Effects of lysophospholipid on rainbow trout (Oncorhynchus mykiss) growth, biochemical indices, nutrient digestibility and liver histomorphometry when fed fat powder diet
Abstract
This research was conducted to evaluate the effects of lysophospholipid (LPL) on growth, biochemical indices, nutrient digestibility, digesta viscosity and liver histology in rainbow trout fed fat powder diets. Three hundred fish with an average individual weight of 25.43 ± 1.93 were divided into five dietary groups each in triplicates. Experimental diets formulated based on addition of four levels of LPL (0, 3, 6 and 9 g kg−1) to a basal diet containing fat powder. There was also a fish oil-based diet as positive control. After 56 days of trial, measuring growth parameters suggested significantly improvement of body weight, specific growth rate and feed conversion ratio in LPL 9 compared to other diets containing fat powder; however, control fish oil diet resulted in the highest values (p < .05). Cholesterol values decreased by addition of LPL to the diets containing fat powder (p < .05). On the contrary, triglyceride concentration increased by LPL administration. Inclusion of fat powder to the diet reduced body fat and fat digestibility (p < .05); however, these parameters were improved by administration of 9 g kg−1 LPL. Also, viscosity of diet and digesta was decreased in LPL 9 (p < .05). Contrarily, ascending trend displayed in lipase activity by increasing LPL levels. Moreover, the replacement of fish oil by fat powder in the diet led to enlargement of hepatocytes nuclei, however, addition of LPL levels reduced nucleus size (p < .05). Also, sinusoids content developed in liver of fish fed fat powder (p < .05). This study suggests that rainbow trout can digest fat powder and also reduces side effects of fat powder with the help of LPL when fat powder is replaced with fish oil.
1 INTRODUCTION
Global fish oil production is expected to be unsustainable because of distinction of ocean stocks and high demand (Natale et al., 2013). These limitations have forced aquaculture industry to replace lipid sources (Oliva-Teles et al., 2015). Among the fat sources, fish oil has been an ideal option to be added in fish diet in case of having unsaturated fatty acids and higher digestibility. On the contrary, using terrestrially based lipids like vegetable oil and fat powder, as alternative fat sources, could decrease the requirement for fish oil but they may reduce lipid digestibility (Amirkolaie et al., 2014). Vegetable oils are more resistant to lipolysis process than fish oil since lipase prefers to use polyunsaturated fatty acids as substrate (Austreng et al., 1979). On the other hand, low cholesterol level of vegetable oils can reduce emulsification and micelle formation effectively through a lower bile synthesis in the liver (Crespo & Esteve-Garcia, 2003). Hence, control these pathways can help improves lipid digestibility.
Biochemical parameters are indicator for health condition of fish (Fazio et al., 2016, 2021). Moreover, fish health may be also affected by the use of alternative sources of fish oil. Liver is an important organ which plays a key role in lipid anabolism and catabolism (Dutta & Datta-Mushi, 1996). Histological changes including degenerations of tissue and accumulation of large lipid vacuoles in hepatocytes have been reported earlier in European sea bass (Dicentrarchus labrax), gilthead sea bream (Sparus aurata) (Alexis, 1997) and rainbow trout (Oncorhynchus mykiss) fed vegetable oil diets (Caballero et al., 2002). Maximum levels of fish oil replacement have been variably achieved between 500 and 600 g kg−1 depend on species and oil sources (Montero et al., 2003). Also, Mosconi-Bac (1990) declared that accumulation of lipids in the liver can cause the liver necrosis in Dicentrarchus labrax using inappropriate fat sources (i.e., high saturated fatty acids or low n-3/n-6 PUFA ratio) after 106 days.
These negative effects can be partially compensated by some health benefit additive like cholic acid (Adhami et al., 2017), bile salts (Yamamoto et al., 2007) and taurine (Takagi et al., 2005, 2011). Phospholipids, also, can improve fat digestion through emulsification, and formation of lipoproteins (Tocher et al., 2008). Hence, oil droplet accumulated in liver can be eliminated. Furthermore, lecithin inclusion in diet can suppress serious health disorders like hepatic lipoidosis or lipoid liver disease in rainbow trout because of protecting cell membrane against oxidation (Honjoh et al., 1967).
Lysophospholipid (LPL) is the final product of phospholipid hydrolysis in which one fatty acid is eliminated by phospholipase (Joshi et al., 2006). It is surface-active and plays an important role as emulsifying agent, thus improve the absorption of lipids and lipid-soluble compounds (Abat et al., 2002). In addition, abnormal fats are inhibited by lysolecithin to accumulate in liver (Leeson & Summers, 2001). On the other hands, there are some reports supporting the idea that blood cholesterol and triglyceride are affected by emulsifiers (Yamamoto et al., 2007). Taghavizadeh et al. (2020) indicated enhancement of growth and feed utilization by addition of 2 g kg−1 LPL to the rainbow trout diet. Furthermore, inclusion of lysolecithin in diet of channel catfish (Ictalurus punctatus) led to lower lipid content in liver and increasing intestinal lipase activity (Liu et al., 2019). The aim of the present study is to clarify liver changes and fat deposition by addition of fat powder with or without LPL in rainbow trout. Also, we attempt to understand whether LPL can compensate the possible negative effect of fat powder on fat digestibility, liver histology, lipolysis and digesta viscosity or not.
2 MATERIAL AND METHODS
2.1 Ethics statement
This experiment was approved by Animal Care and Use Committee of Sari Agricultural and Natural Resources University.
2.2 Fish and experimental system
The current study was conducted at aquaculture experimental facility of Sari Agricultural and Natural Resources University. Three hundred juvenile rainbow trout with an average individual weight of 25.43 ± 1.93 g were obtained from rainbow trout rearing farm (Sari city, Mazandaran, Iran) and moved to the experimental facility. Fish were checked for absence of lesions or deformity and described clinically healthy. Twenty fish per tanks were randomly distributed into 15 300 L-circular fibreglass tanks which were equipped by oxygen supplier aeration systems. The average temperature, dissolved oxygen and pH throughout the trial were 13.36 ± 1.77°C, 7.18 ± 0.49 mg L−1, 7.9 ± 0.0, respectively. A natural 12L:12D photoperiod was applied similarly in all tanks during the experimental period.
2.3 Experimental diet formulation
The formulation and chemical composition of the experimental diets are presented in Table 1. Four doses (0, 3, 6, 9 g kg−1) of lecithin converted lysophospholipid (Sinason Company, Tehran, Iran) were added to a basal diet in which 700 g per kg of liquid oil (canola + fish oil) was replaced by fat powder. Fat powder (Avid Company, Beshel industrial town, Qaemshahr, Iran) was produced of sunflower oilseeds using calcium oxide under high pressure and temperature turning fatty acids to powder. There was also a control diet contained fish oil and canola oil as a positive control without LPL addition. Furthermore, 6 g kg−1 of chromic oxide (Cr2O3) was added to the diets for digestibility measurement. Feed ingredients were thoroughly mixed, and water was added before pelleting in a meat grinder with 2.5 mm mesh size. Then, pellets dried in oven (40°C) and stored at −20°C prior using. Five experimental diets were tested; control (containing canola and fish oil as lipid sources without fat powder), 0, 3, 6 and 9 g kg−1 lysophospholipid (LPL) each in triplicate. Fish were acclimated to experimental conditions for two weeks and fed experimental diets for 8 weeks, after adaptation.
| Diets | Control | LPL 0 | LPL 3 | LPL 6 | LPL 9 |
|---|---|---|---|---|---|
| Ingredients | |||||
| Corn meal | 70 | 70 | 70 | 70 | 70 |
| Fish meal | 380 | 380 | 380 | 380 | 380 |
| Wheat gluten | 120 | 120 | 120 | 120 | 120 |
| Soybean meal | 150 | 150 | 150 | 150 | 150 |
| Wheat meal | 90 | 90 | 90 | 90 | 90 |
| Fish oil | 65 | 17 | 15.5 | 14 | 12.5 |
| Canola oil | 65 | 17 | 15.5 | 14 | 12.5 |
| Fat powder | 0 | 96 | 96 | 96 | 96 |
| lysophospholipid | 0 | 0 | 3 | 6 | 9 |
| Vitamina | 20 | 20 | 20 | 20 | 20 |
| Mineralb | 20 | 20 | 20 | 20 | 20 |
| Binderc | 20 | 20 | 20 | 20 | 20 |
| Chemical analysis (% of dry matter) | |||||
| Fat | 22.34 | 22.36 | 22.24 | 22.24 | 22.27 |
| Protein | 42.00 | 42.00 | 41.90 | 41.95 | 41.90 |
| Ash | 13.52 | 13.85 | 13.39 | 13.36 | 13.35 |
| Moisture | 1.33 | 1.33 | 0.67 | 1.00 | 1.67 |
- a Vitamin premix consisted of (g kg−1 premix): 1,200,000 IU vitamin A, 400,000 IU vitamin D3, 3,000 IU vitamin E, 5400 mg vitamin C, 200 mg vitamin B1, 3,360 mg vitamin B2, 7,200 mg vitamin B3, 9,000 mg vitamin B5, 2,400 mg vitamin B6, 600 mg vitamin B9 and 4 mg vitamin B12, 500 mg.
- b Mineral premix consisted of (g kg−1 premix): 2,600 mg Mn, 600 mg Cu, 6,000 mg Fe, 4,600 mg Zn, 50 mg Se, 100 mg Iu, 50 mg Co and 100,000 mg choline chloride.
- c Corn starch.
2.4 Sampling procedure
Fish fed with experimental diets three times a day (8.00, 12.00 and 17.00) to apparent satiation for 8 weeks. On last day of experiment, the digesta from middle intestine of three randomly selected fish per tank was collected for viscosity measurement 4 h after feeding. At final day of the study, fish were starved for 24 h to empty the intestine. On the sampling day, fish weight and length were measured individually for each tank after anaesthesia with 150 mg L−1 clove oil. Fish were overdosed with 1000 mg L−1 clove powder prior sampling (Taghavizadeh et al., 2020). In order to measure somatic indices, three fish from each tank were randomly dissected, viscera and liver weighed; then, fish livers were transferred to formalin for histological study. Latter three fish were used to determine body proximate composition after evisceration.
Afterwards, four fish randomly were taken from each tank and anaesthetized with 150 mg L−1 clove oil. Blood samples were collected from caudal blood vessel via 2-ml syringe in order to determining biochemical parameters. Then, serum was obtained by centrifuging blood at 5000 g for 10 min after clotting and stored at −18°C for 30 days before analysing. Total protein, glucose, albumin, triglyceride and cholesterol were measured spectrophotometrically using commercial kits (Pars Azmun, Karaj, Iran) following the methods described by Hoseinifar et al. (2016).
2.5 Fish performance

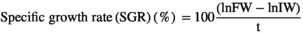

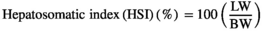
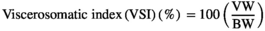
2.6 Enzyme activity
Pancreatic lipase-specific activity was determined using p-nitrophenyl myristate, methoxy ethanol, sodium cholate and Tris-HCl as a substrate. In the following, acetone/n-heptane 5:2 was used to stop the reaction (Iijima et al., 1998). The activity of lipase expressed based on protein soluble as previously described by Bradford (1976).
2.7 Proximate composition
Analysis of fat, crude protein, moisture, and ash in diets, faeces and the body of fish were performed according to AOAC (2005) procedures. Dry matter was measured by drying at oven (105°C, 24 h) until a constant weight was obtained. Ash content was determined through burning in a muffle furnace at 550°C for 6 h. Crude protein was analysed using Kjeldahl method after acid digestion at 200°C, and fat was extracted by petroleum ether in a Soxhlet apparatus after 3–6 h (until transparent solution was observed in upper part). Proximate composition was obtained using dry matter of samples after placed the body and feed at oven 65°C until become dried.
2.8 Apparent digestibility
At the end of the feeding trial, remaining ten fish were kept in the tanks for faeces collection and diet digestibility determination for two week. Fish were anaesthetized by 150 mg L−1 clove oil before faeces collected by stripping from the posterior part of the fish intestine. Faecal sampling continued until collection of desirable amount of sample (10 g).
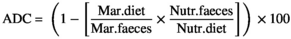
2.9 Viscosity of digesta and diets
Viscosity measurement was done for both diets and intestinal digesta followed the method described before (Refstie et al., 1999). One gram of diet or digesta diluted with 1ml distilled water and centrifuged at 3000 g for 10 min. Then, viscosity (in centipoise, cP = 1/(100 dyne sec × cm2)) of the supernatant was measured by viscometer (BROOKFIELD digital) in 60 rpm using aviCheck method (Finnfeeds International, Marlborough, UK). Absolute viscosity (cP) was represented at shear rate of 11.2 s−1.
2.10 Histology sampling
Liver samples of each fish were taken at the end of the experiment, fixed in 4% buffered formalin, dehydrated in ethanol and embedded in paraffin. Sections (4 mm) were stained with haematoxylin and eosin (H&E), observed under light microscopy (Dino-Lite lens) and finally analysed by Dino-capture 2 software (Martoja & Martoja-Pierson, 1970).
2.11 Statistical analysis
The present experiment was performed based on completely randomized design. The effect of LPL on growth and feed utilization were analyses by one-way ANOVA procedure. SPSS 22 (statistical software) was used to analysis the data after verification for normality using Shapiro–Wilk test. Duncan's post hoc test was used to compare means between groups at 0.05% possibilities. All data were presented as means of each treatment with standard deviation.
3 RESULTS
There were significant differences in final weight, WG, SGR, FCR, conditional factor, hepatosomatic and viscerosomatic indices among fish fed different experimental diets (Table 2; p < .05). Supplementation of 9 g kg−1 LPL in the diet increased WG and SGR comparing with other doses of LPL (p < .05). However, control diet containing fish oil showed higher final weight, WG and SGR than fat powder experimental diets (p < .05). FCR value decreased in LPL 0 and 9 diet among fat powder containing diet, while inclusion of 3 and 6 g LPL resulted in highest FCR (p < .05). Moreover, HSI and VSI values elevated significantly in fat powder diet contained 0 g kg−1 LPL (p < .05). Contrarily, those values reduced by LPL addition (p < .05).
| Diets | Control | LPL 0 | LPL 3 | LPL 6 | LPL 9 |
|---|---|---|---|---|---|
| Initial weight (g) | 23.79 ± 0.94 | 25.62 ± 2.42 | 26.87 ± 2.82 | 25.23 ± 0.96 | 25.66 ± 2.54 |
| Final weight (g) | 106.30 ± 3.78a | 66.57 ± 1.85c | 61.41 ± 1.83c | 62.26 ± 3.63c | 78.35 ± 1.91b |
| Weight gain (%) | 346.88 ± 4.1a | 161.22 ± 22.47c | 129.83 ± 18.7c | 146.96 ± 16.22c | 207.33 ± 18.04b |
| Specific growth rate (%day−1) | 2.67 ± 0.01a | 1.71 ± 0.15c | 1.48 ± 0.14c | 1.61 ± 0.12c | 1.99 ± 0.18b |
| Feed conversion ratio | 1.33 ± 0.02c | 1.82 ± 0.10b | 2.03 ± 0.16a | 2.09 ± 0.07a | 1.69 ± 0.10b |
| Feed intake (g) | 2244.33 ± 109.32a | 1442.90 ± 92.85d | 1378.40 ± 39.08d | 1597.00 ± 79.24c | 1776.50 ± 76.66b |
| Hepatosomatic index (%) | 1.02 ± 0.02e | 1.38 ± 0.03a | 1.30 ± 0.01b | 1.22 ± 0.03c | 1.15 ± 0.02d |
| Viscerosomatic index (%) | 10.00 ± 0.26e | 12.83 ± .50a | 12.19 ± 0.08b | 11.47 ± 0.29c | 10.67 ± 0.40d |
| Survival (%) | 100 | 100 | 100 | 100 | 100 |
Note
- All values are means of three replicates (tanks)/treatment ± standard deviation; different superscript letters show significant differences (p < .05).
Moreover, cholesterol reduction was observed by adding LPL to the diet containing fat powder (p < .05; Table 3). On the contrary, LPL administration caused an increase in triglyceride levels (p < .05); however, control group revealed the highest triglyceride and cholesterol values. Total protein, glucose and albumin concentration were not significantly affected by LPL levels (p > .05).
| Diets | Control | LPL 0 | LPL 3 | LPL 6 | LPL 9 |
|---|---|---|---|---|---|
| Cholesterol (mg/dl) | 214.30 ± 12.94a | 175.85 ± 4.63b | 174.16 ± 3.41b | 170.02 ± 1.95b | 130.01 ± 4.98c |
| Triglyceride (mg/dl) | 359.04 ± 12.51a | 211.11 ± 18.85d | 274.30 ± 9.81c | 282.40 ± 2.32c | 322.67 ± 4.93b |
| Total protein (g/dl) | 5.44 ± 0.53 | 4.98 ± 0.12 | 5.30 ± 0.45 | 5.09 ± 0.93 | 5.54 ± 0.14 |
| Glucose (g/dl) | 50.81 ± 5.71 | 51.87 ± 2.76 | 49.86 ± 5.41 | 50.12 ± 4.19 | 50.60 ± 4.59 |
| Albumin (g/dl) | 4.37 ± 0.06 | 4.16 ± 0.17 | 4.27 ± 0.31 | 4.28 ± 0.24 | 4.17 ± 0.23 |
Note
- All values are means of three replicates (tanks)/treatment ± standard deviation; different superscript letters show significant differences (p < .05).
According to Table 4, fish fed control diet showed a greater body fat (p < .05). Inclusion of fat powder to the diet reduced body fat; however, body fat content increased by administration of 9 g kg−1 LPL to fat powder. In contrast, the lowest body protein achieved in fish fed control and LPL9 diets. Control fish owned higher ash content among treatments. Furthermore, body moisture did not affect by LPL addition (p > .05).
| Diets | Control | LPL 0 | LPL 3 | LPL 6 | LPL 9 |
|---|---|---|---|---|---|
| Fat (%) | 11.85 ± 0.61a | 8.51 ± 0.47cd | 8.12 ± 0.40d | 9.02 ± 0.42c | 9.97 ± 0.16b |
| Protein (%) | 14.73 ± 0.66c | 17.27 ± 0.44ab | 18.25 ± 1.12a | 16.81 ± 1.49ab | 15.82 ± 0.89bc |
| Ash (%) | 0.92 ± 0.08b | 1.35 ± 0.01a | 1.15 ± 0.05ab | 1.16 ± 0.24ab | 1.15 ± 0.07ab |
| Moisture (%) | 73.44 ± 0.85a | 73.55 ± 0.87a | 73.06 ± 1.61a | 73.34 ± 0.81a | 72.43±0.43a |
Note
- All values are means of three replicates (tanks)/treatment ± standard deviation; different superscript letters show significant differences (p < .05).
Fat digestibility was improved by supplementation of 9 g kg−1 LPL to fat powder diet (p < .05; Table 5). Contrarily, addition of fat powder reduced protein digestibility with or without LPL compared to control diet. Moreover, ash digestibility showed no significant differences among different diets (p > .05).
| Diets | Control | LPL 0 | LPL 3 | LPL 6 | LPL 9 |
|---|---|---|---|---|---|
| Fat (%) | 75.44 ± 1.96a | 60.01 ± 1.33c | 60.04 ± 2.32c | 61.90 ± 0.84c | 65.46 ± 0.93b |
| Protein (%) | 87.97 ± 1.22a | 85.37 ± 0.86b | 84.24 ± 0.82b | 85.70 ± 0.73b | 85.50 ± 1.23b |
| Ash (%) | 88.69 ± 0.76a | 87.44 ± 1.33a | 86.14 ± 2.73a | 88.13 ± 0.87a | 87.93 ± 1.36a |
Note
- All values are means of three replicates (tanks)/treatment ±standard deviation; different superscript letters show significant differences (p < .05).
Furthermore, no significant differences have been observed in viscosity of experimental diets (p > .05; Table 6), while midgut digesta viscosity significantly reduced in fish fed LPL 9 (p < .05).
| Control | LPL 0 | LPL 3 | LPL 6 | LPL 9 | |
|---|---|---|---|---|---|
| Dietary viscosity (cP) | 1.12 ± 0.00 | 1.12 ± 0.00 | 1.12 ± 0.00 | 1.12 ± 0.00 | 1.11 ± 0.01 |
| Digesta viscosity (cP) | 1.11 ± 0.00ab | 1.12 ± 0.01a | 1.12 ± 0.00a | 1.12 ± 0.00a | 1.10 ± 0.01b |
Note
- All values are means of three replicates (tanks)/treatment ± standard deviation; different superscript letters show significant differences (p < .05).
- Abbreviations: cP, centipoise.
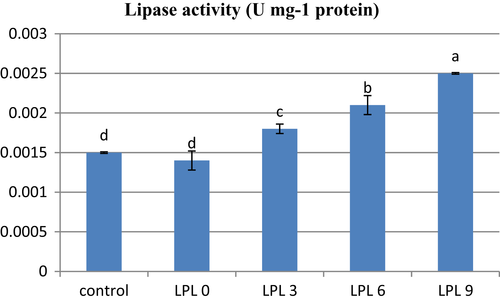
Lipase activity of rainbow trout fed different levels of LPL showed an increasing trend by addition of LPL to fat powder (p < .05; Figure 1). The lowest lipase activity was observed in fish fed control and 0 g kg−1 LPL diets (p < .05).
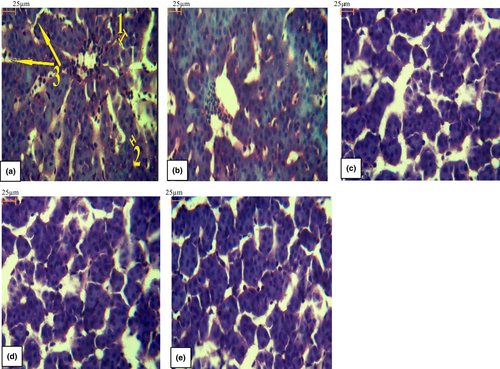
Liver histology of fish fed different diets displayed regular-shaped hepatocytes around sinusoidal spaces (Figure 2). But in some cases, in the liver of fish fed 0 g kg−1 LPL deformed sinusoids were observed. For instance, the sinusoidal space had become more voluminous and hepatocytes nuclei had become jammed tightly (Figure 2b). Also, histomorphometry of liver including hepatocytes size, hepatocytes nuclei size and sinusoids content in rainbow trout fed fat powder supplemented with different levels of LPL are represented in Table 7. LPL levels had no impact on hepatocytes size between different groups (p > .05). Fish oil replacement with fat powder led to an increased hepatocytes nucleus size, whereas this value decreased by addition of LPL levels (p < .05). Sinusoids content was the lowest in control group and increased significantly in fish fed fat powder (p < .05).
| Diets | Control | LPL 0 | LPL 3 | LPL 6 | LPL 9 |
|---|---|---|---|---|---|
| Hepatocytes size (µm) | 28.75 ± 0.61 | 28.29 ± 1.15 | 28.97 ± 0.53 | 29.08 ± 0.50 | 28.77 ± 0.70 |
| Hepatocytes nucleus size (µm) | 11.50 ± 0.21b | 13.18 ± 0.79a | 12.84 ± 0.58a | 12.55 ± 0.54ab | 12.40 ± 0.53ab |
| Sinusoids content (%) | 8.35 ± 0.98b | 15.68 ± 0.72a | 15.59 ± 1.04a | 15.67 ± 0.39a | 14.40 ± 1.07a |
Note
- All values are means of three replicates (tanks)/treatment ± standard deviation; different superscript letters show significant differences (p < .05).
4 DISCUSSION
In the current study, rainbow trout's final weight, WG and SGR were improved by inclusion of 9 g kg−1 LPL to a fat powder diet. LPL is a polar lipid and can emulsify fats and increase activity of lipase to digest lipid droplets thereby improving growth performance. Similarly, addition of 2 g kg−1 Lipidol to the diet showed the best growth and feed utilization in rainbow trout (Taghavizadeh et al., 2020). Li et al. (2019) reported that supplementation of lysolecithin to diet containing 65 g kg−1 fish oil led to a higher growth performance in juvenile turbot (Scophthalmus maximus L.) compared to positive control diet containing 75 g kg−1 fish oil, respectively. In the present study, fat powder used as main fat source in experimental diets compared to control diet (fish oil + canola oil) and also in different levels of substitution (96 g kg−1) which could justify our results. The similar results were obtained by the use of 0.125 g kg−1 and 0.250 g kg−1 lysolecithin in crucian carp (Carassais auratus gibelio) (HongXia Li, Liu, et al., 2010) and the use of 0.5 and 1 g kg−1 LPL in hybrid tilapia diet (Li, Tian, et al., 2010). Optimum level of LPL administration changes has been observed in different research depends on species or LPL type. Azarm et al. (2013) reported higher weight gain and specific growth rate in rainbow trout fry fed diet containing 20–40 g kg−1 chicken egg lecithin. In fish body, phospholipid turns into LPL by the action of enzyme. On the other hand, fish cannot naturally synthesis enough phospholipid and so need it through diet. So, inclusion of LPL in diet leads to energy sparing for synthesis action (Craig & Gatlin, 1997). Still, growth performance was lower in the optimum level of LPL (i.e. 9 g kg−1) than that of positive control diet. A lower feed intake induced by fat powder diets may be also reason for such a lower growth performance. Nevertheless, LPL supplementation at 6 and 9 g kg−1 increased feed intake and improved growth at 9 g kg−1 comparing LPL0.
Furthermore, highest values of HSI and VSI observed in fish fed fat powder but decreased by administration of LPL. HSI increasing may suggest accumulation of fat in liver probably by incomplete digestion of fat powder. Whereas, LPL affects lipid transportation from gut towards the tissues as similarly reported by Salhi et al. (1999) and Fontagne et al. (1998). LPL seems to be the main reason for reduction of lipid content in liver. Also, Kenari et al. (2010) suggested that high fat diet elevates HSI because of accumulation of extra lipid in liver. Furthermore, Babalola and Adebayo (2007) obtained similar result in Planet catfish (Heterobranchus longifilis). Fat powder is resistant against lipolysis because of having high saturated fatty acids, so it might leads to liver steatosis causing high HSI.
In the present study, cholesterol was lower in diet containing fat powder than that of positive control and also the lowest value achieved by adding 9 g LPL to the diet suggesting the removal of cholesterol induced by LPL. LPL probably stimulates liver to synthesis bile salts which ultimate to cholesterol decreasing by turning cholesterol to bile acids. On the contrary, triglyceride level increased by LPL addition to the diet containing fat powder and control diet. This could be related to higher digestion of lipids in those diets. Chylomicrons are secreted by the intestine to transport triglyceride. LPL emulsifies fat and induces absorption of chylomicrons containing triglyceride. Similarly, in the study of HongXia Li, Liu, et al. (2010) and Li et al. (2019) LPL led to decrease cholesterol level in crucian carp and turbot, respectively; However, triglyceride value decreased by inclusion of LPL in turbot. Li, Tian, et al. (2010) reported that the levels of blood triglyceride were not significantly affected by the supplementation of dietary lysolecithin but cholesterol value was higher in tilapia fed 0.125 g kg−1 comparing control or 0.250 g kg−1 lysolecithin. Contradiction in results might happen by utilization of different levels and types of LPL. Moreover, total protein, glucose and albumin as indicators of nutritional and health condition (Fazio et al., 2021) demonstrated no significant changes in fish fed experimental diets (p > .05). Similarly, addition of lysophospholipid did not affect glucose value in earlier study on crucian carp (HongXia Li, Liu, et al., 2010).
Lysophospholipid has greater emulsifier properties compared with phospholipids (Taghavizadeh et al., 2020). So, lipid transferring by synthesis of lipoprotein and moving oil droplet from liver to the tissue will be induced by LPL which led to increase the lipid content of fish body. It can explain increased body lipid in diet containing 6 and 9 g kg−1 LPL. However, control fish showed higher value of lipid among diets since digestibility of fish oil is high and extra fat will be stored in adipose tissue after using as energy source. Seiedzadeh et al. (2015) reported increasing body fat by utilizing 0.4 g kg−1 dietary egg lecithin in Mesopotamichthys sharpeyi species. Similar results have been observed in yellow drum Nibea albiflora and large yellow croaker Larimichthys croceus, indicating the whole-body lipid contents increased by LPL (Wang et al., 2016; Yi et al., 2014). Also, body protein decreased by addition of LPL in the present study. There is a balance between body protein and fat which seems to be preserved in each group by increasing body fat and decreasing body protein in the same fish. Adipocytes take fatty acids and amino acids of diet as substrate and convert them into triglycerides for storage. This could be the reason for adverse relation between protein and fat content of body.
Fish oil is suitable in case of having unsaturated fatty acids and high digestibility while saturated fat like residual products from oil extract shows poor digestibility. LPL supplementation was to increase emulsifying properties because of having two polar and nonpolar heads in their molecular structure. The results represent fat digestibility increasing by adding 9 g kg−1 LPL to the fat powder compared with other doses. Lipid molecules are insoluble in aqueous environment, while LPL as an amphipathic molecule helps stabilizing nonpolar lipids (Carey et al., 1983). LPL emulsifies fats and forms micelles, so more fat molecules will be in contact with lipase. Addition of 9 g kg−1 LPL led to increase fat digestibility but it was still lower than that of control diet containing fish oil. The slight positive impact of LPL on fat powder digestion might show that higher concentration of LPL is needed. In accordance with our results, HongXia Li, Liu, et al. (2010) demonstrated higher apparent digestibility coefficients of nutrients in experimental groups of crucian carp fed with 0.05 and 0.1 per cent LPL. Similar other studies showed enhancement of lipid apparent digestibility in broiler (Zampiga et al., 2016; Zhang et al., 2010).
Another explanation for digestion and absorption enhancement is because of the stimulation of pancreatic secretion. LPL improved lipase activity in the present study confirmed this theory. This finding was also in accordance with studies reported lipase activity increased by phospholipids (Chang et al., 1999; Kenari et al., 2010). It seems that fish are not capable of digesting fat without the supplementation of emulsifier like LPL when fish oil replaced with fat powder. Digestive enzyme accessibility to the digesta will be negatively affected by increasing viscosity in digestive tract (Santos et al., 2004). So, digestion and/or absorption of nutrients interferes by effect of viscous solutions (Marquardt et al., 1979). Viscosity of digesta in the midgut decreased when 9 g kg−1 LPL was added to the diet which probably happened because of emulsification effect of LPL. It has also been reported by Pasquier et al. (1996) that high viscosity reduces lipolysis due to low emulsification. Lipase performance enhances when viscosity of digesta decreases and causes higher digestibility which we observed by addition of 9 g kg−1 LPL in this experiment.
Liver is an organ which is sensitive to nutritional condition in fish (Escaffre & Bergot, 1986). Liver sinusoids, look like empty places under microscope, are types of capillaries placed between liver hepatocytes which joining them directly to the blood cells. Fish oil replacement by fat powder developed irregular sinusoids content in form of big holes that could disturb blood supply. Hepatocytes size did not affect by different diets whereas, enlargement of hepatocyte nuclei distinguished in fish fed fat powder. It could be related to lipid accumulation induced by dysfunction of lipid metabolism and/or increasing glycogen storage. Neutral fats cause a reduction in the lipid transportation (Salhi et al., 1999) via synthesize of chylomicron containing saturated fatty acids leading to accumulation of lipid (Leger, 1985), as we also observed in this experiment which could ultimately resulted to injuries in hepatocytes. Lack of essential fatty acid may lead to increase in hepatocyte volume according to Watanabe et al. (1989). It is also confirmed that phosphatides have protective role against oxidation in lipid structure on the cell membranes preventing hepatic lipoidosis and liver disease (Řehulka & Minařík, 2003). Similarly, lipid accumulation has been reported in gilthead sea bream larvae fed diet containing low n-3 HUFA and/or polar lipid (Salhi et al., 1999). We also found hepatic nuclei size reduced using LPL in diet containing fat powder in this experiment. It might be because of LPL facilitated lipid transportation due to lipoprotein synthesis from liver to adipose tissue. This idea also supported by Fontagne et al. (1998) that secretion of chylomicrons or very low density lipoproteins (VLDL) enhanced by phosphatidylcholine so led to decrease the accumulation of liver lipid, liver weight and hepatocyte volume. Since phospholipid required for lipoprotein synthesize and fish are limited to produce phospholipid (Teshima et al., 1986), LPL addition to the diet can modulate the condition.
In total, fat powder is an accessible alternate for fish oil but decrease fat digestibility and cause damages to the liver. On the other hands, present research suggests that supplementation of 9 g kg−1 LPL led to digest fat powder. It also helps scavenging fatty acids from liver and deposition in adipose tissue as explains low liver weight and increasing body fat. It seems that LPL can reduce side effect of fat powder. However, fish oil, still, seems to be an ideal fat source in fish feed and only can be partially substituted by fat powder in presence of emulsifier like LPL. Inclusion of 9 g kg−1 LPL improves digestibility of fat powder but we do not have any information about further doses; so, larger supplementation of LPL is suggested for future studies since the most effective dose of LPL in this experiment was the higher one.
ACKNOWLEDGMENTS
This research was supported by the Iran National Science Foundation (INSF) (grant number: 98012490).




