Effects of dietary leucine levels on growth performance, hematobiochemical parameters, liver profile, intestinal enzyme activities and target of rapamycin signalling pathway related gene expression in rainbow trout, Oncorhynchus mykiss fingerlings
Funding information
This study was supported by the Department of Biotechnology (DBT), Government of India, New Delhi under the project on nutrient requirement of rainbow trout fingerlings (Grant No. BT/PR10573/AAQ/3/654/2013).
Abstract
A study was designed to investigate the influence of leucine (Leu) levels on growth performance, enzyme activities and relative expression of TOR and 4E-BP genes in rainbow trout, Oncorhynchus mykiss (average initial body weight: 1.60 ± 0.25 g/fish; average initial length 5.20 ± 0.30 cm/fish). Six isonitrogenous (450 g/kg) and energetic (20.90 kJ 100 g−1, gross energy) experimental diets in gradation of 2.5 g/kg of dry diets were formulated to contain graded Leu levels (10.0, 12.5, 15.0, 17.5, 20.0 and 22.5 g/kg, drymatter basis). 20 fish were stocked in triplicate groups in 75 L trough connected with flow-through system (2–2.5 L/min) and were fed twice daily (09:00 and 17:00 hours) at 5% BW day−1. Quadratic regression analysis of live weight gain (LWG%), feed conversion ratio (FCR), protein efficiency ratio (PER) and body protein deposition (BPD) data indicated Leu requirement at 17.70, 17.42, 17.25 and 17.57 g/kg dry diet, respectively. Highest (p < .05) protein, lowest moisture and intermediate fat contents were noted at 17.5 g/kg Leu fed diet. Significant variation in haematological parameters was also observed. While no significant (p ˃ .05) differences in plasma total protein, cholesterol, triglycerides, albumin and globulin were noted. However, alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate transaminase (AST), glucose, urea and uric acid levels produced significant differences. Except amylase, higher enzymatic activities were observed at 17.5 g/kg Leu diet. Higher relative expression level of target of rapamycin (TOR) and eukaryotic translation initiation factor 4E-binding protein (4E-BP) mRNA was observed at 17.5 and 15.0 g/kg Leu fed diets. Based on the above data, it is recommended that 17.45 g/kg Leu, corresponding to 38.77 g/kg of dietary protein would be useful for optimum growth and also regulates relative gene expression of TOR and 4E-BP signalling pathway.
1 INTRODUCTION
Provision of supplementary feed loaded with all the essential nutrients that required by the organism has become a fundamental component of any successful culture system. In enhanced extensive and semi-intensive operations, feed is being formulated rich with essential source of nutrients for direct consumption of fish, while natural food resource on the other hand still remain the major and ideal source of food (Ahmed & Khan, 2004). However, in intensive culture where contribution of natural organisms is not significant with respect to the stocking density, supplementary feeds are used as an alternate source of dietary nutrients that fulfil the nutritional demands of the concerned species.
Since dietary protein constitutes one of the most expensive components of fish feed and is also considered as an initial source of nitrogen waste products that entering into the culture system (Rahimnejad & Lee, 2013). Therefore, optimization of dietary protein levels along with increasing nutrient retention by the fish could not only be helpful in reduction of nitrogen load in their environment, but also positively impact on the overall expenditure in aquaculture (Thoman et al., 1999). However, the knowledge on protein requirement of fish has a limited value without knowing the essential amino acid (EAA) requirement of concerned fish. Proteins are composed of bundles of amino acids, which not only acts as the building blocks of proteins but also serve as key regulators of major metabolic pathways (Jobgen et al., 2006; Meijer, 2003). Among all the amino acids required by the fish for their multidimensional role in the body, ten have been declared essential for growth and development of fish (NRC, 2011; Twibell et al., 2003; Wilson, 1985), therefore, requires an exogenous source in the form of feed that fulfil the demand of these amino acids (NRC, 2011). The deficiency of these EAA may cause reduced growth and poor feed efficiency (Wilson & Halver, 1986), besides their absence might also impact the overall physiology of fish. Therefore, satisfying the essential amino acids requirements of cultured fish is vital to attain the optimum growth of fish.
Branched chain amino acids (BCAAs) comprises of leucine, isoleucine and valine are essential amino acids in animals including fish, they not only act as building blocks for tissue protein but also plays a functional significance in fish such as: protein synthesis, promote insulin release and inhibits protein degradation (Jobgen et al., 2006; Meijer, 2003; Wu, 2013). Among them, leucine (Leu) is largely involved in growth, maintenance and metabolic activities in fish, besides that it is also involved in performing various physiological roles in fish. After absorption, Leu is transaminated into alpha-keto-iso-caproate (α-KIC) and subsequently oxidized as well as decarboxylated in to final product acetyl-CoA, which provide passage to the TCA cycle in order to perform vital biological functions (Zhang et al., 2017; Zhou et al., 2019). Leu also has a significant involvement in the maintenance of blood glucose level, nitrogen stability, somatotrophic hormone, energy metabolism, body adipose deposition and haemoglobin concentration, besides stimulation of protein synthesis (Abidi & Khan, 2007; Ahmed & Khan, 2006; Li et al., 2011; Norton & Layman, 2006).
Improvement of growth in fish mainly depends upon the results of protein synthesis and deposition (Hochachka & Mommsen, 1995). The regulation of protein synthesis is actually achieved by the alterations in peptide chain initiation through changes in the rate of translation of mRNA (Rhoads et al., 2007; Tang et al., 2013). Among all the essential amino acid required by fish, Leu acts as a functional amino acid that stimulate muscle protein synthesis and inhibit proteolysis in mammals, as well as in fish because, it is an activator of target of rapamycin (TOR) (Nakashima et al., 2007; Ahmad et al., 2021). It has capability of up regulating the pathways that implicate muscle protein synthesis through insulin dependent and independent pathways. Leu also have a direct involvement in activation of mTOR in muscle, besides helpful to stimulate downstream phosphorylation of p70S6 kinase and 4E-BP1 signalling RNA translation and protein synthesis (Zhao et al., 2020). It has been suggested that Leu performed an important part has been found to be able to regulate the target of rapamycin (TOR) signalling pathway, gluconeogenesis and lipogenesis in rainbow trout hepatocytes (Lansard et al., 2009, 2010). However, its imbalance (deficiency or excess) in the diets could cause severe biochemical malfunction in the form of growth retardation in many aquaculture species (Ahmed & Khan, 2006; Abidi & Khan, 2007; Choo et al., 1991; Deng et al., 2014; Farhat & Khan, 2014; Fernstrom, 2005; Rodehutscord et al., 1997)
The requirement of Leu for several fish species have been reported from different part of the world and the detailed information have already been reviewed (Ahmed & Khan, 2006), later on many studies are being added such as yellow croaker, Pseudosciaena crocea (Yan et al., 2010); Catla (Zehra & Khan, 2015); Ctenopharyngodon idella (Deng et al., 2014); stinging catfish, Heteropneustes fossilis (Farhat & Khan, 2014); ); Megalobrama amblycephala (Ren et al., 2015); ; Trachinotus ovatus (Tan et al., 2016); Oreochromis niloticus (Gan et al., 2016; Zehra & Khan, 2017); Sciaenops ocellatus (Castillo & Gatlin, 2018); Carassius auratus (Zou et al., 2018); Epinephelus fuscoguttatus × E. lanceolatus (Zhou et al., 2019). Although Leu requirement of rainbow trout have been determined in the past (Ogino, 1980; NRC, 1993); however, these workers did not correlate their growth data with hematobiochemical parameters and relative expression of TOR and 4E-BP levels, which is vital for promoting protein synthesis.
Rainbow trout, Oncorhynchus mykiss is one of the most widely successful cultivable coldwater species in the Indian Himalayan regions hold an important place to that fetch high price commercial value (Ahmed & Ahmad, 2020). It is one of the largest member of the family Salmonidae with fast growth rates, reaching weights up to 2.5 to 3.0 kg under culture conditions. The fish fetch high market demand throughout the globe due to its nutritional quality, which resulted gap between demand and supply that can only be fulfilled through the intensification of this fish species (FAO, 2002). In recent years, trout farming in Kashmir has gained momentum due to the success achieved through its culture. Although some work on the nutritional requirement of rainbow trout fingerling stage has been carried out from this part of the world such as ration size (Ahmed, 2018), protein requirement (Ahmed & Ahmad, 2020), however, amino acid requirement of rainbow trout under similar condition has not been determined yet due to which the overall success in trout farming is still marginal. As the condition for trout culture in this region is little different from other part of the world where rainbow trout is cultured, for example, water temperature of cold tropical Himalayan region especially Jammu and Kashmir were fluctuate from 5–16℃, dissolved oxygen from 6.4–10.9 mg/L and pH from 7.0–8.0, respectively (Ahmed & Ahmad, 2020), hile the climatic condition of trout habitats in rest of the world are slightly different from our region (Sidoruk & Cymes, 2018).
Therefore, the aim of this study was to determine the dietary Leu requirement of rainbow trout by assessing the overall effect of varied Leu levels on growth performance, hematobiochemical composition, enzyme activities, besides to observe the relative expression of target of rapamycin (TOR) and 4E-BP levels.
2 MATERIALS AND METHODS
2.1 Experimental diet
Casein-gelatin based isonitrogenous (450 g/kg) and isoenergetic (20.90 kJ 100 g−1, gross energy (GE) diets ingradation of varied levels of Leu 10.0 (LI), 12.5 (LII), 15.0 (LIII), 17.5 (LIV), 20.0 (LV) and 22.5 (LVI) g/kg were designed using casein (fat-free), gelatin and L-crystalline amino acid premix (Table 1). The level of protein in all the diets was maintained at 450 g/kg as estimated optimum for maximum growth in our previous study (Ahmed & Ahmad, 2020). The level of L-crystalline amino acids was used to memic the amino acid profile of the diets to that of 40% whole egg protein, excluding the test amino acid (Leu). The gradations of L-Leu were added in each diet in the increments of 2.5 g/kg, dry diets. A casein-gelatine ratio used in all the diets was chosen on the criteria that provide the lowest quantity of test amino acids and maximum quantities of other amino acids. In each incremental Leu level, Leu was increased with the replacement of non-essential amino acids, that is, aspartic acid, serine and glycine, so as to maintain the uniform nitrogen level in all the diets. All the diets were set based on the existing literature on rainbow trout (NRC, 1993). Method for preparation of experimental diets used in this feeding experiment was same as detailed earlier (Ahmed, 2014). The vitamin and mineral premixes used in the study were prepared as per Halver (2002). The dough was then passed through a pelletizer attached with a 2 mm die to make pellets, which were later on dried in a hot air oven at 40℃ in order to decrease the moisture content below 100 g/kg. The dry pellets were crumbled, sieved and stored at 4℃ until used.
| Experimental diets | ||||||
|---|---|---|---|---|---|---|
| Ingredients (g/kg, dry diet) | 10.0 | 12.50 | 15.0 | 17.50 | 20.0 | 22.5 |
| Caseina | 90.0 | 90.0 | 90.0 | 90.0 | 90.0 | 90.0 |
| Gelatinb | 22.50 | 22.50 | 22.50 | 22.50 | 22.50 | 22.50 |
| Amino acid mixc | 393.06 | 393.83 | 394.58 | 395.32 | 396.06 | 396.83 |
| Dextrin | 199.70 | 198.60 | 197.40 | 196.30 | 195.20 | 194.0 |
| Corn oil | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 |
| Cod liver oil | 100 | 100 | 100 | 100 | 100 | 100 |
| Mineral mixd | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 |
| Vitamin mixe, f | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 |
| Carboxymethyl cellulose | 70.0 | 70.0 | 70.0 | 70.0 | 70.0 | 70.0 |
| Alpha cellulose | 4.74 | 5.07 | 5.52 | 5.88 | 6.24 | 6.67 |
| Total | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
| Total leucine | 10.0 | 12.50 | 15.0 | 17.50 | 20.0 | 22.50 |
| Calculated crude protein (g/kg) | 450.0 | 450.0 | 450.0 | 450.0 | 450.0 | 450.0 |
| Gross energyg(kJ/g, dry diet) | 20.9 | 20.9 | 20.9 | 20.9 | 20.9 | 20.9 |
| Proximate composition of experimental diet (g/kg) | ||||||
| Dry matter | 910.50 | 915.60 | 922.65 | 918.70 | 916.30 | 912.40 |
| Analysed crude protein | 449.16 | 450.80 | 449.70 | 450.89 | 449.56 | 448.90 |
| Lipid content | 147.50 | 146.05 | 150.30 | 149.75 | 150.90 | 148.70 |
| Ash | 46.84 | 51.70 | 48.57 | 46.63 | 49.39 | 45.16 |
| Estimated gross energy (kJ/g) | 19.95 | 20.62 | 20.71 | 20.50 | 20.08 |
19.95 |
| Leucine | 9.90 | 12.41 | 14.82 | 17.44 | 19.87 | 22.65 |
| Isoleucine | 31.55 | 31.20 | 30.92 | 31.65 | 31.22 | 30.86 |
| Valine | 28.92 | 28.10 | 28.75 | 28.30 | 27.91 | 29.05 |
- a Crude protein (80%),
- b Crude protein (93%), Loba Chemie, India;
- c Essential amino acids (g/kg) arginine 20.53, histidine 5.88, isoleucine 26.73, leucine variable, lysine 20.54, methionine 13.33, phenylalanine 20.30, threonine 13.72, tryptophan 5.28, valine 22.74. Non-essential amino acids cystine 9.24, tyrosine 13.39, alanine 18.05, aspartic acid variable, glutamic acid 7.06, proline 21.22, serine variable, glycine variable (Loba Chemie, India).
- d Halver 2002 mineral (AlCl3·6H2O, 150; ZnSO4·7H2O, 3000; CuCl,100; MnSO4·4-6H2O, 800; KI,150; CoCl2·6H2O,1000 mg/kg; plus USP # 2 Ca (H2PO4)2. H2O, 135.8; C6H10CaO6 327.0; C6H5O7Fe.5H2O, 29.8; MgSO4.7H2O, 132.0; KH2PO4 (dibasic), 239.8; NaH2PO4.2H2O, 87.2; NaCl, 43.5 (g/kg).
- e vitamin mix (choline chloride 5000: thiamin HCL 50; riboflavin 200; pyridoxine HCL 50; nicotinic acid 750; calcium pentothenate 500; inositol 2000; biotin 5.0; folic acid 15; ascorbic acid 1000; menadione 40; alpha-tocopheryl acetate 400; cyanocobalamine 0.1 (g/kg).
- f Calculated on the basis of fuel values 23.10, 20.21, 24.27, 16.02 and 37.65 kJ/g for casein, gelatin, amino acids, dextrin, and fat, respectively (Jauncey, 1982).
2.2 Experimental set up and feeding trial
Rainbow trout, Oncorhynchus mykiss fingerlings of uniform size and in good health condition transported in oxygen filled polythene bags from hatchery unit of State Government Fishery Department, Dachigam, Laribal, Srinagar, Jammu and Kashmir to feeding trial laboratories station at University of Kashmir. The fishes were first given a prophylactic dip of KMnO4 and were reared under continue flow-through system for a fortnight by feeding a mixture of practical diet in the form of dry pellets. Desired number of fingerlings was selected from the lot and was acclimatized for 2 weeks on synthetic diet (Halver, 2002). After acclimatization, 360 fingerlings of rainbow trout (average initial body weight: 1.60 ± 0.25 g/fish; average length: 5.20 ± 0.30 cm/fish) were then randomly distributed in 70 L circular troughs (water volume 60 L) connected with a water flow-through (2–2.5 L/min) system at the rate of 20 fish per trough for each diets in triplicates. The experimental diets were fed at the rate of 5% BW day−1 on dry to wet-weight basis to each group (Ahmed, 2017). The experimental trial was run for 8 weeks. The diets were divided into two equal portions and fed at 09:00 and 17:00 hours. The remaining details of the feeding trial are same as explained previously (Ahmed & Ahmad, 2020).
2.3 Water quality analysis
During the feeding trial, the physico-chemical parameters such as temperature, pH, dissolved oxygen, free carbon dioxide and total alkalinity were analysed on alternate day basis by following the standard methods (APHA, 1992). The water samples for analysis were collected early in the morning hours before routine feeding. Water temperature (12.7–16.2℃) was analysed by using a mercury thermometer, dissolved oxygen (7.1–8.2 mg/L) was estimated through Winkler's iodimetric test, free carbon dioxide (6.1–15.2 mg/L), total alkalinity (70–83 mg/L) through titrimetric methods, respectively. While pH of water samples (6.9–7.5) was monitored by using a digital pH meter (pH ep-HI 98107, USA).
2.4 Chemical analysis
At the beginning of the feeding trial a pooled sample of 30 specimens were sacrificed for initial body proximate analysis. While at the termination of feeding trial, final weight of each group was taken. Among them, nine fish were randomly pooled from each replicate of dietary treatment and three sub-samples of each replicate from the pooled sample (n = 3 × 3) were analysed for final body composition. Proximate analysis of test diets, initial and final body composition were determined by following standard AOAC (1995) methods: for dry matter (oven drying at 105 ± 1℃ for 22 h), crude protein (N-Kjeldhal × 6.25) Kjeltec 8400 (FOSS Denmark), crude lipid (solvent extraction with petroleum ether B.P. 40–60℃) using soxlet extraction technique (FOSS Avanti automatic 2050, Sweden) and ash oven incineration at 650℃ for 2–4 h (Muffle furnace YSPL-532, India). Amino acid content in the diets was analysed from an ISO 9001:2015 certified professional laboratory Aakaar Biotechnologies Private Limitted Lucknow, India by using HPLC technique.
2.5 Haematological analysis and hepatosomatic index (HSI)
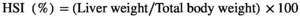
2.6 Digestive enzyme activities
2.6.1 Blood plasma analysis
Before the start of the feeding trial (n = 10) and after the end of experimental trial, pooled blood (n = 2 × 3) samples were collected. Clotted blood in eppendorf tubes were centrifuged for 10 min using a high-speed centrifuge (Tarson MC-02) 4100 × g for 10 min at 4℃. The separated plasma was then analysed for blood enzymes such as alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate transaminase (AST), glucose, urea, uric acid, total protein, albumin, globulin, cholesterol and triglycerides by using veterinary biochemistry analyzer (VS2 Abaxis, USA). For liver profile, pooled liver samples (n = 2 × 3) was homogenized in 10 volumes (w/v) of ice-cold physiological saline solution, then centrifuged at 6000 × g for 20 min at 4℃. The supernatant was used for ALT and AST analysis with the help of biochemistry auto analyzer (Robert Riele GmbH & Co KG. Germany) using standard commercial kits (Erba Diagnostic Mannheim GmbH Mallaustr, Mannheim/Germany).
2.6.2 Intestinal enzyme activities
Intestine samples (n = 2 × 3) was homogenized in 10 volumes (w/v) of ice-cold physiological saline solution and then centrifuged at 6000 × g for 20 min at 4℃. The supernatant was freezed at −20℃ for further analysis. Trypsin and chymotrypsin activities were determined according to Hummel (1959), while amylase and lipase activities were analysed by using the technique given by Furné et al. (2005).
2.7 Growth parameters
The performance of growth parameters of fish fed diets with different Leu levels was calculated as a function of the weight gain by using the standard definitions:
Weight gain (%) = Final body weight – initial body weight/initial weight × 100.
Specific growth rate (SGR%) = 100 × (In final wet-weight (g) − In initial wet-weight g)/duration (days).
Feed conversion ratio (FCR) = Dry weight of feed consumed / Wet-weight gain.
Protein efficiency ratio (PER) =Wet-weight gain (g) / Protein consumed (g, dry weight basis).
Body protein deposition (BPD%) = 100 × (BWf × BCPf) – (BWi ×BCPi) / [TF × CP].
Where BWi and BWf = mean initial and final body weight (g), BCPi and BCFf = mean initial and final percentage of muscle protein, TF = Total amount of diet consumed and CP = Percentage of crude protein of the diet.
2.8 Analysis of target of rapamycin (TOR) and binding protein (4E-BP) gene expression in muscle
The relative gene expressions of TOR and 4E-BP mRNA signalling pathway were analysed by assessing through real-time PCR (Liang et al., 2018). Total RNA was extracted from skeletal muscle by Trizol method (Thermo Fisher Scientific, Darmstadt, Germany) according to the manufacturer´s protocol followed by the quantification and purification of total RNA by spectrophotometer (GENESYS 10S UV-VIS Thermo Scientific). Then complementary DNA (cDNA) was synthesized using a cDNA Synthesis Kit (iScript™cDNA Synthesis Kit Biorad, Hercules, CA), according to the manufacturer's protocol. The identified genes (TOR, 4E-BP and β-actin) were synthesized by using specific primers (TOR: 5՛- CAGCCACACACTTTTACAGACC-3՛ and 5՛- AATCTTGGTGAGGTACGGCTG-3՛); 4E-BP: 5՛- GACCAGGCGGATGACCATAA-3՛ and 5՛- GCAGGAACTTCCGGTCGTAG-3՛; β-actin: 5՛- CCCAAACCCAGCTTCTCAGT-3՛ and 5՛- ATCCGCTGTTTCACCGTTCC-3՛) (Table 2). PCR confirmation of identified genes was done by running 2% agarose gel. Real-time PCRs was performed for TOR and 4E-BP (LightCycler 480 Roche, USA) according to standard protocols with the specific primers already mentioned. PCR amplication was carried out for TOR and 4E-BP using a Chromo 4TM continuous fluorescence detector (Bio-Rad, Hercules, CA, USA) under different thermo cycling conditions. Melting curve analysis was performed by running a gradient from 95 to 50℃ to confirm the presence of single PCR products. The 2−∆∆Ct method was employed to quantitate expression levels for TOR and 4E-BP genes relative to those for β-actin as per the method given by Livak and Schmittgen (2001).
| Target gene | Primer sequence (5′−3′) | Amplicon length (bp) | Annealing temperature (°C) | GenBank accession number |
|---|---|---|---|---|
| Target of rapamycin (TOR) | ||||
| Forward | 5′-CAGCCACACACTTTTACAGACC-3′ | 22 | 60.25 | NM 001124235.1 |
| Reverse | 5′-AATCTTGGTGAGGTACGGCTG-3′ | 21 | 59.82 | |
| ElF4-binding protein (4E-BP) | ||||
| Forward | 5′-GACCAGGCGGATGACCATAA-3′ | 20 | 59.35 | NM 001165149.2 |
| Reverse | 5′-GCAGGAACTTCCGGTCGTAG-3′ | 20 | 61.40 | |
| β-actin | ||||
| Forward | 5′-CCCAAACCCAGCTTCTCAGT-3′ | 20 | 59.35 | XM 021615845.1 |
| Reverse | 5′-ATCCGCTGTTTCACCGTTCC-3′ | 20 | 59.35 | |
2.9 Statistical analysis
The data obtained after the end of feeding trial with respect to various Leu fed diets in the form of LWG%, FCR, PER, SGR% and body composition variables were subjected to one-way analysis of variance (ANOVA) (Snedecor & Cochran, 1967; Sokal & Rohlf, 1981). To determine the significant differences (p ˂ .05) among the treatments, Duncan's multiple range test (Duncan, 1955) was employed. For generating more accurate data in response to the dietary Leu intake, the optimum dietary Leu level was estimated by using second-degree polynomial regression (Y = a + bx + cx2) analysis (Zeitoun et al., 1976). Data for all variables were statistically analysed using Origin software (version 8.5.1; Origin Software, San Clemente, CA).
3 RESULTS
3.1 Growth performance
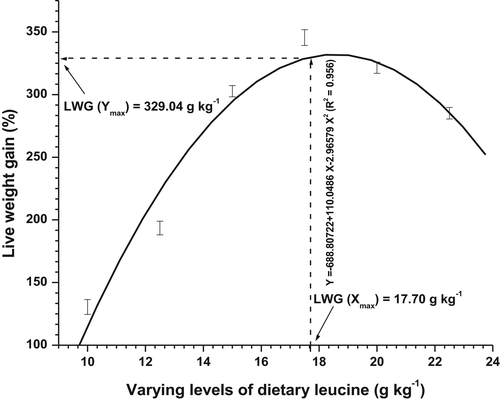
Over the 8 weeks feeding trial fish fed various Leu containing diets had significantly (p < .05) affected live weight gain (LWG%), specific growth rate (SGR), feed conversion ratio (FCR) and other growth parameters (Table 3). Increasing dietary Leu level from 10.0 to 17.5 g/kg resulted an increase in LWG% and SGR (p < .05), but further increases in Leu, that is, 20.0 and 22.5 g/kg did not produced any increase in these parameters. PER and body protein deposition (BPD) showed a similar trend as noted in LWG% and SGR. 17.5 g/kg dietary Leu fed diet supported best-FCR (1.37) followed by 20.0 g/kg Leu containing diet. Except at low Leu containing diets, that is, 10.0 and 12.5 g/kg where higher HSI content was noted. No significant (p > .05) differences in HSI were observed with fish fed >12.5 g/kg Leu diets (Table 4). No diet related mortality or any deficiency signs were seen during the study. The LWG%, FCR, PER and BPD (X) data to dietary Leu level (Y) was subjected to second-degree polynomial regression analysis in order to generate break-points, represented with following equations:
| Parameters | Equations | Break-points (g/kg) |
|---|---|---|
| LWG% | Y = −688.80722 + 110.0486 X − 2.96579 X2 (R2 = 0.956) | 17.70 (Figure 1) |
| FCR | Y = 7.93303 − 0.71596 X + 0.01991 X2 (R2 = 0.938) | 17.42 |
| PER | Y = –1.68477 + 0.34578 X − 0.00973 X2 (R2 = 0.875) | 17.25 |
| BPD | Y = –39.58601+ 6.53718 X − 0.17201 X2 (R2 = 0.830) | 17.57 |
| Dietary leucine levels (g/kg) | ||||||
|---|---|---|---|---|---|---|
| 10.0 | 12.5 | 15.0 | 17.5 | 20.0 | 22.5 | |
| Average initial weight (g) | 1.561 ± 0.02 | 1.575 ± 0.03 | 1.620 ± 0.03 | 1.637 ± 0.03 | 1.591± 0.02 | 1.614 ± 0.02 |
| Average final weight (g) | 3.601 ± 0.14f | 4.628 ± 0.18e | 6.451 ± 0.10c | 7.299 ± 0.25a | 6.707 ± 0.17b | 6.218 ± 0.14d |
| Live weight gain (%) | 130.490 ± 5.90f | 193.51 ± 5.45e | 302.690 ± 4.50c | 345.510 ± 6.31a | 321.41 ± 4.47b | 285.16 ± 4.62d |
| Specific growth rate (%) | 1.49 ± 0.05f | 1.92 ± 0.03e | 2.48 ± 0.02c | 2.66 ± 0.03a | 2.56 ± 0.02b | 2.39 ± 0.02d |
| Feed conversion ratio | 2.65 ± 0.07a | 2.19 ± 0.06b | 1.78 ± 0.05d | 1.37 ± 0.05f | 1.55 ± 0.05e | 1.97 ± 0.06c |
| Protein efficiency ratio | 0.83 ± 0.02e | 1.01 ± 0.03d | 1.24 ± 0.04b | 1.61 ± 0.05a | 1.42 ± 0.04b | 1.12 ± 0.03c |
| Body protein deposition (%) | 10.02 ± 0.40e | 14.20 ± 0.25d | 20.16 ± 0.55c | 30.36 ± 1.05a | 25.23 ± 0.88b | 18.98 ± 0.56c |
| Survival (%) | 100 | 100 | 100 | 100 | 100 | 100 |
Note
- Mean value of three replicates ± SEM; Mean values sharing the same superscript are not significantly different (p > .05).
3.2 Haematological parameters and hepatosomatic index
Significant (p < .05) differences in haematological parameters were seen in fish fed varied levels of dietary Leu (Table 4). Highest haemoglobin (10.15 g/dl) content was noted in fish fed 17.5 g/kg Leu diet, while lowest Hb content was obtained at lower Leu fed diets, that is, (10.0 and 12.5 g/kg). Haematocrit values improved with increasing dietary Leu levels up to 17.5 g/kg and thereafter a reduction in Hct content was recorded. Highest red blood cell (RBC) count 2.45 × 106 mm−3 was also noted at 17.5 g/kg Leu fed diet, while lowest value was estimated at 10.0 g/kg Leu fed diet. Similarly, the fish fed graded levels of dietary Leu also produces significant (p < .05) differences in their leukocyte (WBC) counts with significantly (p < .05) higher WBC 3.22 × 103 mm−3 count was found at lowest Leu level 10.0 g/kg fed diet. Erythrocyte sedimentation rate (ESR) was lowest 2.0 mm/h for the groups reared with diet 22.5 g/kg, whereas it was highest 2.95 mm/h found in those fed 10.0 g/kg Leu diet. Significantly (p < .05) lower mean corpuscular value (MCV) 160.93 and 161.11 fl was calculated in fish fed 15.0 and 22.5 g/kg Leu diet, while other groups indicated relatively higher MCV values. Higher MCHC and MCH were observed in fish fed lower Leu containing diet, that is, 10.0 g/kg.
| Dietary leucine levels (g/kg, dry diet) | |||||||
|---|---|---|---|---|---|---|---|
| Initial | 10.0 | 12.5 | 15.0 | 17.5 | 20.0 | 22.5 | |
| HSI | 2.25 ± 0.12 | 3.15 ± 0.17a | 2.85 ± 0.13b | 1.82 ± 0.19c | 1.89 ± 0.10c | 1.72 ± 0.14c | 1.95 ± 0.17c |
| Hb (g/dl) | 6.85 ± 0.07 | 7.32 ± 0.05e | 7.88 ± 0.07d | 9.20 ± 0.08c | 10.15 ± 0.13a | 9.92 ± 0.10b | 9.45 ± 0.11c |
| Hct (%) | 26.45 ± 0.15 | 28.40 ± 0.13e | 34.60 ± 0.15d | 35.76 ± 0.09c | 42.55 ± 0.18a | 38.85 ± 0.13b | 36.25 ± 0.09c |
| RBC (106/mm3) | 1.50 ± 0.03 | 1.65 ± 0.07d | 1.90 ± 0.05c | 2.15 ± 0.07b | 2.45 ± 0.06a | 2.30 ± 0.04b | 2.25 ± 0.05b |
| WBC (×103/mm3) | 2.52 ± 0.14 | 3.22 ± 0.05a | 2.85 ± 0.05b | 2.40 ± 0.05c | 2.33 ± 0.09c | 2.45 ± 0.08c | 2.77 ± 0.08d |
| ESR (mm/h) | 2.40 ± 0.15 | 2.95 ± 0.12a | 2.70 ± 0.08b | 2.20 ± 0.10c | 2.10 ± 0.08c | 2.18 ± 0.06c | 2.05 ± 0.09c |
| MCV (fl) | 176.33 ± 1.35 | 172.12 ± 1.47b | 182.10 ± 1.22a | 160.93 ± 1.40c | 173.67 ± 1.32b | 168.91 ± 1.65b | 161.11 ± 1.15c |
| MCH (pg) | 45.66 ± 0.32 | 44.36 ± 0.54a | 41.47 ± 0.63b | 42.79 ± 0.28b | 41.42 ± 0.35b | 43.13 ± 0.55a | 42.00 ± 0.20b |
| MCHC (g/dl) | 25.88 ± 0.43 | 28.81 ± 0.30a | 22.77 ± 0.24c | 25.72 ± 0.27b | 23.85 ± 0.43c | 25.53 ± 0.28b | 26.06 ± 0.31b |
Note
- Mean value of three replicates ± SEM; Mean values sharing the same superscript are not significantly different (p > .05).
- Abbreviations: ESR, Erythrocytes sedimentation rate; Hb, Hemoglobin concentration; Hct, Haematocrit; HSI, Hepatosomatic index; MCH, Mean corpuscular haemoglobin; MCHC, Mean corpuscular haemoglobin concentration; MCV, Mean corpuscular volume; RBC, Red blood cell count; WBC, White blood cell count.
3.3 Plasma biochemical indices and liver profile
The effects of graded levels of dietary Leu supplementation on blood plasma biochemical indices and liver profile of O. mykiss were also studied, which indicated that varied levels of dietary Leu had some pronounced effects on biochemical indices Table 5. The contents of total protein (TP), cholesterol (CHO), triglycerides (TG), albumin and globulin in the plasma did not showed any significant (p > .05) differences with fish fed various Leu diets. Contrary to this, plasma alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate transaminase (AST) and glucose level (GLU) showed a decline trend with increase in graded levels of Leu diet up to 17.5 g/kg and thereafter an increasing trend was noted. Plasma urea and uric acid contents also significantly increased with increasing Leu levels and both reached maximum at 22.5 g/kg Leu diet.
| Dietary leucine levels (g/kg, dry diet) | ||||||
|---|---|---|---|---|---|---|
| Serum biochemical indices | 10.0 | 12.5 | 15.0 | 17.5 | 20.0 | 22.5 |
| Total protein (g/L) | 35.12 ± 1.23a | 35.82 ± 1.79a | 35.97 ± 1.45a | 36.45 ± 2.30a | 35.61 ± 2.12a | 34.20 ± 1.24ab |
| Cholesterol (mmol/L) | 10.30 ± 1.74a | 10.22 ± 1.69a | 9.90 ± 1.82a | 10.27 ± 1.43a | 10.54 ± 1.80a | 9.40 ± 1.85a |
| Triglyceride (mmol/L) | 2.41 ± 0.92a | 2.30 ± 0.72a | 2.16 ± 0.62a | 2.09 ± 0.43a | 2.05 ± 0.36a | 2.25 ± 0.49a |
| ALP (U/L) | 68.00 ± 6.42a | 65.04 ± 5.82a | 53.31 ± 5.47c | 50.07 ± 4.89c | 58.07 ± 5.22b | 61.16 ± 5.94b |
| ALT (U/L) | 36.80 ± 3.13a | 34.24 ± 3.34a | 25.49 ± 3.56c | 20.52 ± 3.26c | 29.74 ± 2.92b | 32.44 ± 2.86b |
| AST (U/L) | 134.32 ± 12.32a | 125.74 ± 10.49b | 97.34 ± 9.42c | 81.60 ± 8.16d | 94.21 ± 7.74c | 123.65 ± 9.37b |
| Glucose (mmol/L) | 9.20 ± 1.04b | 9.04 ± 1.15b | 8.41 ± 0.95c | 8.30 ± 0.93c | 9.58 ± 1.05b | 12.19 ± 1.13a |
| Urea (mmol/L) | 39.20 ± 1.92d | 48.36 ± 1.82c | 48.90 ± 1.12c | 53.22 ± 1.50b | 69.01 ± 1.76a | 72.21 ± 1.19a |
| Uric acid (mg/dl) | 5.87 ± 1.08d | 6.35 ± 0.98c | 6.87 ± 0.79c | 8.09 ± 1.02b | 9.86 ± 1.07a | 10.56 ± 1.72a |
| Albumin (g/L) | 25.24 ± 1.82a | 25.70 ± 1.74a | 25.92 ± 1.84a | 26.52 ± 1.98a | 25.58 ± 1.42a | 25.10 ± 1.17a |
| Globulin (g/L) | 9.88 ± 1.53ab | 10.13 ± 1.71a | 10.04 ± 1.75a | 10.32 ± 1.86a | 10.03 ± 1.98a |
9.79 ± 1.93ab |
| Liver profile | ||||||
| ALT (U/L) | 27.80 ± 1.47a | 25.42 ± 1.36b | 22.14 ± 1.56bc | 19.12 ± 1.85d | 23.04 ± 2.01b | 24.18 ± 2.09b |
| AST (U/L) | 218.42 ± 12.82a | 198.90 ± 11.94b | 172.04 ± 9.69c | 142.60 ± 8.57e | 154.48 ± 10.43d |
203.06 ± 9.79b |
Note
- Mean value of three replicates ± SD; Mean values with the same superscript letters are not significantly different (p > .05).
- Abbreviations: ALP, Alkaline phosphatase; ALT, Alanine transferase; AST, Aspartate transferase.
| Intestinal enzymatic activities | Dietary leucine levels (g/kg) | |||||
|---|---|---|---|---|---|---|
| 10.0 | 12.5 | 15.0 | 17.5 | 20.0 | 22.5 | |
| Trypsin (µ/g tissue) | 4.28 ± 0.72d | 4.61 ± 0.82d | 6.01 ± 0.43c | 10.43 ± 0.47a | 8.37 ± 0.52b | 5.90 ± 0.62c |
| Chymotrypsin (µ/g tissue) | 3.24 ± 0.41c | 4.12 ± 0.92b | 4.39 ± 0.82b | 5.78 ± 0.72a | 5.18 ± 0.58a | 3.97 ± 0.64c |
| Amylase (µl/min g) | 9.14 ± 0.97b | 9.48 ± 1.02b | 10.17 ± 1.06a | 8.75 ± 0.94bc | 8.03 ± 1.04c | 10.44 ± 0.93a |
| Lipase (µl/min g) | 0.69 ± 0.01c | 0.72 ± 0.04c | 0.78 ± 0.04ab | 0.82 ± 0.07b | 0.88 ± 0.09a | 0.59 ± 0.10d |
Note
- Mean value of three replicates ± SD; Mean values with the same superscript letters are not significantly different (p > .05).
ALT and AST activities in liver tissue of rainbow trout linearly decreased with the increase of Leu diet up to 17.5 g/kg and thereafter an increase in ALT and AST were also seen. However, both ALT and AST activities were reported maximum in fish-fed lower Leu containing diet (LI) (Table 5).
3.4 Intestinal enzyme activities
The digestive intestinal enzyme activities of rainbow trout fed varied levels of Leu showed remarkable changes (Table 6). Higher activities of trypsin, chymotrypsin, and lipase in intestine were seen with the increase of each dietary Leu levels only up to 17.5 g/kg Leu fed diet and thereafter a significant (p < .05) decrease in these activities were noted, while as maximum amylase activity was observed for fish fed higher Leu diet, that is, 22.5 g/kg compared to all other groups.
3.5 Whole body composition
Whole body composition of rainbow trout fingerlings fed diets providing varied Leu levels had significantly (p < .05) affected (Table 7). Body protein content was found significantly (p < .05) highest with fish fed 17.5 g/kg Leu containing diet, and beyond that a slightly decline trend in protein content was observed. Moisture content decreased linearly with increasing dietary Leu levels up to 17.5 g/kg and thereafter an incremental increase in moisture content was noted. Fat content increased linearly with the increase of each dietary Leu levels and reached its peak at 22.5 g/kg Leu fed diet. Except at lowest Leu fed diet, that is 10.0 g/kg, where highest ash content was observed, no significant (p > .05) difference in whole body ash content was noted among the fish fed varied levels of dietary Leu.
| Dietary leucine levels (g/kg) | |||||||
|---|---|---|---|---|---|---|---|
| Initial | 10.0 | 12.5 | 15.0 | 17.5 | 20.0 | 22.5 | |
| Moisture (%) | 78.68 ± 0.34 | 77.30 ± 0.37a | 75.86 ± 0.28b | 74.24 ± 0.18c | 72.16 ± 0.29d | 72.98 ± 0.30d | 72.50 ± 0.27c |
| Protein (%) | 13.31 ± 0.16 | 12.53 ± 0.15f | 13.76 ± 0.12e | 15.45 ± 0.9c | 17.52 ± 0.8a | 16.62 ± 0.7b | 15.92 ± 0.9d |
| Fat (%) | 3.63 ± 0.09 | 5.63 ± 0.06a | 6.24 ± 0.05b | 6.64 ± 0.04c | 6.80 ± 0.05e | 7.10 ± 0.08de | 7.43 ± 0.06d |
| Ash (%) | 2.74 ± 0.07 | 3.35 ± 0.06a | 2.45 ± 0.04b | 2.13 ± 0.05c | 2.04 ± 0.08c | 2.20 ± 0.04c | 2.22 ± 0.07c |
Note
- Mean value of three replicates ± SEM; Mean values with the same superscript letters are not significantly different (p > .05).
3.6 Relative gene expression of target of rapamycin (TOR) and binding protein (4E-BP) in fish muscle
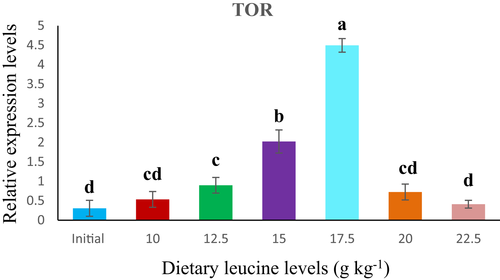
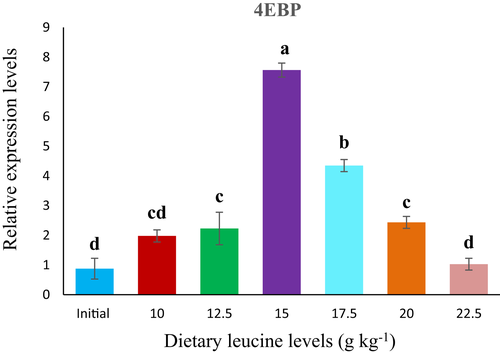
In the present study, dietary Leu levels had progressively affected the relative expression of TOR and 4E-BP in the muscle of rainbow trout fingerling. TOR mRNA expression level in muscle significantly elevated with increasing Leu levels up to 17.5 g/kg and gradually decreased thereafter. Significantly (p < .05) highest TOR level was observed for fish fed 17.5 g/kg Leu diet (Figure 2) . Contrary to TOR mRNA level, the relative expression of 4E-BP mRNA level in the muscle results a plateau-like response up to 15.0 g/kg Leu fed group, thereafter it showed decreased trend from 17.5 g/kg onwards (Figure 3).
4 DISCUSSION
Leucine being an indispensable amino acid for the protein synthesis plays an essential role in growth and well-being of fish (Kim & Lee, 2013; Zehra & Khan, 2015), thus inclusion of appropriate amount Leu in the diet of fish is imperative in order to achieve optimal fish growth (Ahmed & Khan, 2006). Results of the present study indicated that optimum dietary Leu requirement for maximum LWG% of rainbow trout was estimated to be 17.45 g/kg dietary Leu, corresponding to 38.77 g/kg of dietary protein, which is lower than the requirement reported for rainbow trout 44.0 and 92.0 g/kg, respectively (Choo et al., 1991; Ogino, 1980). The dietary Leu requirement recommended in the present study is almost similar to the requirement reported for other fish species such as Oncorhynchus tshawytscha 39 g/kg (Chance et al., 1964), Cirrhinus mrigala 38.5 g/kg (Ahmed & Khan, 2006), Labeo rohita 39.0 g/kg (Abidi & Khan, 2007) and is lower than the requirement reported for other species, that is, C. idella 42.4 g/kg (Deng et al., 2014), H. fossilis 43.4 g/kg (Farhat & Khan, 2014), C. catla 47.9 g/kg (Zehra & Khan, 2015), O. niloticus 43.1 g/kg (Gan et al., 2016), Trachinotus ovatus 71.5–80.2 g/kg (Tan et al., 2016) and S. ocellatus 42.4 g/kg (Catillo & Gatlin, 2018) and slightly higher than the requirement reported in Cyprinus carpio 37.7 g/kg (Wu, 2014) of dietary protein. Our study clearly indicates that Leu is one of the essential amino acid for the growth and maintenance of rainbow trout fingerling and will possibly make an excellent use for formulation of Leu balanced diet for aquaculture of this species.
The wide variation among these requirements may be due to several factors including differences in dietary protein sources (Forster & Ogata, 1998), fish heritable differences (Deng et al., 2010), feed formulation, discrepancies in age and size, feeding regimes, ecological conditions (Ahmed & Khan, 2004; Ruchimat et al., 1997; Zehra & Khan, 2017) and use of different mathematical methods (Baker, 1996; Deng et al., 2010; Encarnacao et al., 2004; Zehra & Khan, 2015).
The fish fed inadequate Leu levels (10.0 and 12.5 g/kg) and higher Leu level (22.5 g/kg) showed reduction in WG compared to intermediate Leu levels 17.50 and 20.0 g/kg which confirmed that insufficiency and excess levels of Leu in the diets could cause a poor growth performance of rainbow trout fingerling. The deficiency of Leu and its toxic effects, when supplied excess in the diet on growth of fish have been reported in the past in several fish species such as, rainbow trout (Choo et al., 1991); Indian major carp, C. mrigala (Ahmed & Khan, 2006); grass carp, C. idella (Deng et al., 2014); H. fossilis (Farhat & Khan, 2014), C. catla (Zehra & Khan, 2015) and S. ocellatus (Catillo & Gatlin, 2018). Negative impact on growth may be attributed due to an imbalanced amino acid content of the diets (Peres & Oliva-Teles, 2008) and also might be due to mal-absorption or underutilization of other amino acids which induce toxic and stress effect on the fish (Zhou, Wu, et al., 2020). Another reason for the depressed growth presumably is due to the result of antagonism effects of BCAAs, which has clearly reported in mammals typically arises from an excess of Leu over isoleucine and valine (NRC, 1993), although, studies on antagonisms among BCAA in fish are not apparent and are incoherent between species (Ahmed & Khan, 2006).
The study of haematological parameters are widely established as a convincible indicators for the well-being and biological conditions of fish both in the cultured and wild condition (Ahmed et al., 2019; Dawood et al., 2015; Kader et al., 2010; Lemaire et al., 1991). These variables are also helpful to assess the functional activities of oxygen carrying capacity of the bloodstream (Ahmed, 2012; Shah & Altindag, 2005). In the present study, haematological parameters showed remarkable differences in response to varying concentrations of dietary Leu. Hb, HCT and RBC values showed an increased pattern up to the fish fed 17.5 g/kg Leu diet, which is in conformity with the results of other fish species, that is, H. fossilis (Farhat & Khan, 2014); C. catla (Zehra & Khan, 2015). The higher values for Hb, HCT and RBCs noted at fish fed 17.5 g/kg Leu diet in the present study might be related to enhanced fish growth, providing for an efficient level of the blood oxygen carrier system. Erythrocytes sedimentation rate (ESR) is considered as one of the important non-specific haematological variable, which elevates during stress, nutritional toxicity, severe infections and metal poisoning. During the study it has been observed that ESR value proportionally decreased with each incremental Leu level and was recorded lowest with fish fed 22.5 g/kg Leu diet, which may be due to the possible elevated blood viscosity, resultant an enhancement in RBCs count (Ahmed et al., 2020). Erythrocyte indices characterize the size of red blood cells, as well as haemoglobin content in the peripheral blood (Ahmed et al., 2019). The higher MCV values at lower and higher Leu containing doses recorded in the present study may be because of obliteration of erythrocytes leading to anaemia (Johnsson-Sjobeck & Larson, 1979; Sakthivel, 1988; Wang et al., 2017). Nevertheless, no differences in MCHC and MCH data were obtained.
Blood plasma variables are known as convincible indicators for the well-being and biological conditions of fish (Ahmad et al., 2021; Han et al., 2014; Kader et al., 2010; Lemaire et al., 1991). Until today, there is little available information on the effects of dietary Leu on blood plasma, liver profile and digestive intestinal enzyme activities of fish species. The total protein in plasma plays a vital role in fish blood and performs functions of the defence, osmoregulation and transportation of various substances within an organism (Rudneva & Kovyrshina, 2011; Sharaf & Khan, 2020). While cholesterol plays an important role in the synthesis of cell membranes, bile and hormones within animal bodies including fish (Tan et al., 2016). Although fish has a capability to synthesize cholesterol and most of it in blood is from the liver and digestive tract. During the study, we tried to evaluate the effect of graded concentration of Leu on rainbow trout and our findings indicated that the contents of plasma total protein (TP), cholesterol (CHO), triglyceride (TG), albumin and globulin did not show any significant differences with respect to the inclusion of Leu in the diets, which is in conformity with the study on blunt snout bream, M. amblycephala (Ren et al., 2015), in which no significant differences among the plasma biochemistry profile of the fish were recorded when fed graded levels of dietary Leu. Several other studies have obtained the same results as reported in present study (Wang et al., 2017; Zhou et al., 2007). The other parameters including ALP, ALT and AST (plasma) decreased by increment of dietary Leu up to 17.5 g/kg diet, thereafter increased trend was noted with increase of dietary Leu. Similar findings were also observed in juvenile red sea bream, Pargus major fed varied levels of valine diet (Rahimnejad & Lee, 2013). Usually, increments in plasma ALT level indicate liver abnormal function (Habte-Tsion et al., 2013; Rahimnejad & Lee, 2013). During the present study, plasma glucose level showed same pattern as indicated in plasma ALT and AST and reached higher at 22.5 g/kg Leu fed diet. Maximum glucose level found in higher Leu fed diets may be due to the use of oxidative regulation of glucose by skeletal muscle (Li et al., 2011), which is in agreement with the findings in M. amblycephala (Ren et al., 2015). However, urea and uric acid contents constantly showed an increased trend with increasing Leu levels and both reached maximum in 22.5 g/kg Leu fed group, which is in accordance with the results for golden pompano, T. ovatus (Tan et al., 2016).
Alanine transaminase (ALT) and aspartate transaminase (AST) in liver are two important transaminase enzymes and play a crucial role in amino acid metabolism, and also helpful in understanding the condition of liver (Liang et al., 2018; Rahimnejad & Lee, 2013). Significant decrease in ALT and AST (liver) was observed with fish fed up to 17.5 g/kg dietary Leu, which is in accordance with the study of Rahimnejad and Lee (2013). This indicates that 17.5 g/kg dietary Leu has a positive effect on maintaining normal functions in liver, while as, deficient or excess Leu levels may weaken the liver condition in rainbow trout fingerling. Similar results were also reported in other fish species such as juvenile red sea bream, P. major (Rahimnejad & Lee, 2013); juvenile olive flounder, Paralichthys olivaceus (Rahimnejad & Lee, 2014); M. amblycephala (Liang et al., 2019) and T. ovatus (Tan et al., 2016; Zhou, Lin, et al., 2020).
Intestine has not only a digestive property but also plays a vital role in immune system (Gao et al., 2017; Lokesh et al., 2012; Rombout et al., 2011). It has direct contact with intestinal contents due to that nutrients gets easily absorbed, and concurrently it protects against the interference of harmful entities like toxins and bacteria (Furness et al., 1999; Zhou, Lin, et al., 2020). In fish, trypsin, chymotrypsin, lipases and amylases are very important digestive enzymes, which play a crucial role in the digestion of protein, fat and carbohydrate, respectively (Suzer et al., 2007; Zhao et al., 2012). In the present study, the contents of trypsin, chymotrypsin and lipase in intestine significantly elevated with increasing dietary Leu levels up to 17.5 g/kg Leu diet and reduced thereafter, while as amylase reached maximum for fish fed 22.5 g/kg Leu diet. This is in conformity with the findings of Zhao et al. (2012), who suggested that inclusion of isoleucine in juvenile Jian carp, C. carpio diet had pronounced effects on digestive amylase activities. Dong et al. (2012) who also reported that the activities of trypsin, chymotrypsin, amylase and lipase in the intestine were enhanced with the increment levels of dietary valine. The increment of these digestive intestinal enzyme activities confirmed that appropriate supplementation of Leu in the diet improved the digestive ability of fish, sustaining the protein retention values (Dong et al., 2012).
Leucine supplementation improved both body protein and fat contents in this study, which clearly indicated that Leu promotes the accretion of nutrients in fish body (Deng et al., 2014; Zehra & Khan, 2015). Minimum moisture and maximum protein contents were evident in fish fed 17·5 g/kg dietary Leu. Similar trend was also observed in body protein deposition, which is in conformity with the findings on other fish species such as C. mrigala (Ahmed & Khan, 2006), M. amblycephala (Ren et al., 2015) and C. catla (Zehra & Khan, 2015). The elevation in whole body protein content affected by varying levels of dietary Leu might be due to involvement of different proteins or amino acid transamination reactions occurring in the body of the fish (Abidi & Khan, 2007). Whole body fat content ameliorated with the incremental levels of dietary Leu. Higher fat content reported with each incremental Leu level might be due to the formation of muscle tissue carbon skelton of ketogenic amino acid Leu, which is converted into acetyl-CoA as well as acetoacetate, that are mainly used to synthesize fatty acids (Erwan et al., 2009; Hyun et al., 2007; Zehra & Khan, 2015). No interactive effects were found on body ash contents among the treatment levels, except at lower Leu containing diets where high body ash content was recorded. In the present study, higher HSI values was recorded in fish fed diets with lower levels of Leu compared to higher Leu fed groups, indicated that fish possess fatty liver, as fish could not receive balance amount of nutrients, which ultimately affects its health.
Protein synthesis is a crucial component of the process involved in growth response (Anthony et al., 2001), where the target of rapamycin (TOR) pathway mainly plays a vital role (Holz et al., 2005; Wang & Proud, 2006). It is a key regulator of stability between protein synthesis and degradation in response to nutrition eminence and quantity (Wullschleger et al., 2006; Wu et al., 2017). While as eukaryotic initiation factor 4E-binding proteins (4E-BPs) are notorious downstream effectors of TOR signalling pathway (Anand & Gruppuso, 2006). Branched chain amino acids (BCAAs) especially Leu, has an important role in the activation of the anabolic signal, with the most potent growth promoting effect (Neinast et al., 2019). In the present study, relative expression of TOR mRNA was significantly affected by dietary Leu levels and showed an increased trend up to fish fed 17.5 g/kg Leu containing diet, and decreased thereafter, indicating that an appropriate amount of dietary Leu could up-regulate the TOR signalling pathway to improve the protein synthesis in rainbow trout fingerlings. Similar results were also reported in other studies in which supplementation of amino acids particularly Leu to diets had a pronounced impact on TOR gene expression in different fish species (Seiliez et al. 2008; Zhao et al., 2012). While studies on rainbow trout, O. mykiss (Lansard et al., 2009, 2010; Seiliez et al., 2011) and Jian carp, C. carpio var. Jian (Feng et al., 2013; Tang et al., 2013; Zhao et al., 2012) have also confirmed that proper nutrition plays an important role in regulating the TOR signalling pathway in fish similar to mammals. However, in fish, the key mediator effects of Leu have been established on various aspects of physiology as well as the activating of mTOR complex regulation (NRC, 2011). Ren et al. (2015) observed that TOR mRNA expression level was considerably exaggerated by dietary Leu level and recorded an insignificant pattern with whole body protein in juvenile blunt snout bream, which is in conformity with the present study signifying that balanced Leu levels could up-regulate the TOR signalling pathway to improve the protein synthesis in fish species. More recently, it was found that balanced Leu diet elevated TOR and S6K1 mRNA levels, as well as phosphorylation of these pathways in the muscle of hybrid catfish, Pelteobagrus vachelli × Leiocassis longirostris (Zhao et al., 2020). However, in the present study, 4E-BP mRNA levels in muscle also showed an elevated trend at a certain points and reached utmost at 15.0 g/kg Leu diet, which is slightly on lower side when compared to TOR mRNA level, as it was reported higher with fish fed 17.5 g/kg dietary Leu level. Similar observation was recorded in zebrafish fed graded Leu levels, where it is found to augment the phosphorylation of 4E-BP1 and trigger the expression of TOR mRNA signalling pathway (Payne et al., 2012). Our study is supported with the mechanism of protein synthesis via activating the mTOR signalling pathway including the phosporylation of S6K1 and eukaryotic translation initiation factor (elF-4E-BP) assembly (Kimball & Jefferson, 2006), where Leu plays a crucial role. Zou et al. (2018) also reported that dietary Leu up-regulates TOR, S6K1 and elF-4E-BP mRNA levels in the hepatopancreas in the muscle of gibel carp, C. auratus. Our findings also specify that optimum dietary Leu level could regulate TOR mRNA signalling pathway in fishes in the similar manner as in mammals (Li et al., 2011). However, the mechanism of TOR signalling pathway regulation by dietary Leu is complex and the information is inadequate, which needs further exploration.
5 CONCLUSION
The present study provided evidence that optimal dietary Leu supplementation significantly increased growth parameters in O. mykiss fingerlings and based on second-degree polynomial regression analysis of growth parameters, the optimum Leu level for maximum growth of fingerling rainbow trout, O. mykiss was estimated to be 17.45 g/kg dietary Leu, corresponding to 38.77 g/kg of dietary protein and insufficiency or excess dietary Leu resulted negative effects on growth parameters. The dietary Leu levels also influenced haematological parameters, enzymatic variables in the plasma and intestine of the fish, as well as improved muscle protein synthesis by activating TOR signallingpathway. Data from this study would be helpful in developing nutritionally balanced complete diets for the intensive culture of rainbow trout.
ACKNOWLEDGEMENTS
The authors are highly grateful to the Head, Department of Zoology, University of Kashmir, Hazratbal, Srinagar, India, for providing the laboratory facilities. We are also pleased to Department of Fisheries, Seed Hatchery, Dachigam, Laribal, Srinagar (Jammu & Kashmir) for providing trout fingerlings to carry out this experiment.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest to disclose.
ETHICAL STATEMENT
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed during the present study. All the protocols used in the present study have been approved by Animal Ethical Committee registered under R. No. 801/Go/RE/S/2003/CPCSEA.
Open Research
DATA AVAILABILITY STATEMENT
The data used in this study are included within the manuscript.




