Quality of life in patients with glioblastoma and their relatives
Abstract
Objectives
Glioblastoma is the most aggressive primary brain tumour in adults. The rapid decline of physical and cognitive functions is likely to affect patients and relatives during the entire course of disease. The aim of this study was to describe and compare (a) health-related quality of life (HRQoL) and psychological symptoms between patients with glioblastoma and their relatives, and (b) HRQoL between patients and a general population over time.
Methods
At baseline, 63 patients and 63 relatives were included. The participants completed the Short Form Health Survey (SF-36) and the Hospital Anxiety and Depression scale (HADS) at seven different occasions from pre-surgery until two years post-surgery. A comparison of SF-36 was made between patients and an age- and gender-matched control group. Descriptive analysis, effect size and Wilcoxon signed-rank test were used.
Results
Relatives scored lower health-related quality of life (HRQoL) and higher symptoms of anxiety than patients, whilst patients scored worse in the physical parts of the SF-36. Three weeks post-surgery, relatives scored their lowest HRQoL and had the highest risk of anxiety symptoms. Comparing patients with controls, the patients rated worse in both the mental and physical component summaries in HRQoL at most time points.
Conclusion
Both patients and relatives showed deterioration of HRQoL. In addition, relatives showed high frequency of anxiety symptoms. Our data reveal that relatives of patients with glioblastoma need attention throughout the disease trajectory and they also need support at the right time point.
1 INTRODUCTION
Glioblastoma is the most aggressive and most common malignant brain tumour that affects adults. The mean age at diagnosis is around 65 years old and the incidence rate is 3.23 cases per 100,000. The average life expectancy is 10–15 months1-4 and the estimated 2-year survival rate is 8%–25%.2, 4, 5 Glioblastoma affects many aspects of a person's life and causes a variety of progressive, and usually concurrent, symptoms such as headache, hemiparesis, cognitive problems, personality changes and communication problems.6 The complexity of symptoms and problems can have a negative impact on the health-related quality of life (HRQoL)—not only that of patients, but also relatives.7, 8 Consequently, patients with glioblastoma are a vulnerable group, with more severe symptoms of depression and illness disturbance compared to patients with other malignant diseases.7 As such, they experience low HRQoL prior to surgery,9 early after surgery and two months after surgery.8, 10 A low HRQoL score early after surgery is a known negative prognostic factor for survival.7, 8 Relatives of patients with glioblastoma report distress pre-surgery9 and at the 2-month follow-up.10
In a previous study, we reported that relatives of patients with glioblastoma have worse mental HRQoL and more frequent symptoms of anxiety and depression prior to surgery than the patients.9 Apart from studies on patients with malignant glioma (with WHO grade III and IV grouped together), longitudinal descriptions and comparisons of patients with glioblastoma and their relatives in the literature are sparse. The few longitudinal studies of HRQoL focussing on both patients and relatives usually do not exceed six months post-surgery,10 even though six-month survival in this patient group is estimated at 60%–65%,4, 5 and 8%–25% in the case of two-year survival.2, 4, 5 Thus, there is a lack of data on how HRQoL and psychological symptoms in patients with glioblastoma change—compared to their relatives—over the entire course of disease. The aim of this study was to describe and compare (a) HRQoL and psychological symptoms between patients with glioblastoma and their relatives, and (b) HRQoL between patients and a control sample from the general population over time.
2 METHODS
2.1 Design and participants
This study was designed as a prospective longitudinal cohort study. Inclusion criteria were established as: patients aged 18 years old or over, with radiologically verified glioblastoma, identified in a population-based study at the University Hospital in Gothenburg, Sweden.11 Glioblastoma was defined according to the 2016 WHO classification,12-14 as described in our previous study.9 The majority of tumours (>80%) in the cohort were IDH-wildtype. Exclusion criteria were emergency surgery, operated at another hospital, non-conversant in Swedish, reoperation, other histological diagnosis after surgery and patients without a relative. For recruitment of relatives, the patients were asked to select the person closest to them, aged 18 years old or over, regardless of whether they were living together or not. At baseline, the paired participants comprised 63 patients and 63 relatives. Further description and a flow chart of the enrolment process, as well as baseline demographic data of the study cohort, have been presented in our earlier study.9
2.2 Questionnaires
2.2.1 Health-related quality of life assessment
To measure and compare patients’ and relatives’ subjective experience of HRQoL over time, the generic Short Form Health Survey (SF-36) questionnaire was chosen as a validated instrument.15 SF-36 is divided into eight multi-item scales: physical functioning (PF), role limitations due to physical problems (RP), bodily pain (BP), general health perceptions (GH), vitality (VT), social functioning (SF), role limitations due to emotional problems (RE) and mental health (MH). The raw scores from the eight multi-item scales are converted via a specific algorithm to a 0–100 grade, where higher scores represent better health state.16, 17 The items in SF-36 can be summarized by two general health indices, Physical Component Summary (PCS) and Mental Component Summary (MCS), covering the main dimensions of physical health and mental health. An age- and gender-matched samples for each patient who participated in the study were manually retrieved from the SF-36 general population data.17 This was done at every measured timepoint.
2.2.2 Psychological symptoms assessment
To evaluate and compare patients’ and relatives’ psychological symptoms over time, the Hospital Anxiety and Depression scale (HADS) was used. HADS is a validated generic measuring instrument that assesses two main areas, symptoms of anxiety (HADa) and depression (HADd).18 Each main area is divided into seven questions, each scored 0–3, resulting in total range of 0–21 for each main area. Scores <8 indicate the probable absence of clinically meaningful degrees of anxiety or depression, scores ≥8 indicate the possible presence of clinically meaningful degrees of depression or anxiety, and scores ≥11 indicate the probable presence of clinically meaningful degrees of depression or anxiety.19, 20
2.3 Data collection
Data was collected between 2012 and 2018. Patients and their relatives completed the self-reported questionnaires pre-surgery, 3 weeks post-surgery (when patients received radiation), 12 weeks post-surgery (when receiving chemotherapy) and then every 6 months until 2 years post-surgery. At baseline, a research assistant distributed the questionnaires to the participants. At the follow-ups, questionnaires were sent to the participants by mail. Demographic data, molecular tumour characteristics and post-surgery treatment were collected from patients’ electronic medical records and are presented in Table 1).
| Patients | n (%) |
|---|---|
| Total sample | 63 |
| Male/Female | 40(63)/23(37) |
| md (range) | |
|---|---|
| Age (in years) at surgery | 62 (37−77) |
| n (%) | |
|---|---|
| Localization of tumour | |
| Unilateral | 44 (69.8%) |
| Bilateral | 2 (3.2%) |
| Central | 17 (27.0%) |
| Operation side | |
| Right | 32 (50.8%) |
| Left | 25 (39.7%) |
| Bilateral | 6 (9.5%) |
| Comorbidity | |
| Yes | 33 (52.4%) |
| No | 28 (44.4%) |
| Unknown | 2 (3.2%) |
| % | |
|---|---|
| Molecular tumour characteristics | |
| MGMT promoter methylation | 46% |
| IDH-gene mutation | 6% |
| Post-surgery treatment | |
| Primary oncological treatment | 83% |
| Radiotherapy and chemotherapy | 51% |
| Radiotherapy | 13% |
2.4 Statistics
Descriptive statistics were presented as means, standard deviation (SD), percentages and 95% confidence intervals (CI) at every time point. Descriptive data are presented until 2 years post-surgery and comparative data were analysed until eighteen months post-surgery due to the small sample size at the 2-year time point. Wilcoxon signed-rank test was used to evaluate the difference between patients and their relatives and between patients and the age-and gender-matched sample. A p-value ≤.05 (two sided) was considered significant. To evaluate the extent of difference between patients and the general population sample, the effect size was calculated and interpreted with Cohens 0.2–0.5 (small effect), 0.5–0.8 (moderate effect) and >0.8 (large effect).21 Since the parameter age was not known for the group of relatives, this specific analysis could not be made between relatives and the general population. Analyses were performed in SPSS version 26 (SPSS Statistics; IBM, Armonk, NY, USA).
2.5 Ethical approval
This study was designed and performed according to the principles of the Declaration of Helsinki. Approval was granted by the Regional Ethical Review Board in Gothenburg, Dnr. 559–12. Licence number for SF-36: QM037698. Before data collection, all participants received oral and written information and gave informed consent. All participants consented to publication. Informed consent and consent to publish was obtained from all participants in this study.
3 RESULTS
The results presented describe the differences over time in HRQoL and psychological symptoms between patients and their relatives and HRQoL between patients and an age- and gender-matched population.
The results at baseline (pre-surgery) have been described in our previous study9 but are presented here for the sake of completeness, that is to compare HRQoL and psychological symptoms between patients and their relatives over the entire course of the disease. After patients and relatives were paired, the sample comprised 63 pairs (patients/relatives) pre-surgery, 42 pairs three weeks post-surgery, 32 pairs twelve weeks post-surgery, 25 pairs six months post-surgery, 12 pairs one year post-surgery, 11 pairs eighteen months post-surgery and 6 pairs two years post-surgery. Regarding the longitudinal dropout, 24 patients discontinued participation in connection with their date of death. Due to ethical considerations, we have no data on why the remaining participants chose to discontinue.
3.1 Health-related quality of life (SF-36)
3.1.1 SF-36 over time in patients and relatives
To clarify trends in HRQoL over time, the sample was divided into groups and participants were allocated to a specific group according to when study participation ended, that is at three weeks, twelve weeks, six months, one year or eighteen months. The groups of patients who ended participation at twelve weeks, six months and eighteen months post-surgery had a downwards trend (a deterioration) of the SF-36 MCS. In addition, patients generally had a downwards trend for PCS regardless of when they ended their participation in the study. (Figure 1). The MCS was most affected at eighteen months post-surgery. Furthermore, three weeks post-surgery, patients had a noticeably low mean score for SF-36 item RP (Table 2).
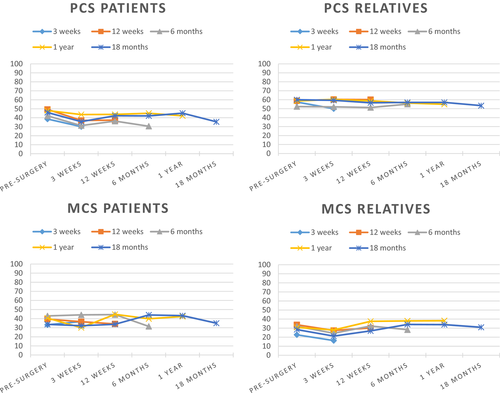
| Pre-surgery | 3 weeks | 12 weeks | 6 months | 1 year | 18 months | 2 years | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Relatives | Patients | Relatives | Patients | Relatives | Patients | Relatives | Patients | Relatives | Patients | Relatives | Patients | Relatives | |
|
n = 63 mean (sd) |
n = 63 mean (sd) |
n = 42 mean (sd) |
n = 42 mean (sd) |
n = 32 mean (sd) |
n = 32 mean (sd) |
n = 25 mean (sd) |
n = 25 mean (sd) |
n = 12 mean (sd) |
n = 12 mean (sd) |
n = 11 mean (sd) |
n = 11 mean (sd) |
n = 6 mean (sd) |
n = 6 mean (sd) |
|
| SF-36 | ||||||||||||||
| PCS | 43.2 (11.0) | 57.3 (7.9) | 34.5 (10.9) | 55.6 (10.3) | 39.6 (10.4) | 56.5 (8.1) | 38.6 (12.0) | 56.1 (8.8) | 43.9 (6.5) | 56.2 (6.7) | 35.5 (12.3) | 53.4 (10.3) | 32.9 (13.1) | 56.6 (11.4) |
| PF - physical functioning | 70.7 (29.9) | 91.0 (17.4) | 53.0 (33.7) | 88.1 (19.4) | 63.8 (32.9) | 91.6 (10.1) | 57.0 (33.6) | 91.4 (11.9) | 76.7 (19.3) | 89.6 (13.7) | 57.7 (34.2) | 88.1 (14.3) | 44.2 (43.6) | 92.5 (14.1) |
| RP - role limitations, physical | 26.1 (38.6) | 76.6 (35.6) | 6.0 (19.8) | 59.5 (43.1) | 24.2 (34.5) | 69.5 (37.4) | 30.0 (39.5) | 80.0 (36.1) | 39.6 (45.8) | 83.3 (30.8) | 27.3 (41.0) | 65.9 (42.2) | 20.8 (33.2) | 66.7 (51.6) |
| BP - bodily pain | 73.6 (29.8) | 84.2 (23.7) | 69.3 (30.2) | 83.0 (24.2) | 78.1 (23.4) | 84.3 (23.5) | 77.6 (23.5) | 81.8 (24.3) | 91.2 (13.9) | 85.6 (22.1) | 62.2 (30.7) | 75.0 (28.7) | 78.0 (17.7) | 87.0 (31.8) |
| GH - general health | 66.9 (19.8) | 76.7 (17.0) | 48.7 (16.6) | 70.7 (20.8) | 49.7 (22.8) | 74.3 (19.2) | 44.2 (22.8) | 74.2 (21.7) | 57.3 (19.7) | 76.8 (17.1) | 42.1 (20.9) | 69.6 (17.8) | 40.6 (23.8) | 68.0 (24.1) |
| MCS | 37.2 (13.3) | 29.2 (14.7) | 37.2 (13.5) | 22.6 (14.3) | 39.3 (12.3) | 31.0 (15.9) | 38.5 (12.8) | 32.8 (15.7) | 44.3 (10.9) | 35.7 (14.3) | 35.4 (7.7) | 30.9 (11.8) | 42.4 (12.3) | 25.6 (18.1) |
| VT – vitality | 51.7 (25.7) | 50.2 (22.9) | 46.3 (19.3) | 38.9 (24.8) | 50.0 (24.7) | 49.7 (24.4) | 46.3 (25.5) | 55.4 (21.6) | 54.6 (21.8) | 59.2 (25.5) | 42.0 (25.6) | 47.0 (17.0) | 47.5 (32.4) | 43.3 (26.4) |
| SF - social functioning | 58.9 (27.7) | 60.1 (27.1) | 44.3 (26.7) | 47.9 (25.7) | 61.3 (30.0) | 60.2 (24.7) | 60.0 (31.9) | 64.5 (29.9) | 70.8 (24.0) | 74.0 (28.4) | 50.0 (27.4) | 64.8 (21.5) | 56.3 (32.4) | 52.1 (31.0) |
| RE - role limitations, emotional | 41.9 (45.5) | 39.2 (43.3) | 45.6 (45.8) | 23.0 (36.4) | 44.4 (46.6) | 40.0 (45.0) | 43.1 (45.6) | 41.7 (43.1) | 63.9 (48.1) | 50.0 (41.4) | 39.4 (49.0) | 30.3 (45.8) | 44.4 (50.2) | 33.3 (51.6) |
| MH - mental health | 63.0 (22.8) | 52.6 (20.6) | 58.6 (23.1) | 44.5 (21.9) | 67.0 (22.4) | 57.0 (22.4) | 63.0 (24.0) | 59.1 (21.1) | 77.0 (14.9) | 61.0 (21.7) | 61.6 (15.3) | 55.6 (11.7) | 70.9 (14.8) | 48.7 (27.4) |
| HADS | ||||||||||||||
|
Anxiety mean (sd) |
6.8 (4.7) | 9.4 (4.7) | 6.3 (4.2) | 10.7 (5.1) | 6.5 (5.0) | 9.0 (4.9) | 7.0 (4.9) | 8.7 (5.4) | 4.6 (3.3) | 7.8 (4.2) | 7.4 (4.8) | 9.9 (3.1) | 5.8 (4.3) | 10.4 (4.7) |
| Probable presence n(%) | 13 (21.3) | 23 (37.7) | 9 (22.0) | 21 (53.2) | 6 (18.8) | 10 (31.3) | 6 (26.1) | 9 (39.1) | 1 (8.3) | 1 (8.3) | 2 (20.0) | 4 (40.0) | 1 (25.0) | 1 (25.0) |
| Possible presence n(%) | 10 (16.4) | 17 (27.9) | 8 (19.5) | 12 (29.8) | 9 (28.1) | 12 (37.5) | 5 (21.7) | 4 (17.4) | 2 (16.7) | 7 (58.3) | 5 (50.0) | 5 (50.0) | 1 (25.0) | 2 (50.0) |
| Probable absence n(%) | 38 (62.3) | 21 (34.4) | 24 (58.5) | 8 (17.0) | 17 (53.1) | 10 (31.3) | 12 (52.2) | 10 (43.5) | 9 (75.0) | 4 (33.3) | 3 (30.0) | 1 (10.0) | 2 (50.0) | 1 (25.0) |
|
Depression mean (sd) |
5.4 (4.8) | 6.7 (4.8) | 6.6 (4.4) | 7.9 (4.6) | 6.2 (5.0) | 6.5 (4.8) | 6.5 (5.2) | 6.5 (5.5) | 5.5 (4.7) | 5.5 (4.5) | 8.0 (5.3) | 6.9 (3.5) | 6.5 (5.1) | 7.0 (5.3) |
| Probable presence n(%) | 11 (18.0) | 16 (26.2) | 7 (16.7) | 14 (29.8) | 5 (15.6) | 6 (9.5) | 4 (17.4) | 5 (21.7) | 1 (8.3) | 2 (16.7) | 3 (30.0) | 2 (20.0) | 2 (40.0) | 1 (20.0) |
| Possible presence n(%) | 5 (8.2) | 7 (11.5) | 13 (31.0) | 15 (31.9) | 8 (25.0) | 10 (15.9) | 7 (30.4) | 5 (21.7) | 2 (16.7) | 3 (25.0) | 3 (30.0) | 3 (30.0) | 0 (0.0) | 1 (20.0) |
| Probable absence n(%) | 45 (73.8) | 38 (62.3) | 22 (52.4) | 18 (38.3) | 19 (59.4) | 16 (25.4) | 12 (52.2) | 13 (56.5) | 9 (75.0) | 7 (58.3) | 4 (40.0) | 5 (50.0) | 3 (60.0) | 3 (60.0) |
For the groups of relatives who ended participation at three weeks, one year and eighteen months post-surgery, a downward trend (deterioration) of the SF-36 PCS was noted. In addition, MCS in relatives showed a downward trend in the groups who ended their participation at three weeks, six months and eighteen months post-surgery (Figure 1). Relatives rated all the mental items of SF-36 the lowest (worst) three weeks post-surgery compared to the other time points measured. Furthermore, relatives scored all the mental items of SF-36 highest one year post-surgery (Table 2).
3.1.2 Psychological symptoms (HADS)
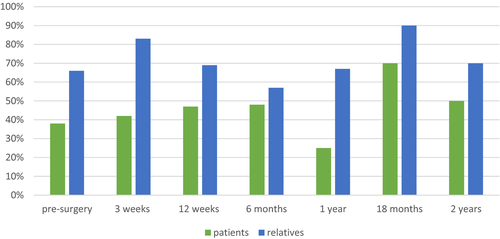
Patients scored probable absence of anxiety symptoms at all time points measured and had the highest mean at eighteen months post-surgery. They did not exceed the threshold for possible presence of depressive symptoms, except at eighteen months post-surgery. Relatives scored possible or probable presence of anxiety symptoms on all but one occasion (one year post-surgery). Their highest risk of symptoms of anxiety appeared to be at two time points: three weeks post-surgery, when 83% of the relative's scored presence of symptoms of anxiety and eighteen months post-surgery, when this figure increased to 90% (Figure 2, Table 2).
3.2 Comparisons between patients’ and relatives’ HRQoL and HADS
Prior to surgery and three weeks post-surgery, patients scored worse than relatives on all physical components of SF-36. Furthermore, patients were more physically affected than relatives regarding the SF-36 summary PCS and the items PF and GH at all time points measured (Table 3).
| Pre-surgery | 3 weeks | 12 weeks | 6 months | 1 year | 18 months | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 63 | n = 42 | n = 32 | n = 25 | n = 12 | n = 11 | |||||||
| Mean difference (95%CI) |
Wilcoxon p-value |
Mean difference (95% CI) |
Wilcoxon p-value |
Mean difference (95% CI) |
Wilcoxon p-value |
Mean difference (95% CI) |
Wilcoxon p-value |
Mean difference (95% CI) |
Wilcoxon p-value |
Mean difference (95% CI) |
Wilcoxon p-value |
|
| SF-36 | ||||||||||||
| PCS | −14.1 (−17.6, −10.6) | <.001a | −21.1 (−25.4, −16.8) | <.001a | −16.9 (−21.1, −12.6) | <.001a | −17.6 (−22.9, −12.2) | <.001a | −12.2 (−16.0, −8.5) | .002a | −17.8 (−29.3, −6.3) | .017a |
| MCS | 8.0 (3.8, 12.3) | <.001b | 14.5 (9.1, 20.0) | <.001b | 8.4 (2.7, 14.0) | .006b | 5.7 (−1.4, 12.8) | .039b | 8.6 (1.3, 15.9) | .041b | 4.5 (−5.6, 14.7) | .33 |
| PF | −20.3 (−28.7, −11.9) | <.001a | −35.2 (−46.2, −24,1) | <.001a | −27.7 (−39.0, −16.5) | <.001a | −34.4 (−47.4, −21.4) | <.001a | −12.9 (−21.7, −4.1) | .004a | −30.4 (−56.0, −4.8) | .05a |
| RP | −50.5 (−63.0, 38.0) | <.001a | −53.6 (−68.1, −39,0) | <.001a | −45.3 (−60.7, −29.9) | <.001a | −50.0 (−68.1, −31.9) | <.001a | −43.8 (−69.2, −18.3) | .14 | −38.6 (−71.7, −5.6) | .04a |
| BP | −10.7 (−19.7, −1.7) | .047a | −13.6 (−24.8, −2.5) | .03a | −6.2 (−18.4, 6.0) | .23 | −4.2 (−14.5, 6.1) | .36 | 5.6 (−10.3, 21.4) | .50 | −12.8 (−39.4, 13.7) | .16 |
| GH | −9.7 (−16.1, −3.4) | .006a | −22.1 (−29.7, −14.4) | <.001a | −24.7 (−33.2, −16.2) | <.001a | −30.1 (−39.2, −20.9) | <.001a | −19.4 (−33.2, −5.5) | .02a | −27.5 (−43.1, −12.0) | .01a |
| VT | 1.5 (−6.4, 9.5) | .80 | 7.4 (−2.2, 17.0) | .15 | 0.3 (−9.8, 10.5) | .81 | −9.1 (−21.4, 3.1) | .15 | −4.6 (−18.6, 9.4) | .47 | −5.0 (−25.4, 15.4) | .81 |
| SF | −1.2 (−8.8, 6.4) | .71 | −3.6 (−11.6, 4.5) | .39 | 1.2 (−9.2, 11,6) | .92 | −4.5 (−19.6, 10.6) | .57 | −3.1 (−20.4, 14.2) | .72 | −14.8 (−39.6, 10.1) | .14 |
| RE | 2.7 (−12.0, 17.3) | .77 | 22.6 (6.5, 38.7) | .009b | 4.4 (−15.1, 24.0) | .62 | 1.8 (−19.6, 22.3) | .72 | 13.9 (−13.9, 41.6) | .22 | 9.1 (−35.8, 54.0) | .92 |
| MH | 10.3 (4.2, 16.5) | <.001b | 14.1 (5.5, 22.7) | .004b | 10.0 (0.3, 19.7) | .045b | 3.8 (−8.0, 15.7) | .21 | 16.0 (5.3, 26.7) | .013b | 6.0 (−9.0, 21.0) | .24 |
| HADS | ||||||||||||
| Anxiety mean (sd) | −2,6 (−4.0, −1.3) | <.001b | −4.4 (−6.1, −2.8) | <.001b | −2.3 (−4.3, −0.2) | .048b | −1.7 (−3.9, 0.6) | .04b | −3.2 (−5.1, −1.3) | .005b | −2.0 (−5.6, 1.6) | .21 |
| Depression mean (sd) | −1.3 (−2.6, −0.1) | .02b | −1.3 (−2.9, 0.2) | <.001b | −0.3 (−2.4, 1.7) | .77 | −0.1 (−2.9, 2.7) | .53 | 0.0 (−2.8, 2.8) | .89 | 1.0 (4.0, 6.0) | .84 |
- a Patients scored worse than relatives.
- b Relatives scored worse than patients.
- Significance of Bold values indicates p < .05.
Relatives scored significantly worse than patients on the SF-36 MCS on all occasions, except at eighteen months post-surgery. They also scored lower for the SF-36 item MH than patients at all time points measured, except at six months post-surgery. At three weeks post-surgery, relatives scored worse than patients for several mental components and items of SF-36, MCS (p < .001, RE p = .009 and MH p = .004). Relatives reported a higher risk of symptoms of anxiety and depression compared to patients on many of the occasions measured. Prior to patient surgery, relatives scored worse than patients for both HADa and HADd. Furthermore, relatives scored worse than patients did on HADa at all the time points except eighteen months post-surgery (Table 3).
3.3 Comparison between patients and general population
Comparing patients’ SF-36 PCS and MCS with an age- and gender-matched population, patients scored significantly lower for MCS than the general population at all time points except at one year. PCS was significantly lower at all time points, except before surgery and one year after surgery. The overall effect sizes were moderate (Table 4).
| PCS | MCS | |||||||
|---|---|---|---|---|---|---|---|---|
| Patients (mean) |
General populationa (mean) |
p-value | Effect size b |
Patients (mean) |
General population a (mean) |
p-value | Effect size b | |
| Pre-surgery (n = 63) | 43.2 | 45.7 | .165 | 0.1 | 37.2 | 50.8 | <.001 | 0.5 |
| 3 weeks (n = 42) | 34.5 | 45.5 | <.001 | 0.5 | 37.2 | 51.0 | <.001 | 0.5 |
| 12 weeks (n = 32) | 39.6 | 45.9 | .010 | 0.3 | 39.3 | 46.3 | .02 | 0.3 |
| 6 months (n = 25) | 38.6 | 47.1 | .009 | 0.4 | 38.5 | 49.4 | .003 | 0.4 |
| 1 year (n = 12) | 43.9 | 46.7 | .239 | 0.3 | 44.3 | 51.1 | .136 | 0.2 |
| 18 months (n = 11) | 35.5 | 47.4 | .022 | 0.5 | 35.4 | 51.0 | .013 | 0.6 |
- a Age- and gender-matched normative population.
- b Effect size according to Cohen: 0.2–0.5 (small effect), 0.5–0.8 (moderate effect) and >0.8 (large effect).
4 DISCUSSION
We present evidence suggesting that HRQoL and psychological symptoms in patients with glioblastoma and their relatives are affected throughout the disease trajectory. In patients the physical HRQoL was impaired, whilst relatives in our study scored impaired mental HRQoL and psychological symptoms on almost all occasions. Furthermore, patients scored worse physical and mental HRQoL than an age- and gender-matched reference population. Patients and relatives estimated low HRQoL preoperatively9 and throughout the disease trajectory up until two years post-surgery. These results highlight the importance of support both before surgery and during the entire disease process.
Other studies have shown that increased support can reduce the anxiety of relatives of patients with other cancer types and that supportive conversations have a positive effect on the relatives’ distress.22, 23 Relatives of patients with glioma perceive lack of emotional support, and too much focus on physical care, as negative effect on their psychological symptoms.23 One possible way to support relatives could be psychoeducation and cognitive behavioural therapy, as has been described for patients with high-grade gliomas and their relatives.24
The group of relatives in our study, experienced symptoms of anxiety at all time points except one year post-surgery. This indicates that relatives also need targeted support throughout the course of the disease and suggests that it would be worthwhile to screen relatives on several occasions to identify symptoms of anxiety. In addition, our results revealed two time points that proved particularly stressful for the relatives in terms of symptoms of anxiety; at three weeks and at eighteen months post-surgery, when 83% and 90% of the relatives displayed symptoms of anxiety, respectively. It should be noted; however, that the sample was small (n = 11) at eighteen months post-surgery.
The few previous studies that have, to date, longitudinally monitored relatives of patients with glioblastoma show a high level of anxiety and low HRQoL close to diagnosis and at follow-up two months later.10 Furthermore, relatives of patients with a more mixed diagnostic group of high-grade gliomas report highest distress close to diagnosis, with high levels maintained until six months after patients received chemotherapy.25 Possible explanations for the pronounced symptoms of anxiety in relatives might be that they are, to a greater extent, reminded of the impending death and the patient's worsening symptoms. Another possible explanation might be that relatives of patients with brain tumours experience increased psychological symptoms due to the patient's suffering, struggle to survive22 and changed personality.22, 23, 26
Three weeks post-surgery proved to be a particularly challenging period for the relatives, when they appeared to be highly emotionally affected. Previous studies show that patients themselves have a difficult time throughout the course of the disease.7, 27, 28 In our study, relatives show signs of emotional struggle with a peak at three weeks post-surgery. At this time point, 83% of the relatives rated symptoms of anxiety, compared to 42% of the patients. Furthermore, relatives’ MCS showed a downward trend and relatives rated all mental components of SF-36 lowest at this time point. One way to address their vulnerability could be to screen relatives and offer targeted support to those with high levels of emotional stress.10
To analyse the results, we divided the patients into groups based on when the participants ended their engagement in the study. Examining the dynamic process at the different time points showed a downward trend for patients’ MCS at all measured time points. This result is consistent with a previous study that reported worse levels of HRQoL in patients closer to death.29 The patients’ SF-36 PCS showed a downward spiral, when ending participation in the study, which occurred probably shortly before they died. These data are consistent with the symptom pathway previously described, with increasing physical symptoms closer to death.26, 30
When relatives were divided into groups based on the time point at which they ended participation, the SF-36 MCS showed a downward trend at three weeks, 6 months and 18 months post-surgery. A previous study showed that relatives of patients with high-grade glioma displayed high levels of anxiety up until nine months post-surgery.31
Comparisons between the patients and relatives showed that patients generally rated worse than relatives in terms of the physical parts of SF-36 at all measured time points. Due to the symptoms of the disease trajectory, patients are expected to be more physically affected than their relatives.7 More interesting was the fact that relatives rated worse than patients on the mental parts of SF-36 and for symptoms of anxiety and depression. Relatives also rated worse on HADa and for symptoms of anxiety at most of the measured time points. This result is in agreement with earlier studies where relatives of patients with cancer reported more symptoms of anxiety than the patients themselves10, 22—also the case when the patient was close to death.22 In addition, another study showed that relatives of patients with high-grade glioma experienced symptoms of anxiety, in contrast with patients who did not report symptoms of anxiety at any time point from radiotherapy until one year post-surgery.32
The HRQoL of patients, presented as PCS and MCS in SF-36, compared with an age- and gender-matched control group, showed that the patients had lower PCS and MCS than the general population at all time points measured. In addition, the patients’ mental HRQoL was affected in connection with oncological treatment, which has been described previously,7, 27, 28 although to date there has been only limited data regarding long-term follow-up. It is alarming that patients with glioblastoma rate HRQoL worse than a matched control group and also have worse HRQoL than patients with other types of malignancies such as breast cancer and lung cancer.7 This highlights that patients with glioblastoma and their relatives are in need of specially tailored support throughout the illness trajectory.
5 CONCLUSION
We explored HRQoL and psychological symptoms over time for both patients with glioblastoma and their respective relatives. The main finding was relatives’ emotional difficulties throughout the course of the disease, especially at three weeks post-surgery. Relatives had high levels of anxiety and their mental HRQoL was compromised. They also rated their anxiety and mental component summary worse than patients did. Patients need both physical and emotional support, whilst relatives need mainly emotional support, and it should be noted that targeted support needs to be adapted throughout the illness.
6 STRENGTHS AND LIMITATIONS
The study design, with pairwise comparisons between patients and their respective relatives and encompassing a period as long as two years post-surgery, represents a clear strength. A limitation is the large dropout over time, which is, however, unavoidable and inherent to the study design. Indeed, the dropout reflects the severity of symptom development in patients with glioblastomas and is a strong argument for investigating the long-term effects of the disease, monitoring HRQoL and psychological symptoms over time. Another limitation is that we only presented descriptive data longitudinally without statistical analysis, again due to the large dropout over time. In addition, a comparison between relatives and an age- and gender-matched control group was not possible, since age as a parameter was missing in the relatives’ demographics data.
ACKNOWLEDGEMENTS
The authors would like to thank all patients and their relatives who participated in this study. Furthermore, we would like to thank Dr. Boglarka Fekete for data material, Anneli Johansson, Lena Nilsson, Stina Svensson and Ünzüle Yildiz also Nils-Gunnar Pehrsson for statistical consultation and Nick Guthrie for language review. This study was funded by: Sahlgrenska Academy and Institute of Health and Care Science, University of Gothenburg; Agreement between the Swedish government and the county councils, ALF-agreement, ALFGBG-717021; Health Medical Care Committee at Västra Götaland Regional FoU-support, VGFOUREG-750851; AFA Research Foundation; and the Ulrica Cronés Foundation.
CONFLICT OF INTEREST
The authors have no competing conflicts of interest to declare that are relevant to the content of this study.
AUTHOR CONTRIBUTIONS
Conceptualization and Data curation [P.S, B.R, A.O], Formal analysis [P.S, I.H, A.O], Investigation [P.S, B.R, A.O], Methodology and Validation [P.S, I.H, A.S, B.R, A.O], Visualization [P.S, I.H, A.O], Writing – original draft [P.S, A.O], Writing – review & editing [P.S, I.H, A.S, B.R, A.O]. All authors read and approved the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The datasets analysed during the current study are available from the corresponding author on reasonable request.




