Usefulness of Morphology-Voltage-P-wave duration (MVP) score as a predictor of atrial fibrillation recurrence after pulmonary vein isolation
Abstract
Background
Atrial fibrillation (AF) is known to be the most common arrhythmia, and the successful rate of long-term ablation can vary comparatively. Therefore, a clinical scoring system to predict rhythm outcome remains a critical unmet need. The electrocardiographic (ECG) risk score which is named Morphology-Voltage-P-wave duration (MVP) score was reported to be useful for predicting new-onset AF. The goal of the current study was to investigate whether the MVP score was a useful scheme in the prediction of rhythm outcome following pulmonary vein isolation (PVI) in paroxysmal atrial fibrillation (PAF).
Methods
We retrospectively analyzed baseline characteristics, risk scores, and rates of AF recurrence 12 months postablation in the medical records of 207 consecutive patients with PAF undergoing PVI in General Hospital of Ningxia medical University from 2010 to 2018.
Results
Two hundred and seven patients (71 females, median age 58.7 years) with symptomatic PAF underwent PVI. From the cohort, 32.3% (67) had a recurrence of AF within 1 year of the PVI. The area of the MVP score under the curve in the receiver operating characteristics (ROC) analysis was 0.789 (95% CI 0.730–0.840, p < .001). A score cut-off value of >3 showed the best predictive ability for AF recurrence within 1 year after PVI, with sensitivity (53.03%) and specificity (89.87%).
Conclusions
The results of our study suggest that the easy-to-measure ECG MVP score can be used to predict recurrence of PAF after PVI.
1 INTRODUCTION
Atrial fibrillation (AF) is known to be the most common cardiac arrhythmia, and the healthcare costs have been progressively increased in the worldwide. (Chugh et al., 2014) The economic burden of AF may grow continually because of hospitalizations, stroke, and loss of productivity. (Kim et al., 2011) And after the PAF is diagnosed, most of it cannot be curable and can develop into chronic AF (CAF) as the end result. (Kato et al., 2004) AF can be managed with pharmacotherapy, but percutaneous radiofrequency ablation is used to symptomatic patients who wish to avoid, have not responded to, or cannot tolerate medication. Despite its benefits, radiofrequency ablation is still an invasive and expensive procedure, and the recurrence rate occurs is up to 50% in 1 year. (Bhatti, Oakeshott, Dhinoja, & Grapsa, 2019) The ability to predict postablation recurrence would have implications on patient outcomes and healthcare cost at meantime.
There are several risk scores have been developed for predicting recurrence. Based on the previous study, most of them included mixed patients about paroxysmal and persistent AF, and the number of PVI carried out for PAF has risen. Therefore, a clinical scoring system to predict arrhythmia recurrence of PAF remains a critical unfulfilled need. Existing risk scores for AF recurrence after ablation include some clinical variables such as larger left atrial (LA) size, older age of patients at the time of ablation, and female sex (Bhargava et al., 2009; Winkle, Mead, Engel, & Patrawala, 2011). Nowadays, we found some P-wave variables which reflect the process of electrical and structural remodeling are useful predictors of arrhythmia recurrence after ablation procedure for AF. The ECG risk MVP score including some P-wave variables was reported to be useful for predicting new-onset AF. (Alexander et al., 2019) We retrospectively evaluate the score's ability to predict arrhythmia recurrence after PVI procedure for PAF.
2 METHODS
2.1 Patient population
Utilizing data from General Hospital of Ningxia medical University, the participants were consecutive refractory symptomatic PAF patients. These patients who were retrospectively enrolled presented to our institution for PVI from January 2010 to December 2018. PAF was defined according to current guidelines. (JCS Joint Working Group 2014) Paroxysmal AF was defined as self-terminating within 7 days after onset documented by previous routine electrocardiograms (ECG) or Holter ECG. Exclusion criteria included the following basis: (a) had a history of previous catheter ablation, cardiac surgery, serious valvular heart disease, or cardiac pacemakers; (b) with low-quality ECG, or without a recorded sinus ECG or echocardiogram before the procedure; (c) were not followed up with a 12-lead ECG or 24-hr Holter recordings at the1st, 3rd, 6th, and 12th month after the ablation procedure; and (d) those patients who did catheter ablation other than radiofrequency ablation such as surgical ablation, cryoballoon. In all, 207 consecutive patients with documented symptomatic were identified and included in the study. All patients underwent transesophageal and transthoracic echocardiography images prior to the procedure for excluding the patients who have intra-atrial thrombus or structural heart disease.
Baseline demographics, medical history, and laboratory data (including preablation age, gender, diabetes mellitus [DM], coronary artery disease [CAD], hypertension, obstructive sleep apnea [OSA], previous stroke or transient ischemic attack [TIA], drug treatment, left atrial [LA] anteroposterior diameter, left ventricular ejection fraction [LVEF], P-wave duration [PWD], interatrial block [IAB], and P-wave amplitude) were all extracted from the hospital electronic medical records. The CHADS2 (Gage et al., 2001), CAAP-AF (Winkle et al., 2016), and MVP scores were calculated for each patient.
2.2 MVP and CAAP-AF score assignment
In Table 1, the MVP ECG risk score was calculated in each patient being assigning 0–2 point for each of the following factors: PWD, IAB, and P-wave amplitude. The CAAP-AF score ranges from 0 to 13 points using baseline characteristics by the CAD, LA size, age, persistent AF, the number of antiarrhythmic drugs failed, and female gender.
| CAAP-AF | Clinical parameter | Score | MVP | Variable | Value | Score |
|---|---|---|---|---|---|---|
| C | Coronary artery disease | 1 | M | Morphology in inferior leads | Nonbiphasic (<120 ms) | 0 |
| Nonbiphasic (≥120 ms) | 1 | |||||
| Biphasic | 2 | |||||
| A | Left atrial diameter (cm) | V | Voltage in lead I | >0.20 mV | 0 | |
| <4 | 0 | 0.10–0.20 mV | 1 | |||
| 4 to < 4.5 | 1 | |||||
| 4.5 to < 5.0 | 2 | <0.10 mV | 2 | |||
| 5.0 to < 5.5 | 3 | |||||
| ≥5.5 | 4 | |||||
| A | Age (years) | P | P-wave duration | <120 ms | 0 | |
| <50 | 0 | |||||
| 50 to < 60 | 1 | 120–140 ms | 1 | |||
| 60 to < 70 | 2 | |||||
| ≥70 | 3 | >140 ms | 2 | |||
| P | Persistent or long-standing AF | 2 | ||||
| A | Antiarrhythmics failed | |||||
| 0 | 0 | |||||
| 1 or 2 | 1 | |||||
| ≥2 | 2 | |||||
| F | Female gender | 1 |
2.3 ECG measurement
A standard 12-lead ECG (25 mm/s, 10 mm/mV) was obtained before the ablation procedure. These images were amplified × 10 and measured using software Image J. Each ECG was reviewed blindly by two electrophysiologists. The P-wave onset was identified as the point of initial upward or downward deflection from the baseline, and the offset was defined as the returning point of the waveform to the isoelectric line. P waves will be analyzed after we found the isoelectric interval exists and the P waves had no fusion with the preceding QRS complex or T wave (Figure 1): PWD was measured in leads II, III, and aVF of the 12 surface leads. The wave's peak or nadir to the isoelectric line was measured as the amplitude of the P wave in the lead I. Each ECG lead was measured with at least five consecutive beats as an average. PWD ≥ 120 ms was considered as prolongation. Partial IAB was defined PWD ≥ 120 ms. Advanced IAB was defined as both prolongation and a biphasic(±) morphology in the inferior leads.
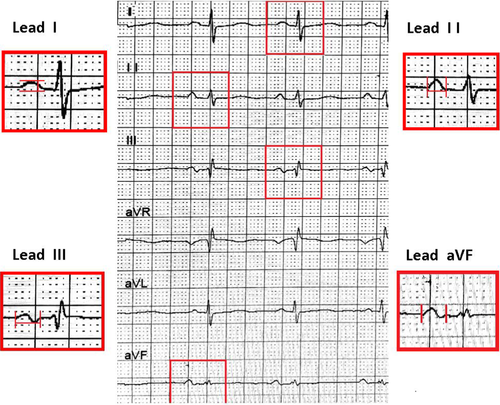
2.4 Radiofrequency ablation procedures and follow-up
In all patients, catheter ablation was performed following our standard approach in institutional ablation protocol. Written informed consent was obtained in all patients after discussing the risks and benefits of the radiofrequency ablation procedure. In all cases, the PVI was done using irrigated tip catheter guided by circular mapping catheter and the 3D mapping system, and the energy was delivered with a target temperature of 45°C and a maximal power limit of 40 W. The procedural endpoint was complete absence of all PV spikes during sinus rhythm documented with the two Lasso catheters within the ipsilateral PVs at least 30 min after PV isolation. Linear ablation of the cavotricuspid and/or mitral isthmus was underwent in patients who had right or LA isthmus flutter.
Patients included in this study were followed up with a 12-lead ECG and 24-hr Holter recordings at the 1st, 3rd, 6th, and 12th month after the ablation procedure, and the records were extracted from the hospital electronic medical records. If the patient was symptomatic, a new ECG or 24-hr Holter recording was obtained. After the procedure, all patients received antiarrhythmic drugs if there were neither contraindications nor intolerance. If no recurrent atrial tachyarrhythmia occurred after 2 or 3 months, the drug treatment was discontinued. AF recurrence was defined as documented symptomatic or asymptomatic any atrial arrhythmia lasting more than 30s between 3 and 12 months after ablation.
2.5 Statistical analysis
Data were collected in EpiData (version 3.1 for Windows) and then were imported into IBM SPSS (version 26.0 for Windows) and MedCalc (version 15.2.2 for Windows) for statistical analysis. Data are reported as the means ± standard deviation (SD) for continuous variables and as number of patients and proportions for categorical data. The Kolmogorov–Smirnov test was used to test the distribution of continuous variables. When compared patients with AF recurrences and those who remain in sinus rhythm regarding baseline clinical characteristics, treatment, and outcome, we used independent t test and Mann-Whitney U for continuous variables and chi-square tests (Pearson or Fisher's exact as appropriate) for categorical variables.
Multivariate logistic regression analysis was used to test the independent association of CHADS2, MVP, and CAAP-AF scoring systems with PVI outcome. ROC curves were generated for graphical illustration of these scores’ performance in predicting rhythm outcome, with the area under the curve (AUC) being equivalent to the c index for determining the predictive value for a score. The c indices (i.e., areas under the ROC curves) for the 2 scores were compared by using DeLong's method. Kaplan–Meier analysis was conducted to determine the difference in AF recurrences and those who remain in sinus rhythm within different score categories, with survival curves generated to illustrate survival time between groups. A two-sided p < .05 was considered to be statistically significant.
3 RESULTS
3.1 Population demographics and electrocardiogram characteristics
Our retrospective analysis included 207 patients with clinical PAF underwent PVI totally. Table 2 includes the demographics and clinical characteristics of the patients who developed AF recurrence and those who remain in sinus rhythm. Of these patients, 67 (32.3%) developed recurrent AF over the course of the one-year follow-up period (Table 2). There was a significant difference between left atrial size (36.9 ± 5.1 versus 39.0 ± 5.0; p = .009) and AAD failed (61.5 versus 38.5; p = .030) in patients who had recurrence of AF relative to those without recurrence. The prevalence of cardiovascular risk factors such as diabetes, CAD, hypertension, and OSA did not differ between 2 groups. The ECG characteristics of patients with and without recurrence are shown in Table 2. The patients with recurrence had longer P-wave durations (108.8 ± 14.4 versus 104.3 ± 11.0; p < .001) and higher prevalence of advanced IAB (p < .001; Table 2). Patients with recurrent AF also had a higher baseline of CAAP-AF and MVP score. We found that, in general, the higher the CAAP-AF and MVP score, the higher the percentage of patients who had recurrence of AF at 1 year. AF recurrence rates progressively increased with parallel increasing in MVP and CAAP-AF scores. The CHADS2 score was not significantly different between the 2 groups.
| Characteristic | Overall (207) | AF Free (141) | AF Recurrence (66) | p Value |
|---|---|---|---|---|
| Age (years) | 58.7 ± 10.9 | 57.8 ± 11.0 | 60.4 ± 10.4 | .086 |
| Female | 71 (34.3%) | 48 (67.6%) | 23 (32.4%) | .909 |
| CAD | 36 (17.4%) | 23 (63.9%) | 13 (36.1%) | .549 |
| Hypertension | 105 (51.0%) | 68 (64.8%) | 37 (35.2%) | .246 |
| Diabetes mellitus | 50 (24.3%) | 33 (66%) | 17 (34%) | .669 |
| OSA | 10 (4.9%) | 7 (70%) | 3 (30%) | .915 |
| COPD | 7 (3.4%) | 6 (85.7%) | 1 (14.3%) | .310 |
| Stroke/TIA | 24 (11.8%) | 17 (70.8%) | 7 (29.2%) | .763 |
| EF, % | 66.4 ± 6.8 | 66.6 ± 6.9 | 65.7 ± 6.5 | .262 |
| Left atrial size (mm) | 37.6 ± 5.1 | 36.9 ± 5.1 | 39.0 ± 5.0 | .009 |
| BMI | 25.3 ± 4.4 | 25.1 ± 3.2 | 25.9 ± 6.3 | .910 |
| AAD failed | 109 (52.7%) | 67 (61.5%) | 42 (38.5%) | .030 |
| None | 98 (47.3%) | 74 (75.5%) | 24 (24.5%) | |
| 1 or 2 | 86 (41.5%) | 53 (61.6%) | 33 (38.3%) | |
| P-wave duration | 108.9 ± 13.9 | 108.8 ± 14.4 | 104.3 ± 11.0 | <.001 |
| <120 ms | 165 (79.7%) | 133 (80.6%) | 32 (19.4%) | |
| 120–140 ms | 35 (16.9%) | 8 (17.1%) | 29 (82.9%) | |
| >140 ms | 5 (2.4%) | 2 (40%) | 3 (60%) | |
| P-wave voltage | 0.12 ± 0.04 | 0.12 ± 0.03 | 0.13 ± 0.04 | .591 |
| >0.20 mV | 3 (1.4%) | 1 (33.3%) | 2 (66.7%) | |
| 0.10–0.20 mV | 157 (75.8%) | 113 (72.0%) | 44 (28.0%) | |
| <0.10 mV | 47 (22.7%) | 27 (57.4%) | 20 (42.5%) | |
| P-wave morphology | ||||
| No interatrial block | 104 (50.3%) | 83 (79.8%) | 21 (20.2%) | <.001 |
| Partial interatrial block | 76 (36.7%) | 53 (69.7%) | 23 (30.3%) | |
| Advanced interatrial block | 27 (13.0%) | 5 (18.5%) | 22 (81.5%) | |
| CHADS2 score | 1.03 ± 1.05 | 1.09 ± 1.10 | 1.09 ± 0.94 | .295 |
| CAAP-AF Score | 3.07 ± 1.67 | 2.84 ± 1.60 | 3.56 ± 1.72 | .010 |
| MVP score | 2.51 ± 1.27 | 2.09 ± 1.08 | 3.4 ± 1.15 | .001 |
- Abbreviations: AAD, antiarrhythmic drug; AF, atrial fibrillation; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; OSA, obstructive sleep apnea; TIA, transient ischemic attack.
3.2 Risk score for atrial fibrillation
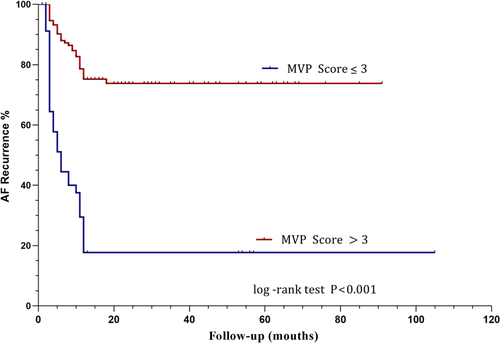
Using the multivariate logistic regression analyses all three scores in Table 3, the MVP was significant predictors of AF recurrences (OR 2.749 95%CI 1.965–3.847 p = .000). The area of the MVP score under the curve in the ROC analysis was 0.789 (95% CI 0.730–0.840, p < .001) for all patients included in the study (Figure 2). The MVP score was a predictive factor of AF recurrence after PVI with a cut-off value of >3 (sensitivity = 53.03%, specificity = 89.87%) (Table 4). In view of the ROC curves based on the MVP, and CAAP-AF scores in predicting events, the differences between the areas under the curves reached statistical significance. MVP score is better than CAAP-AF and Chads2 in predicting the recurrence of AF. Kaplan–Meier curves show recurrence of atrial fibrillation in patients with different MVP score (Figure 3). Patients with scores above 3 had a higher rate of recurrence of atrial tachyarrhythmia (p < .001 by log-rank test).
| Score | p Value | OR | 95% CI |
|---|---|---|---|
| MVP score | .000 | 2.749 | 1.965–3.847 |
| CAAP-AF score | .086 | 1.225 | 0.971–1.544 |
| CHADS2 score | .581 | 0.905 | 0.635–1.290 |
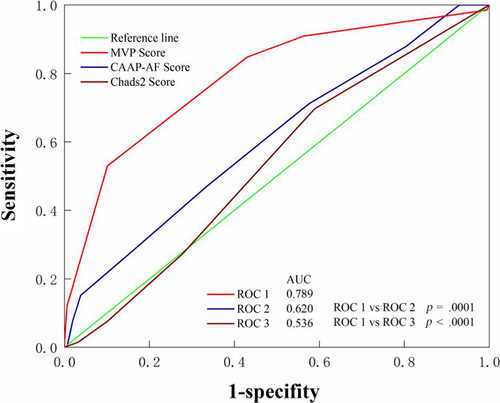
| Score | AUC | Cutoff value | Sensitivity | Specificity | p Value |
|---|---|---|---|---|---|
| MVP score | 0.789 | >3 | 53.03% | 89.87% | <.0001 |
| CAAP-AF score | 0.620 | >3 | 47.76% | 67.88% | .0026 |
| CHADS2 score | 0.536 | >0 | 68.66% | 42.42 | .3496 |
4 DISCUSSION
To the best of our knowledge, this is the first study demonstrating the predictive value of a surface ECG scoring system for predicting recurrence of PAF after PVI. Our major finding was that the MVP score was able to predict arrhythmia recurrence after the PVI.
Atrial fibrillation is known to be the most common cardiac arrhythmia, and the recurrence occurs is up to 50% in 1 year after ablation procedure. In previous studies, there were several risk scores which combined clinical parameters including heart failure, hypertension, advanced age, diabetes mellitus, and so on have been developed for predicting recurrence, such as APPLE (Kornej et al., 2015) and CAAP-AF (Winkle et al., 2016) score. These clinical variables could not evaluate the atrial function properly.
In contrast to other scores, our study was the first to evaluate the predictive value of the ECG score which is composed only of easy-to-measure ECG variables in recurrence of AF. It is well known that the P wave is an ECG marker reflecting the process of atrial depolarization conduction. As many studies showing, PWD has been established as a sign for the electrical conduction of the atria. Prolonged PWD in sinus rhythm before ablation may be associated with atrial substrate remodeling (Redfearn, Lane, Ward, & Stafford, 2006) and left atrial scarring (Chen et al., 2019), and it was dependently associated with higher recurrence rates of AF after PVI (Caldwell et al., 2014; Mugnai et al., 2016; Pranata, Yonas, & Vania, 2019). In addition to PWD, Park et al. (Park et al., 2016) found low P-wave amplitude in lead I, which reflects the low LA voltage, slow intra-LA conduction velocity, and displaced interatrial conduction pattern, is also associated with clinical recurrence after PVI in patients with PAF. Bayés de Luna et al. (Bayés de luna et al., 1988) put forward IAB was a marker of a higher incidence of paroxysmal supraventricular tachyarrhythmia in 1988. Recently, the presence of advanced IAB has been considered as a significant risk factor of recurrence of AF after PVI (Enriquez et al., 2014; Wu et al., 2016). Our study findings are in agreement with the abovementioned studies. In our study, the patients with recurrence had longer P-wave durations and higher prevalence of advanced IAB. However, there had no significant differences in P-wave voltage between two groups. Maybe it's because we did not analyze according to P-wave voltage in different height. The MVP score evaluates the degree of atrial dysfunction comprehensively including electrical remodeling, atrial inhomogeneous propagation of the electrical stimulus, and decreased myocardial mass due to atrial scarring. These risk factors will not only increase the risk of AF, but also associate with higher recurrence rates of AF after PVI.
Some encouraged inspirations perhaps had come to us, although the MVP score has some limitations for predicting recurrence of AF after PVI in patients with PAF. Further study about investigating the predictive model for predicting recurrence of AF after ablation for PAF can include some ECG variables. This study demonstrates that a simple risk score which is composed of easily obtainable ECG variables is highly suitable to a wide variety of populations who underwent PVI for PAF. The MVP score can help to select patients most likely to benefit from the procedure. Patients with higher MVP scores require a more individualized advanced ablation protocols (surgical or hybrid ablations) and they may be less likely to remain in sinus rhythm at 1 year.
5 STUDY LIMITATIONS
We acknowledge certain important limitations in the current study. First, this was an observational, retrospective, and single-center study. Asymptomatic episodes of AF and symptomatic AF recurrences that were detected by other institutions may have not been included .Therefore, the rate of AF recurrence may be underdetected. Second, the MVP score was derived retrospectively and our data were also collected retrospectively. Further prospective studies with continuous rhythm monitoring are needed to validate and confirm our findings. The study was not able to provide useful insights into the different energy sources or contact catheters. Hence, recurrences may have many explanations beyond patient characteristics, such as operator, ablation protocols, or periprocedural management. Finally, the small sample size may have introduced statistical bias.
6 CONCLUSIONS
The MVP score is independently associated with clinical recurrence of AF after PVI. It may provide a useful complementary clinical tool to evaluation of the likelihood of future recurrences. In conclusion, the MVP risk score can identify patients who are at high risk of recurrence after PVI.
ACKNOWLEDGMENTS
This work was supported by Key Research and Development Projects in Ningxia, China (GrantNo. 2018BEG02006).We thank Bintao Wu and Xiaobin Zhang for providing us helpful advice and inspiration.
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
Na Yang, Ning Yan and Shaobin Jia conceived the study. Mohan Wang and Na Yang collected data. Guangzhi Cong and Zhen Yang performed the ECG measurements. All statistical analysis was done by Guangzhi Cong,Shaobin Jia,Na Yang, Ning Yan,Mohan and Zhen Yang participated in discussions and contributed to the design and implementation of the research, to the analysis of the results and to the writing of the manuscript. All authors reviewed and commented on the manuscript.
ETHICS
The study was granted approval by the institutional General Hospital of Ningxia Medical University Research Ethics Board.




