGTCGTG | ||
| MSTN | Sense | GGGATGACAGTAAGGATGGAGC | GQ214770.1 |
| Anti-sense | TCGTATTCGTATGTGCCTTCCT | ||
| MyoD | Sense | CTGACGCACGGAATACTAAA | AB012882.1 |
| Anti-sense | ATCCCGAGCCTGCTGTTG |
2.9 Statistical analysis
Experimental data are analysed by SPSS 19.0 (SPSS Inc., Chicago, USA) using one-way ANOVA test and independent sample t test after testing the normality and homogeneity of variances of data. The significant difference level is set at p < .05. The data are showed as means ± standard error of the mean.
3 RESULTS
3.1 Proximate composition in muscle
Table 3 showed effects of replacing fish meal with YH on proximate composition in muscle of juvenile Jian carp. Crude protein of fish in G2 and G3 was significantly higher (p < .05) than in G1. No significant differences (p > .05), however, for moisture, ash and crude lipid were observed among all groups.
| Items | Control group | Treatment groups | |||
|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | |
| Moisture | 723.8 ± 2.1 | 727.0 ± 6.8 | 713.8 ± 3.6 | 718.0 ± 7.1 | 721.9 ± 1.8 |
| Crude protein | 166.3 ± 0.9a | 175.1 ± 1.1bc | 178.5 ± 1.2c | 173.6 ± 1.2ab | 169.2 ± 1.8a |
| Crude lipid | 67.1 ± 2.3 | 64.2 ± 1.6 | 63.7 ± 1.1 | 63.4 ± 1.3 | 63.3 ± 2.1 |
| Ash | 32.1 ± 1.2 | 32.8 ± 1.3 | 33.8 ± 2.0 | 33.2 ± 1.1 | 33.3 ± 0.9 |
Note
- Values are means ± SEM of four replications. Means in the same line with different superscripts are significantly different (p < .05).
- Abbreviations: FM, fish meal; G1, dietary fish meal replacement by 0g/kg YH; G2, dietary fish meal replacement by 10g/kg YH; G3, dietary fish meal replacement by 30g/kg YH; G4, dietary fish meal replacement by 50g/kg YH; G5, dietary fish meal replacement by 70g/kg YH; YH, yeast hydrolysate.
3.2 Effect of YH on enzymes activity in liver and plasma of Jian carp
As can be seen from Table 4, AST and ALT activities in treatment groups were significantly higher (p < .05) than in the control group. No significant differences (p > .05) for these two enzymes activity in plasma among all groups, however, were observed.
| Group | Enzyme activities in liver | Enzyme activities in plasma | |||
|---|---|---|---|---|---|
| AST (U g−1) | ALT (U g−1) | AST (U L−1) | ALT (U L−1) | LDH (U L−1) | |
| G1 | 22.28 ± 0.37a | 6.59 ± 0.21a | 12.21 ± 0.93 | 10.09 ± 0.82 | 98.83 ± 3.53 |
| G2 | 32.34 ± 0.35b | 11.49 ± 0.23b | 11.73 ± 0.78 | 9.79 ± 0.89 | 99.75 ± 4.33 |
| G3 | 41.43 ± 0.36c | 15.45 ± 0.29c | 12.32 ± 1.10 | 10.18 ± 0.74 | 100.67 ± 3.87 |
| G4 | 27.40 ± 0.32d | 11.56 ± 0.30b | 11.89 ± 0.92 | 9.93 ± 0.95 | 97.94 ± 3.42 |
| G5 | 26.51 ± 0.27d | 9.17 ± 0.22d | 12.14 ± 0.87 | 10.01 ± 0.72 | 98.47 ± 4.22 |
Note
- Values are means ± SEM of four replications. Means in the same column with different superscripts are significantly different (p < .05).
3.3 Effect of YH on enzymes activity in hepatocytes
As can be seen from Table 5, YH had no effects (p > .05) on AST and ALT activities in conditioned medium of hepatocytes, but increased AST and ALT contents of hepatocytes (p < .05) compared with the control group.
| Group | Enzyme activities in hepatocytes | Enzyme activities in conditioned medium | |||
|---|---|---|---|---|---|
| AST (U g−1) | ALT (U g−1) | AST (U L−1) | ALT (U L−1) | LDH (U L−1) | |
| Control | 13.92 ± 0.22a | 15.19 ± 0.13a | 9.41 ± 0.19 | 15.26 ± 0.31 | 92.05 ± 1.57 |
| YH | 37.84 ± 0.23b | 34.78 ± 0.18b | 9.33 ± 0.31 | 15.16 ± 0.48 | 91.21 ± 1.32 |
Note
- Values are means ± SEM of four replications. Means in the same column with different superscripts are significantly different (p < .05).
- Abbreviations: GOT, aspartate aminotransferase; GPT, alanine aminotransferase; YH, yeast hydrolysate.
3.4 MyoD and MSTN expression in muscle and hepatocytes
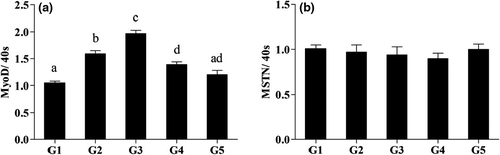
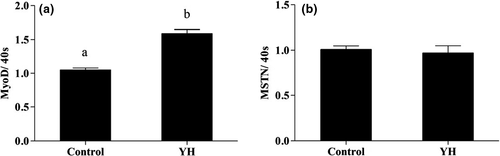
As can be seen from Figures 1 and 2, expression of muscle-related genes including MyoD in G2, G3 and G4 were significantly higher than in G1 (p < .05). Furthermore, YH significantly increased MyoD expression level in hepatocytes compared with the control group. No significant difference (p > .05), however, was observed for MSTN expression level among all groups.
3.5 TOR signal pathway genes expression in muscle and hepatocytes
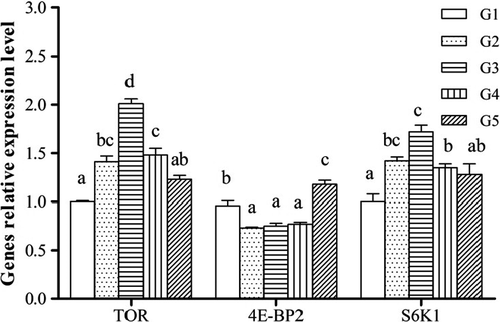
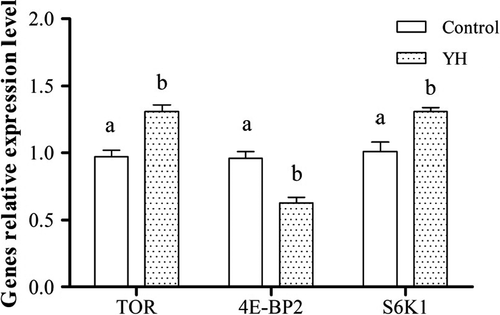
The relative expression of TOR pathway genes in muscle was showed in Figure 3. TOR and S6K1 mRNA levels in G2 and G3 were significantly higher than in G1 (p < .05). 4E-BP2 mRNA level in G2, G3 and G4 was, however, lower than in G1 (p < .05). In addition, the relative expression levels of TOR pathway genes in hepatocytes were presented in Figure 4. Dietary YH significantly increased TOR and S6K1 mRNA levels, and decreased 4E-BP2 mRNA level (p < .05).
4 DISCUSSION
Proximate composition of muscle in fish influences product quality, consequently affecting the product-purchasing behaviour of consumers based on early research (Gatlin et al., 2007). The muscle composition is influenced by exoteric factors, such composition of diets and environment (Kiessling et al., 1991; Lo´pez-Albors et al., 2010). Previous study reported that wild fish has excellent quality and firmer texture compared to farmed fish fed with artificial diets (Izquierdo et al., 2003). In the present study, 10g/kg and 30g/kg YH significantly increased crude protein in muscle of fish compared with the control diet. This suggested that replacing fish meal by YH at proper level increased crude protein content of muscle. Taking this into consideration, we speculated that protein synthesis could be regulated by TOR signal pathway. Therefore, this experiment was conducted to study TOR signal pathway and muscle growth mechanism.
ALT and AST are the crucial metabolic enzymes in body (Cai et al., 2020), which could reflect the indication of tissue damage in animal. In this study, YH had no effect on AST and ALT activities in plasma, indicated that fish meal replacement with YH had no burden on the liver function. A reasonable explanation is that increasing plasma ALT and AST activities was only observed in damaged liver (Li et al., 2014). Interestingly, YH increased AST and ALT activities in liver cell compared with the control group, suggesting that YH supplementation enhanced amino acid metabolism of liver, which could improve protein synthesis. Furthermore, LDH exists widely in many tissues and plays an important role as an indicator of injured tissue (Diamantino et al., 2001). In this experiment, YH have no effects on LDH activity in plasma and the conditioned medium of hepatocytes, suggesting that YH addition did not damage hepatocellular function again. This result was reasonable since an increase in LDH activity was only observed in cell lysis (Lemaire et al., 1991). Therefore, we make further study regarding the effects of replacing fish meal by YH on muscle growth mechanism and TOR signalling pathway of Jian carp based on the above results.
Myogenic regulatory factors (MRFs) are muscle development and growth-related transcription factors, which containing myogenic differentiation factor (MyoD), muscle regulatory factor 4 (Mrf4) and myogenic factor 5 (Myf5). In this study, expression of muscle-related genes including MyoD in G2, G3 and G4 was significantly higher than in G1. This result indicated that replacing fish meal by YH at appropriate level could improve the development of fish muscle. This result might be ascribed to growth-promoting function of MOS in yeast (Dimitroglou et al., 2010). Similar findings were observed in different species, such as sea bream (Sparus aurata) (Dimitroglou et al., 2010), sunshine bass (Gause & Trushenski, 2011) and Nile tilapia (oreochromis niloticus) (Desaleb et al., 2008). Furthermore, YH significantly increased the MyoD expression level in hepatocytes compared with the control group in this study, suggesting that YH could improve growth again.
TOR signal pathway could not only act in response to intracellular sensing of amino acid availability, but also regulate protein metabolism (Laplante & Sabatini, 2012). In order to evacuate the role of TOR pathway in protein synthesis, the relative expression levels of TOR signalling pathway genes were measured. The result showed that dietary 10g/kg and 30g/kg YH significantly increased TOR mRNA level, but decreased with dietary YH supplement level increasing, indicating that YH at proper levels increased protein synthesis by up-regulate expression levels of TOR pathway. This was reasonable because that down-regulated TOR expression decreased protein synthesis (Zhou et al., 2017). Similar findings were reported in several fish species, such as Wuchang bream (Zhou et al., 2017) and turbot (Scophthalmus maximus L.) (Wang et al., 2016). Furthermore, it is reported that TOR signal pathway regulates cell growth and proliferation by stimulating protein synthesis via 4EBPs and S6Ks, downstream proteins of TOR pathway, in animal (Sarbassov et al., 2005). S6Ks control cell growth and apoptosis through regulating translation of ribosomal protein S6 (rpS6) and eukaryotic initiation factor 4B (eIF4B) (Roux & Topisirovic, 2012). In this study, S6K1 mRNA levels in G2, G3 and G4 were significantly higher than in G1, indicating that increased S6K1 could activate protein synthesis. It is interested that YH significantly increased TOR and S6K1 mRNA levels in hepatocytes. This result confirmed that fish meal replacement by YH at proper levels activated TOR pathway genes expression. In addition, in the present study, 4E-BP2 mRNA level of fish fed 10g/kg, 30g/kg and 50g/kg YH was significantly lower than in the control diet. This result was reasonable because that up-regulated expression of 4E-BP2 led to inhibition of mRNA translation, consequently decreased protein synthesis. In fact, the first step of mRNA translation initiation in animal is assembly of the eIF4F complex on mRNA cap structure is (Jackson et al., 2010). As small-molecular weight translational repressors, 4E-BP interfere with assembly of the eIF4F complex by competing with eIF4G for binding to eIF4E (Roux & Topisirovic, 2012). Hence, high expression levels of 4E-BP2 mRNA inhibited mRNA translation and protein synthesis.
In conclusion, dietary 10g/kg and 30g/kg YH raised AST and ALT activities in liver, and increased crude protein content in muscle via up-regulating expression levels of TOR pathway genes of fish.
ACKNOWLEDGEMENT
This study was funded by the China Agriculture Research System (CARS-45-14) in collaboration with Initial Scientific Research Fund of Huaiyin Institute of Technology (Z301B19576).
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The authors declare that the data used to support the findings of this study are included within the article.




