National screening for colorectal cancer is associated with stage shift to earlier diagnosis
Abstract
Background
Australia's National Bowel Cancer Screening Program consists of an immunohistochemical faecal occult blood test, targeting adults aged 50–74. Existing literature supports the principle of early detection of colorectal cancer (CRC) via national screening, but little is known about the association between colonoscopy or polypectomy rates and CRC stage over time. The aim of this study is to identify the longitudinal change to colonoscopy and polypectomy rates, and any stage shift associated with this screening program.
Methods
A retrospective data-linkage study was performed using the Australian national health database (Medicare) to obtain colonoscopy and polypectomy rates between 1998 and 2017. A second prospective database of CRC resection specimens was analysed for this period. The cohort was divided based on time intervals related to the National Bowel Cancer Screening Program: pre-commencement 1998–2006 (Period A), immediately post-commencement 2007–2011 (Period B), and subsequent years 2012–2017 (Period C). Linear regression was used to test relation between annualized predictor and response variables.
Results
Annual colonoscopy rates doubled, and polypectomy rates tripled during the study (P < 0.001). Annual colonoscopy rate correlated to a lower T-stage (P = 0.038) and lower N-stage (P = 0.026), and there was a 7% increase in early CRC (stage I–II) in Period C (P < 0.001). Across the study period there was also a significant increase in right-sided tumours, and concurrent MMR deficiency and BRAF mutation.
Conclusion
Polypectomy and colonoscopy rates increased after the introduction of the National Bowel Cancer screening program. There was a clinically significant shift to earlier CRC stage which manifested 5 years after its implementation.
Introduction
Colorectal cancer (CRC) is prevalent world-wide and the global burden is expected to continue to increase due to an ageing population and rise in Western lifestyle and behaviours. It ranks as the third most common cancer globally, with the highest incidence seen in North America, Europe, Australia and New Zealand.1, 2 In Australia, it is responsible for 11% of cancer deaths annually.3
Stage at diagnosis is the single most important prognostic factor, which over the last two decades has stimulated the focus on screening tests to reduce the risk of CRC.4-6 Stage shift is a modelling concept whereby screening causes the time of cancer diagnosis to be expedited so that the stage is shifted to a lower category. This provides a pragmatic mortality benefit but also a reduced need for adjuvant chemotherapy.7
To facilitate early detection and removal of precursor colonic lesions in Australia, the National Bowel Cancer Screening Program was introduced in 2006, targeting asymptomatic adults aged 50–74. Two stool samples undergo immunohistochemical faecal occult blood testing which is performed in the patient's home, and a positive result is an indication to proceed with a free-of-charge colonoscopy. Variations of this screening program exist in other Western countries, for example in America the screening age is 45–75 years old, and testing can be through direct endoscopic visualization of the colon without the need for prior stool assessment.8 If colonoscopy screening is effective, one would expect a shift in the stage-specific incidence of CRC in the years that followed commencement of that respective screening program. Existing Australian and European literature supports this principle of bowel cancer screening but has not investigated the association between changes in colonoscopy or polypectomy rates and CRC stage over time.8-10 The aim of this study is to identify the longitudinal change to colonoscopy and polypectomy rates, and any stage shift in association with Australia's National Bowel Cancer Screening program.
Methods
Study design
A retrospective data-linkage study was performed using Australia's national healthcare database (Medicare) to obtain records of colonoscopy and polypectomy rates (based on item numbers for all screening colonoscopies, or where a polypectomy was performed) in the state of New South Wales from 1998 to 2017. This included all proceduralists whether physician or surgeon. A second database (Department of Anatomical Pathology Royal North Shore Hospital Sydney) of prospectively collected surgical pathology of CRC resections with curative intent in the Northern Sydney Health district was used to determine CRC stage during the corresponding years. The database is estimated to represent the majority of all CRC histopathology specimens which serviced the single tertiary public hospital and also the largest private hospital in this health district. Pathology reporting (synoptic since 2006) included location of tumours (left or right colon), size of primary tumour, differentiation grade, T-stage (depth of tumour invasion into colon wall), N-stage (number of involved lymph nodes), Lymph Node ratio (LNR, represents the number of involved lymph nodes out of the total number of lymph nodes resected), apical lymph node involvement, M-stage (presence of distant metastasis discovered during surgery), microsatellite instability (MSI) and BRAF status as well as baseline patient demographics. Cases were initially staged according to the version of the American Joint Committee on Cancer classification (AJCC) that was current at the time of reporting, but all malignancies were retrospectively reviewed and restaged according to the current eighth edition of the AJCC staging system. A surgical oncology multidisciplinary meeting was responsible for coordinating patient management, with stable panel members throughout the study period. Lastly, New South Wales CRC incidence data from 1998 to 2017 were obtained from the Cancer Institute NSW website.11 These were independent and retrospectively reviewed.
Outcomes
To compare the longitudinal trend of colonoscopy (total and those with polypectomy) and polypectomy ratio (colonoscopies with polypectomy divided by total number of colonoscopies) on CRC stage (where early stage is defined as AJCC I and II), the total cohort was divided into three groups based on time intervals related to the national bowel cancer screening program: before commencement years 1998–2006 (Period A), immediately after commencement years 2007–2011 (Period B), and then subsequent years 2012–2017 (Period C).
Statistical methods
Statistical analysis to correlate longitudinal data trends was done with SPSS version 24 (IBM, USA). Comparison of means and standard deviation (SD) between Periods was performed using student t-test, for comparison of means (SD) across all three Periods ANOVA was used, and categorical variables were analysed with Chi square test. Simple linear regression (R2, F (df regression, df residual)) was used to test relation between annualized predictor and response variables. The significance value was P < 0.05.
Ethics considerations
The research protocol obtained ethics approval from the local health district as low/negligible risk.
Results
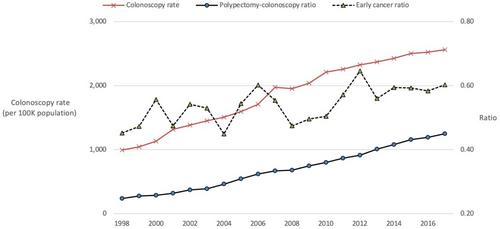
Over 8.1 million colonoscopies were performed in Australia between 1998 and 2017. In the state of New South Wales there were approximately 2.6 million which are summarized by Period in Table 1; the annual colonoscopy rate per 100 000 population was 1348 in Period A, 2086 in Period B, and 2451 in Period C. The annual polypectomy rate per 100 000 was 380 in Period A, 733 in Period B, and 1028 in Period C. Colonoscopic polypectomy and colonoscopy rates, as well as colonoscopy to polypectomy ratio (0.28 in Period A, 0.35 in Period B and 0.42 in Period C) significantly increased (all P < 0.001). The annual trend is illustrated in Figure 1. Overall incidence of CRC per 100 000 population was similar in Period A and Period B, but was significantly lower in Period C (P < 0.001).
| Period A | Period B | Period C | |||
|---|---|---|---|---|---|
| P-value (A versus B) | P-value (B versus C) | ||||
| Mean annual colonoscopy rate (SD) | 1348 (249) | <0.001 | 2086 (139) | 0.001 | 2451 (93) |
| Mean polypectomy-to-colonoscopy ratio (SD) | 0.28 (0.03) | <0.001 | 0.35 (0.02) | 0.001 | 0.42 (0.03) |
| Mean CRC incidence (SD) | 63.9 (1.5) | ns | 63.8 (1.9) | 0.002 | 58.9 (1.8) |
- Abbreviation: ns, non-significant.
From 1998 to 2017 inclusive, 4506 patients underwent colon cancer resections with curative intent – 1750 patients in Period A, 1166 in Period B, and 1590 in Period C. The mean age of the cohort was 71.7 years and 50.9% were male, which was similar between all Periods. Patient demographics, cancer stage, and tumour location are compared in Table 2. Periods A and B had similar percentage of patients diagnosed with early CRC (stages I and II), however Period C had a significant 7% increase. Stage I and IIa patient ratios (those definitely not requiring adjuvant chemotherapy) significantly increased from 0.44 to 0.45 to 0.55 in Periods A, B and C respectively (P = 0.003).
| Period A | Period B | Period C | |||
|---|---|---|---|---|---|
| P-value (A versus B) | P-value (B versus C) | ||||
| Number | 1750 | 1166 | 1590 | ||
| Mean age (SD) | 71.3 (12.2) | 71.3 (12.5) | 71.9 (13.2) | ||
| Males n (%) | 893 (51.0) | 594 (50.9) | 805 (50.6) | ||
| AJCC† Stage n (%) | ns | 0.001 | |||
| I | 308 (17.6) | 200 (17.2) | 327 (20.6) | ||
| II | 603 (34.5) | 407 (34.9) | 612 (38.5) | ||
| III | 758 (43.3) | 510 (43.7) | 573 (36.0) | ||
| IV | 81 (4.6) | 49 (4.2) | 78 (4.9) | ||
| Early cancer‡ n (%) | 911 (52.1) | ns | 607 (52.1) | 0.001 | 939 (59.1) |
| Tumour location (%) | |||||
| Right versus. left colon | 45.9 versus. 54.1 | ns | 47.9 versus. 52.1 | ns | 50.6 versus. 49.4 |
- Abbreviation: ns, non-significant.
- † American Joint Committee on Cancer Eight Edition.
- ‡ Early stage defined as AJCC Stage I and II.
Histopathology results of the cancer specimens are described in Table 3. CRC specific data (size, differentiation, location, AJCC eight edition stage, LNR, apical node, microsatellite instability and BRAF status) completeness was 97.9% in Period A, 99.1% in Period B and 99.7% in Period C. Annual colonoscopy rate correlated to a lower T-stage (P = 0.038) and lower N-stage (P = 0.026). Period A and B had similar mean tumour sizes of 43.0 mm and 43.9 mm respectively, but in Period C size was significantly smaller at 40.2 mm (P < 0.001). Period C also had a lower number of positive lymph nodes (P = 0.001), and less frequent positive apical lymph nodes (P = 0.004). Significantly more tumours in Period C where poorly differentiated (P = 0.004). Across the study Periods there was a significant increase in proportion of right sided tumours (P = 0.026), with concomitant increase in microsatellite instability/MMR deficiency (P = 0.001) and BRAF mutation (P = 0.001).
| Period A | Period B | Period C | |||
|---|---|---|---|---|---|
| P-value (A versus B) | P-value (B versus C) | ||||
| Mean tumour size (SD) | 43 mm (20.4) | ns | 43.9 mm (21.8) | <0.001 | 40.2 mm (20.0) |
| Poorly differentiated (%) | 20.2 | ns | 20.8 | 0.004 | 25.5 |
| T stage (%) | 0.005 | 0.07 | |||
| 1 | 5.4 | 7.9 | 7.9 | ||
| 2 | 17.1 | 14.4 | 17.0 | ||
| 3 | 53.0 | 50.6 | 51.8 | ||
| 4 | 24.5 | 27.1 | 23.3 | ||
| Mean LN resected (SD) | 16.0 (8.8) | <0.001 | 18.4 (10.9) | <0.001 | 17.0 (7.8) |
| Mean LN positive (SD) | 1.8 (3.6) | ns | 1.9 (3.5) | <0.001 | 1.3 (2.9) |
| Mean LN ratio (SD) | 0.12 (0.21) | ns | 0.11 (0.19) | <0.001 | 0.08 (0.16) |
| Apical LN positive (%) | 9.0 | ns | 7.9 | 0.045 | 5.9 |
| MMR deficient (%) | 16.4 | 0.03 | 19.5 | 0.009 | 23.7 |
| BRAF gene mutation (%) | 21.5 | ns | 20.1 | 0.001 | 23.7 |
- Abbreviations: LN, lymph nodes; MMR, mismatch repair; ns, non-significant.
All of the above findings remained significant in subgroup analysis of patients with lymph node resection of more than 11 (nodes >11 examined in Periods A, B and C: 68%, 78.5%, and 81.2%, respectively).
Simple linear regression (Tables 4 and 5) shows a significant and strong correlation between an increase in early stage CRC over the study period, and total number of colonoscopies, colonoscopies with polypectomy and particularly with the polypectomy ratio. Figure 1 visualizes these significant correlations over the years. Similarly, a significant reduction in CRC incidence showed a significant and strong correlation with total number of colonoscopies, polypectomy ratio and particularly colonoscopies with polypectomy.
| R2 | F (df 1, df 18) | P-value | |
|---|---|---|---|
| Effector variable | |||
| Total number colonoscopies NSW | 0.417 | 12.86 | 0.002 |
| Colonoscopies with polypectomy NSW | 0.427 | 13.43 | 0.002 |
| Polypectomy ratio | 0.438 | 14.1 | 0.001 |
| R2 | F (df 1, df 18) | P-value | |
|---|---|---|---|
| Effector variable | |||
| Total number colonoscopies NSW | 0.466 | 15.72 | 0.001 |
| Colonoscopies with polypectomy NSW | 0.541 | 21.24 | <0.001 |
| Polypectomy ratio | 0.516 | 19.18 | <0.001 |
Discussion
The prevailing pathophysiological understanding of CRC, known as the adenoma to carcinoma sequence, postulates that it arises from the progressive accumulation of genetic mutations within benign precursor adenomatous polyps.12 Indirect evidence and observational studies support the use of faecal occult testing, and colonoscopic polypectomy to reduce CRC incidence. Although no randomized trials exist, current practice dictates the removal of adenomas and sessile serrated polyps, as they harbour the greatest malignant potential.13, 14 Our longitudinal study has demonstrated an increasing rate of colonoscopies and polypectomies which occurred in parallel with the maturation of the national screening program, and an associated shift towards earlier CRC stages. Although the participation rate of the screening program has only incremented to 40% of the target population, the increasing annual numbers of colonoscopies and polypectomy-to-colonoscopy ratios correlated with an increased ratio of early (stage I and II) cancers and reduced overall incidence of CRC.
We found that the significant increase in colonoscopy and polypectomy rates in each period was associated with more frequent detection of smaller tumours and right sided tumours. Larger tumours are well known to negatively impact survival.15 Traditional teaching is that cancers of the right colon are identified at a more advanced stage due to an absence of red flag symptoms such as rectal bleeding or altered bowel habit.16, 17 At colonoscopy polyp precursors of right sided CRC are often more difficult to appreciate as they frequently have a flat morphology in the early stages of carcinogenesis rather than classic polypoid lesions of the sigmoid colon or rectum.18 Right sided tumours have a shorter time to recurrence and lower overall survival rates compared to left sided tumours.16, 19 It is also not surprising that our results show an increased detection of CRC with BRAF mutation, which is strongly associated with MMR deficiency, location in the proximal colon, female gender, increased peritoneal recurrence rate, and characteristically poorer overall survival.20 Earlier detection of right sided, smaller cancers with genetic mutations via increasing screening colonoscopies would therefore be expected to contribute to remarkable prognostic improvement in the long-term.
There are two noteworthy benefits of stage shift in CRC. The first is a reduction in CRC mortality through CRC detection at an earlier stage. Cancer specific mortality was not available in our study but stage shift alone has been used as a proxy for reduced mortality in the literature.8 We propose our findings are excellent markers to support improved mortality rate due to stage shift: less frequent involvement of any lymph nodes, higher rate of negative apical nodes, and lower LNR. Lymph node resection is a critical step in the treatment of CRC, and a well-established staging tool.21-23 Furthermore, LNR is an important prognostic factor24 and the apical node intermittently incites interest as an additional prognostic marker.25-27
The second benefit of stage shift is the potential to reduce the requirement for adjuvant chemotherapy after potentially curative resections. Most CRC multi-disciplinary teams would recommend adjuvant treatment in fit patients with later stage CRC: typically stage III and some high-risk stage II identified by risk factors such as lymphovascular or perineural invasion, inadequate lymph node resection (i.e., 11 or less), T4 depth invasion, or positive margins.28, 29 Currently 29%–60% of high-risk stage II patients receive adjuvant chemotherapy. Some have argued that this may be over-treatment and it is specifically in younger patients where a convincing shift to Stages I and IIa (definitely not requiring adjuvant chemotherapy) as shown in our study would confer long-term benefits.29, 30 Mainly because almost 90% of patients undergoing chemotherapy experience at least one side-effect and more than one in four experience severe complications requiring hospital admission.31 The financial burden on health care systems of chemotherapy costs, nursing care and unplanned admissions to manage complications would be alleviated and therefore doubly advantageous from a public health financial perspective.32, 33 The shift to earlier CRC stages established in our study resulted in fewer patients potentially needing adjuvant chemotherapy, which is a major, and underreported, benefit achievable through increased uptake of colonoscopy and CRC screening programs.
As a public health measure, our findings support the use of a screening colonoscopy program which after 5 years of implementation, was associated with increased detection of early stage cancer by a 7%. A recent study similarly reported significantly reduced risk of CRC within 10 years of population-wide screening colonoscopy by 18% in patients aged over 55.34 Furthermore, there is evidence of a detrimental effect of not screening; modelling based on the delays caused by the SARS-COV-2 pandemic showed that colonoscopy screening delays beyond 7 months significantly increase advanced (stages III–IV) CRC by 3%–7%, and delays beyond 12 months increase mortality rate by 12%.35 In contrast to this, recent studies have challenged the presumed benefits of colonoscopy screening programs as perhaps being overly positive. Bretthauer et al. report screening colonoscopy in their European cohort reduced the risk of CRC compared to usual care to a lesser extent than previously reported and without any change to overall mortality.10 Our study provides evidence that stage shifting leading to diminished use of adjuvant chemotherapy is an important factor to take into consideration.
It is important to acknowledge that a national screening program which increases colonoscopy usage is not without its risks. Beyond the rare colonoscopy complications of major bleeding or perforation – less than 1/1000 and 1/3000 respectively – there is a moderate level of short-term psychological distress to patients especially just before and after colonoscopy, or if an initial faecal occult test produces a false-positive result.36, 37 Appropriate counselling prior to undertaking screening, and involving a patient's primary care physician are essential steps to ensure the potential harm is outweighed by the benefits of early CRC detection.
A strength of this study is the reliability of the analysed data, which was collected from separate and independent databases, providing significance and meaning to the correlations found between these databases. An important future direction to build upon our findings would be Australian analysis of screening cost-effectiveness and burden on healthcare infrastructure, particularly if screening age may be lowered to 45 years.33 A limitation was encountered as precise colonoscopy data, repeat colonoscopies, nor demographics were available for our specific Northern Sydney Health District, but due to the historically high correlation with state colonoscopy and polypectomy rates, these numbers were felt to be an accurate representation. Another limitation to this study is the difficulty in measuring the effect of public health awareness campaigns or proceduralist learning curves as a possible confounding variables during the study period. Government initiatives not only increase the uptake of a bowel cancer screening program, but also raise overall awareness of symptoms and signs which may prompt patients to present earlier and hence the increased use of diagnostic colonoscopy.38 While this may be difficult to quantify in any analysis, it is an additional benefit to a well-orchestrated screening program. A final limitation to our research is that the novel correlation identified cannot conclusively be said to be the single causative factor. As our research only examined data of histopathological resections, the possibility of a temporal increase in both early and late/non-operative CRC cannot be completely ruled out, albeit very unlikely in the context of an overall reduced CRC incidence.
Conclusion
The introduction of Australia's National Bowel Cancer Screening program was associated with increasing rates of colonoscopy and polypectomy. There was a clinically significant shift to earlier CRC stage which manifested 5 years after its implementation.
Author contributions
Mina Sarofim: Data curation; formal analysis; investigation; writing – original draft; writing – review and editing. Amir Ashrafizadeh: Data curation; formal analysis; validation; writing – review and editing. Anthony J. Gill: Data curation; formal analysis; project administration; writing – review and editing. Keshani de Silva: Data curation; methodology; writing – review and editing. Justin Evans: Data curation; investigation; project administration; writing – review and editing. Stephen Clarke: Data curation; methodology; supervision; writing – review and editing. Nick Pavlakis: Data curation; supervision; writing – review and editing. Ian Norton: Formal analysis; investigation; writing – review and editing. Alexander Engel: Conceptualization; data curation; formal analysis; supervision; writing – review and editing.
Acknowledgement
Open access publishing facilitated by The University of Sydney, as part of the Wiley - The University of Sydney agreement via the Council of Australian University Librarians.
Conflict of interest
None declared.