Cotinine levels influence the risk of rupture of brain aneurysms
Abstract
Cotinine, the primary metabolite of nicotine, is currently regarded as the best biomarker of tobacco smoke exposure. We aim to assess whether cotinine levels are associated with (1) intracranial aneurysm and (2) intracranial aneurysm rupture.
Methods
We performed a single-center case–control study. Cases were consecutive patients admitted with diagnosis of brain aneurysm (ruptured or unruptured). We randomly selected controls without intracranial aneurysm from the same source population that produced the cases. Smoking data were collected by questionnaire, and serum levels of cotinine were used as an objective measure of nicotine exposure. Logistic regression models were used to assess the relationship between cotinine levels and aneurysm rupture.
Results
We included 86 patients with intracranial aneurysm and 96 controls. Smoking status (p < .001), cotinine levels (p = .009), and female sex (p = .006) were associated with diagnosis of intracranial aneurysm. In the multivariate analysis, controlling for sex, smoker status and age, levels of cotinine were independently associated with aneurysm rupture (OR 1.53, 95% CI 1.10–2.13, p = .012).
Conclusions
Our results suggest that high cotinine levels in smokers with brain aneurysm are significantly associated with high rupture risk, independently of smoker status, age, and sex.
1 INTRODUCTION
Smokers have a total life expectancy at least 10 years shorter than never-smokers.1, 2 Smoking is an independent risk factor for intracranial aneurysm rupture.3-5 The prevalence of smokers among patients with ruptured aneurysm exceeds 70%,6, 7 but if former smokers are included, it reaches almost 80%.8-10 Similar prevalence is reported in those with unruptured intracranial aneurysm.8-17 Nicotine is one of the main harmful chemicals in tobacco. The variable number and quality (light, mild, or regular) of daily smoked cigarettes, as well the pH of the smoke (pH 8 allows a ~ 2.5-fold higher nicotine concentration than pH 7, and fourfold higher than pH 6) and the smoking alternatives (electronic cigarette, cigar, pipe, plain tobacco, hookah, oral tobacco) make it difficult to determine exactly how much nicotine is consumed by each smoker.18 Nicotine is metabolized in the liver to cotinine, and determination of the cotinine level, the major metabolite of nicotine and index of exposure to tobacco smoke, can be performed to ascertain how much nicotine is absorbed. Experimental evidence from mouse models suggest that nicotine increases the aneurysm rupture rate by acting on the α7*-nAChR of vascular smooth muscle cells.19 However, there are no studies assessing the level of nicotine in patients with intracranial aneurysm.
In the present study, we examine the cotinine level in patients with recent diagnosis of intracranial aneurysm, aiming to establish whether cotinine levels are associated with (1) diagnosis of intracranial aneurysm and (2) intracranial aneurysm rupture.
2 MATERIALS AND METHODS
According to the Helsinki principles and previous IRB approval (CE 5224, January 29, 2019), a single-center, cross-sectional, observational, case–control study was conducted in patients with intracranial ruptured or unruptured aneurysm with regard to their smoker or non-smoker status. Patients were consecutively recruited from the Emergency Department and the Neurosurgery ward at Hospital within 8 h of arrival, or from the outpatient clinic between January 2019 and November 2020. The control group was composed of randomly selected individuals submitted to neuroradiological assessment (CT angiography, MRI, or angiography) during the same period but without intracranial aneurysm. Smokers and former smokers were considered together in both groups. Based on their size, intracranial aneurysms were classified as small (<7 mm), medium (7–12 mm), large (13–25 mm), and giant (>25 mm). Patients with two or more aneurysms were considered to have multiple aneurysms. Cotinine levels were assessed in salivary samples. In comatose patients who were not able to cooperate urinary samples were also accepted. A fast test strip that gives semi-quantitative results (levels 0–6: 0 to >1000 ng/ml) was employed (NicAlert, Nymox Pharmaceutical Corporation, St. Laurent). This test has a sensitivity of 93%, a specificity of 95%, a positive predictive value of 95%, and a negative predictive value of 93%.20, 21 Cotinine levels are classified from 0 to 6, with 0 being considered a non-smoker, corresponding to a cotinine content <10 ng/ml, and 6 is associated with a heavy smoking, corresponding to cotinine content>1000 ng/ml. Cotinine levels remain elevated for longer, detectable in urine and blood samples for up to 7 days. Inclusion criteria for both groups were age ≥ 25 and neuroradiological imaging demonstrating or excluding an intracranial aneurysm. Exclusion criteria were presence of neurodegenerative diseases, cerebral hemorrhage in the absence of aneurysm, and pregnancy.
2.1 Statistical analysis
A t-test was used to evaluate the difference in age between the two groups. The chi-squared test was used to evaluate the relationship between two categorical variables. The Mann–Whitney test was used to assess the cotinine distribution between the two groups. Receiver operating characteristic (ROC) analysis was performed to determine the ability of different cotinine level thresholds to discriminate the outcome of interest. Given the low number of outcome events (aneurysm rupture), Firth penalized likelihood regression was used to assess the association between cotinine levels and aneurysm rupture. The variables selected for this multivariate analysis were sex, smoking status, and age. Statistical analyses were performed using Stata software 14.1. A p-value <.05 was considered statistically significant.
3 RESULTS
Cotinine levels were collected from 182 participants. We included 86 patients with ruptured or unruptured aneurysms, of whom 61 were women and 25 were men (F to M ratio 2.4:1) (Table 1). The average age of patients with ruptured aneurysms was 57 years, and in patients with unruptured aneurysms, it was 62 years. There was a female preponderance in both the ruptured aneurysm group and the unruptured aneurysm group. The average size of the ruptured aneurysms was 6.94 mm, and that of the unruptured aneurysms was 7.04 mm (p = .468). In smokers with aneurysm, the median cotinine level was 4 (interquartile range 1–5), while in non-smoking cases, it was 1 (interquartile range 1–1) (p < .021).
| Baselines features | Subjects with intracranial aneurysm (n = 86) | Control group (no intracranial aneurysm (n = 96) | |
|---|---|---|---|
| Average age (years) | 60.3 (range 28–84) | 61.7 (range 31–81) | – |
| Female/Male | 61F/25M | 49F/47M | p = .006 |
| Smoker/ex smoker | 58 (67.5%) | 27 (28%) | p < .001 |
| Smoker median cotinine level | 4 | 4 | – |
| No smoker | 28 (32.5%) | 69 (72%) | – |
| No smoker median cotinine level | 1 | 1 | – |
| Single aneurysm | 61 (71%), 40 smokers (65.5%) | – | – |
| Multiple aneurysms ≥2 | 25 (29%), 18 smokers (72%) | – | – |
| Multiple aneurysms smoker | 18 (72%) | – | – |
| Size | Small: 46 (37.4%) | – | – |
| Medium: 60 (48.8%) | |||
| Large: 16 (13%) | |||
| Giant: 1 (0.8%) | |||
| Cotinine level 0–6: n° (%) | 0: 5 (5.8) | 0: 9 (9.4) | p = .009 |
| 1: 40 (46.5) | 1: 61 (63.5) | ||
| 2: 3 (3.5) | 2: 1 (1.0) | ||
| 3: 8 (9.3) | 3: 3 (3.1) | ||
| 4: 12 (14) | 4: 9 (9.4) | ||
| 5: 13 (11.1) | 5: 12 (12.5) | ||
| 6: 5 (5.8) | 6: 1 (5.8) | ||
| Ruptured | 34 (39.5%) | Unruptured | 52 (60.5%) |
| Ruptured smoker | 24 (70.6%) | Unruptured smoker | 34 (65.4%) |
| Ruptured no smoker | 10 (29.4%) | Unruptured no smoker | 18 (34.6) |
| mean age | 57 years | mean age | 62 years |
| sex | 21F/13M | sex | 40F/12M |
| Mean size (mm) | 6.9 mm | Mean size (mm) | 7.0 mm |
| Cotinine level 0–6: n° (%) | 0: 0 (0.0) | Cotinine level 0–6: n° (%) | 0: 5 (9.6) |
| 1: 13 (38.2) | 1: 27 (51.9) | ||
| 2: 1 (2.9) | 2: 2 (3.8) | ||
| 3: 6 (11.5) | |||
| 3: 2 (5.8) | 4: 7 (13.4) | ||
| 4: 5 (14.7) | 5: 4 (7.6) 23% | ||
| 5: 9 (26.4) 53% | 6: 1 (1.9) | ||
| 6: 4 (11.7) p = .002 | |||
| No smoker (10): median cotinine level | 1 | No smoker (18): median cotinine level | 1 |
More than 70% of patients with ruptured aneurysms (24/34) were smokers. Having multiple aneurysms was not associated with smoker status (p = .418).
The control group included 96 individuals without aneurysm, of which 49 were women and 47 were men (F to M ratio 1:0.96). The average age was 61.7 years (31–81 years). Among the controls, 27 (28%) were smokers and in those the median cotinine level was 4 (interquartile range 4–5), and in non-smoking controls it was 1 (interquartile range 1–1).
3.1 Association between cotinine levels and presence of aneurysm
The distribution of smokers and non-smokers in the two groups confirms the association between smoking and presence of a brain aneurysm (Table 1).
The proportion of women (70.9% versus 51%) was higher in cases than in controls (p = .006). Considering all individuals together (182 subjects), cotinine level 1 was the most represented (101 subjects, 55.5%, of which 97 were nonsmokers), followed by levels 5 (25 subjects, 13.7%), 4 (21 subjects, 11.6%), 3 (11 subjects, 6%), 6 (6 subjects, 3.3%), 0 (14 subjects, 7.7%), and 2 (4 subjects, 2.2%). There was a significant difference (p = .009) in the distribution of cotinine between cases and controls (Figure 1).
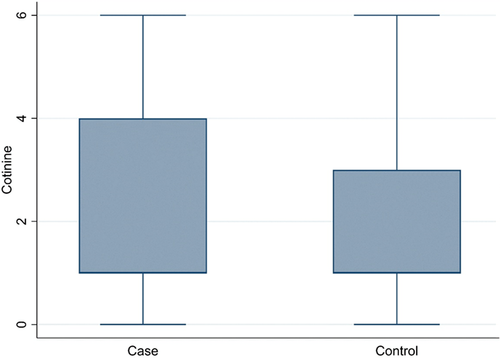
3.2 Association between cotinine levels and aneurysm rupture
The median cotinine level was higher in patients with ruptured aneurysms than it was in those who have an unruptured aneurysm (p = .001) (Figure 2). The area under ROC curve value for cotinine levels for predicting aneurysm rupture was 0.67 (95% confidence interval [CI]: 0.56–0.77). A cut-point for levels of cotinine of 5 correctly classified the largest proportion of subjects (68.60%). However, the sensitivity was low (37.14%). Therefore, the cotinine value was 4 selected as cutoff point (66.28% of subjects correctly classified, sensitivity 76.47%, specificity 51.43%) (Figure 3).
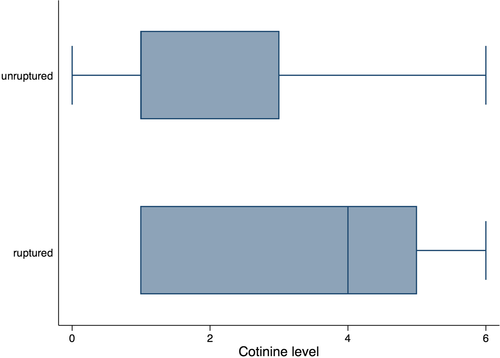
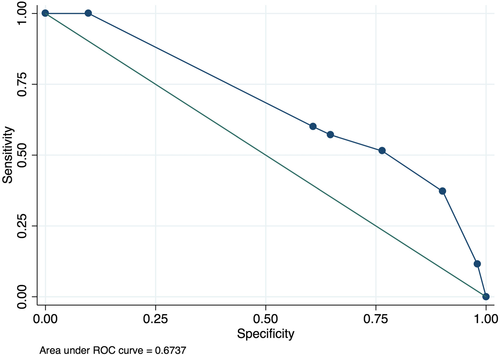
Smoker patients with high cotinine levels (≥4) had a significantly higher proportion of ruptured (18 patients, 52.8%) versus unruptured aneurysms (12 patients, 23%) (p = .002). Patients with a cotinine value between 4 and 6 had a fourfold higher probability of rupture of a cerebral aneurysm than those with a cotinine value between 0 and 3 (unadjusted OR = 3.75, p = .005). No significant relationship between the level of cotinine and the size of the aneurysm was found, considering the entire group (p < .115), or only smokers (p = .269), former smokers (p = .67), or nonsmokers (p = .992).
Cotinine level declined with increasing age (Table S1).
In the multivariate analysis, controlling for sex, smoker status and age, levels of cotinine were independently associated with rupture (OR 1.53, 95%CI 1.10–2.13, p = .012). Considering all the aneurysms, subjects with cotinine levels of 4–6 had a greater probability of having a rupture than those with levels of 0–3 (OR, 5.55; 95% CI, 1.08–28.5; p = .040).
4 DISCUSSION
In this case–control study, we found that higher cotinine levels are associated with a fourfold increased rupture risk, independently of smoker status, sex, and age. Moreover, we found that patients with higher cotinine levels have a higher prevalence of intracranial aneurysm.
Smoking is a dangerous addiction that causes vascular damage in the brain and is significantly related to abdominal and thoracic aortic, iliac, and renal aneurysms.22-24 It is known that the approximate number of cigarettes consumed, packs per day, or years of smoking are often inaccurate in determining the real nicotine absorption by the smoker.25 However, it was previously unknown whether measuring the level of salivary or urinary cotinine can have added value in patients with intracranial aneurysms.
These results suggest that quantitative data provided by cotinine levels may be of added value in determining the risk of aneurysm rupture. However, even a cotinine level of 1 cannot exclude either the presence of an aneurysm or its rupture. An unexpected result was that 72 nonsmokers from both groups had a cotinine level of 1 (10–30 ng/ml), instead of 0. This low cotinine level cannot be neglected. Among these, 23 patients had an aneurysm, and 8 of them a ruptured aneurysm. A cotinine level of 1 could result from secondhand smoke, but only 12/72 (17%) nonsmokers with a cotinine level of 1 claimed a possible home or work exposure to secondhand smoke. Indeed, it has been previously suggested that continuous exposure to secondhand smoke increases the risk of developing cerebrovascular and cardiovascular diseases. However, a retrospective study of 385 women with intracranial aneurysms could not find an association between passive smoking and increased risk of rupture of an intracranial aneurysm.26
Other possible explanations include diet and/or pollution. Low levels of nicotine are present in a number of frequently consumed vegetables, more specifically of the Solanaceae and Brassicaceae family (eggplant, bell peppers, chili peppers, potatoes, tomatoes, tomatillos, and cauliflower), as well tea leaves.27, 28 On average, each fresh fruit of this vegetables contains nicotine at 2–7 μg/kg, and dietary sources may provide a maximum intake of 2.25 μg/day. Consumption of these plants for many years could explain the low cotinine level detected in our nonsmoker participants, who reported daily consumption of these foods. Finally, it cannot be excluded that level 1 is the limit of the cotinine strip test in reliably identifying traces of the nicotine metabolite. Sampling of cotinine in the serum, as for other drug abuse, could better detect/determine low cotinine levels and could be routinely performed on a large scale.
Our study has several limitations. We did not collect samples within an hour of when the smokers last consumed nicotine, since we considered the cotinine result from the strip test to represent their daily consumption. Moreover, we have just assessed one point in time and the number of packs-year was not considered. Finally, the small sample size limits multivariate analysis.
5 CONCLUSIONS
This study suggests that the evaluation of cotinine levels may be of added value in assessing the risk of brain aneurysms and aneurysm rupture. Higher cotinine levels are significantly associated with rupture in patients with intracranial aneurysm, independently of smoking status, age, and sex.
ACKNOWLEDGMENTS
We thank all patients or individuals who agreed to participate in this study. Open Access Funding provided by Universita degli Studi di Roma La Sapienza within the CRUI-CARE Agreement.
AUTHORS CONTRIBUTION
All authors have made substantial contributions to all of the following:
(1) The conception and design of the study, or acquisition of data, or analysis and interpretation of data: Paolo Missori, Diana Aguiar de Sousa, Angela Ambrosone, Antonio Currà, Sergio Paolini, Giorgio Incarbone, Elena Amabile, Francesco Biraschi,
(2) Drafting the article or revising it critically for important intellectual content: Paolo Missori, Diana Aguiar de Sousa, Simone Peschillo, Sergio Paolini, Francesco Diana.
(3) Final approval of the version to be submitted: Paolo Missori, Diana Aguiar de Sousa, Angela Ambrosone, Antonio Currà, Simone Peschillo, Sergio Paolini, Giorgio Incarbone, Elena Amabile, Francesco Biraschi, Francesco Diana.
FUNDING INFORMATION
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICTS OF INTEREST
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
ETHICAL APPROVAL
Institutional Review Board approval (CE 5224, January 292,019).
PATIENT CONSENT STATEMENT
Informed consent was obtained from all subjects or their next of kin.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




