Real-life use of oral disease-modifying treatments in Austria
Funding information
The Austrian MS Treatment Registry is supported by unrestricted grants of Biogen Austria, Novartis Pharma Austria and Genzyme Austria.
Abstract
Objectives
To compare the efficacy, frequencies and reasons for treatment interruption of fingolimod, dimethyl fumarate (DMF) or teriflunomide in a nationwide observational cohort using prospectively collected data.
Materials and methods
Two cohorts of patients with relapsing-remitting multiple sclerosis (RRMS) starting treatment with fingolimod, dimethyl fumarate or teriflunomide documented in the Austrian MS Treatment Registry (AMSTR) since 2014 and either staying on therapy for at least 12 months (12m cohort) or having at least one follow-up visit (total cohort). The 12m cohort included 664 RRMS patients: 315 in the fingolimod, 232 in the DMF and 117 in the teriflunomide group. Multinomial propensity scores were used for inverse probability weighting to correct for the bias of this non-randomised registry study.
Results
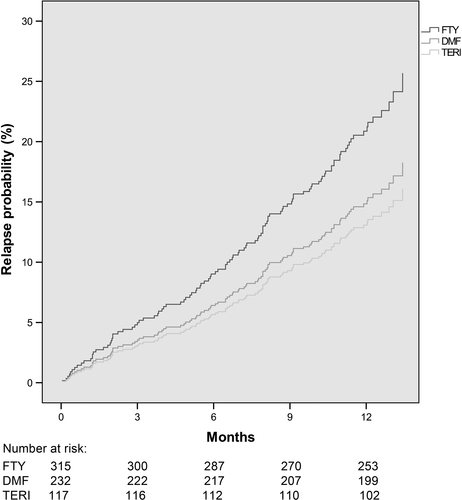
Estimated mean annualized relapse rates (ARR) over 12 months were 0.21 for fingolimod, 0.20 for DMF and 0.19 for teriflunomide treatment, causing an incidence rate ratio (IRR) of 1.01 for fingolimod vs DMF (P = 0.96) and 0.92 for teriflunomide vs DMF (P = 0.84). No differences were found regarding the probability for experiencing a relapse, EDSS change, EDSS progression and EDSS regression, except regarding less sustained EDSS progression for 12 weeks concerning DMF vs fingolimod (P = 0.02). The hazard ratio for treatment interruption comparing fingolimod vs DMF was 1.03 (P = 0.86) and 1.07 comparing teriflunomide vs DMF (P = 0.77).
Conclusions
In the AMSTR, there was no difference concerning ARR, probability for a relapse, EDSS change, treatment interruption, EDSS progression or regression between oral DMTs, except regarding less sustained EDSS progression for 12 weeks concerning DMF vs fingolimod.
1 INTRODUCTION
Treatment efficacy of fingolimod (FTY), dimethyl fumarate (DMF) and teriflunomide (TERI) for relapsing-remitting multiple sclerosis (RRMS) has been proven in randomized trials.1-6 In comparison with placebo groups, fingolimod reduced the annualized relapse rate (ARR) by 48%-54%,1, 2 DMF by 44%-53%3, 4 and teriflunomide by 32%-36%.5, 6 In addition, fingolimod showed a reduction in the ARR by 52% vs interferon beta-1a.7 In post hoc comparisons of DMF vs glatiramer-acetate differences were not significant except for new and/or enlarging T2-weighted hyperintense lesions.4 No difference in ARR between teriflunomide and IFNβ-1a was seen in TENERE.8
Studies matching the clinical efficacy provided conflicting results.9-15 These discrepancies ask for further investigations to confirm or rebut the published findings.
The objective of our study was, first, to compare the efficacy of fingolimod, DMF or teriflunomide and, second, to analyse the probability for stopping, pausing or switching (treatment interruption) either therapy in a nationwide observational cohort using prospectively collected data from a real-life setting.
2 MATERIALS AND METHODS
2.1 Data collection
The Austrian MS Treatment Registry (AMSTR),16 established in 2006 to maintain quality control and comply with reimbursement regulations of the Austrian sick funds, allows to obtain clinical data, to assess indications, the clinical profiles of the treated patients and to monitor safety in real life. The AMSTR is part of the dense MS network in Austria, which is constituted by all MS clinics from neurological departments and some dedicated neurological practices. In addition, prescriptions of DMTs for MS are exclusively reserved for MS centres. Thus, prescriptions and treatment documentations are evenly distributed across Austria. The AMSTR is compliant with Austrian laws on bioethics, and it was also approved by the ethical committee of the Medical University of Vienna (EC number 2096/2013).
AMSTR documents anonymous baseline data, including MS onset and duration, relapses in the prior 12 months, EDSS, gross MRI activity and previous disease-modifying therapies (DMT). Follow-up data (relapses, EDSS, adverse events [AE's], change or discontinuation of treatment) are required to be documented every 3-6 months, mean follow-up 4 months for fingolimod, 4 months for DMF and 3.9 months for teriflunomide. Each relapse had to be confirmed by a neurologist at the MS centre and documented in the AMSTR. Documentation required relapse onset, EDSS and use/dose of iv methylprednisolone treatment. Besides the fact that applying the AMSTR is mandatory for reimbursement, a special quality-related feature of the AMSTR is an external and independent data monitoring to improve data management in terms of completeness and plausibility of documented data.
In 2011, the European Medicines Agency (EMA) approved fingolimod along the same indication criteria as natalizumab. Reimbursement for fingolimod in Austria adheres to this approval. Thus, fingolimod-treated patients in Austria had to have either at least one relapse in the prior 12 months despite treatment with interferon beta or glatiramer-acetate and at least 9 T2 lesions or at least one Gadolinium-enhancing lesion on recent brain MRI (“indication A”), or two or more severe relapses in the preceding treatment-naïve 12 months and one or more Gadolinium-enhancing lesions on brain MRI or a significant increase in T2 lesion load as compared to a previous recent MRI (“indication B”).
In 2013, teriflunomide and in 2014, DMF were approved by the EMA with the indication for the treatment of adult patients with RRMS.
We investigated a total cohort of 1165 patients, who started treatment with fingolimod, DMF or teriflunomide in the AMSTR at any time since 2014. The coverage of the AMSTR for the three oral agents is approximately 70% of the total prescription in Austria. For the purpose of this study, we analysed the data of these patients in two separate cohorts. The first cohort stayed on therapy for at least 12 months (12m cohort), and this group was analysed for comparing the efficacy. The second cohort was the total cohort, defined with at least one follow-up visit, also including the 12m cohort. This group was analysed for the frequency, cause and risk of interruption (total cohort).
The primary outcome measure was the ARR under treatment with fingolimod, DMF or teriflunomide over 1 year after initiation of therapy. Relapses were defined as new or worsening neurological symptoms lasting for at least 24 hours in the absence of fever.
Further outcome measures were the total number of relapses, EDSS progression or regression confirmed after 3 and 6 months, and EDSS changes during the 1-year period (difference between EDSS at baseline and at the last visit). Sustained disability progression or regression was defined as an increase or decrease from baseline of at least 1.0 point in the EDSS score (or at least 0.5 points for patients with a baseline EDSS score greater than 5.5) that persisted for at least 12 or 24 weeks.
For analyses of the treatment interruption, three causes were defined, namely (a) stopping treatment as permanent treatment interruption in the AMSTR; (b) pausing treatment as treatment interruption and restarting with the same treatment; and (c) finally switching treatment as treatment interruption and starting with a new medication in the AMSTR.
2.2 Statistical methods
All effects estimated in comparing treatment groups were average treatment effects (ATE). To control the bias for non-randomised assignment to the treatment groups, we used inverse probability weighting. As we compared three groups, we used the estimation of multinomial propensity scores as described by McCaffrey.17 Propensity scores for treatment with fingolimod, DMF and teriflunomide were estimated for all patients with the baseline parameters age, duration of disease, number of relapses 12 months prior baseline, EDSS, presence of at least 9 MRI T2 lesions and at least one contrast-enhancing MRI T1 lesion, and previous therapy as independent variables. These variables were included in the model because of their clinical meaning, independent from their significance as a predictor in the model. Therefore, we tried to overcome the problem of being misled by false-positive predictors in a multiple testing situation as well as missing relevant variables by abandoning them in a beta failure decision. Treatment groups were balanced for all variables after scoring.
A generalized linear model (GLM) with relapse count as Poisson-distributed dependent variable was used to estimate the treatment effect on the relapse rate in the 12 months observation period.
A linear regression model was used to analyse the change in EDSS from baseline to the last visit in the 12 months observation period.
Cox proportional hazards models were used analysing EDSS progression and regression confirmed after 3 and 6 months, and the relapse hazard in the 12 months observation period.
Cox proportional hazards models were also used analysing treatment interruptions in the patient cohort with at least one follow-up visit.
All models included treatment as categorical factor and inverse multinomial propensity scores as weights regarding the survey character of the study.
For all Cox models, the proportional hazards assumption had been verified by non-significant deviations from the proportional hazards assumption using chi-square test.
As statistical programmes, we used IBM SPSS Statistics for Windows, Version 24.0 (Armonk, NY: IBM Corp.) and Stata Statistical Software, Release 15 (College Station, TX: StataCorp LP.), R package twang version 1.5.
3 RESULTS
The 12 months continuous treatment cohort included 664 RRMS patients: 315 in the fingolimod, 232 in the DMF and 117 in the teriflunomide group. The baseline data of the 664 patients are summarized in Table 1 and show certain imbalances for some baseline variables.
|
Fingolimod N = 315 |
DMF N = 232 |
Teriflunomide N = 117 |
All N = 664 |
|||
|---|---|---|---|---|---|---|
| Female | N | 210 | 161 | 73 | 444 | |
| % | 66.7% | 69.4% | 62.4% | 66.9% | ||
| Age* | Mean | 39.7 | 38.0 | 42.6 | 39.6 | |
| SD | 10.4 | 10.8 | 10.5 | 10.7 | ||
| Duration of MS at baseline (y)* | Mean | 9.5 | 7.0 | 9.3 | 8.6 | |
| SD | 7.7 | 7.2 | 8.6 | 7.8 | ||
| EDSS at baseline* | Mean | 2.5 | 1.7 | 2.0 | 2.1 | |
| SD | 1.7 | 1.3 | 1.5 | 1.5 | ||
| Relapse rate within 12 mo prior treatment start | Mean | 1.3 | 1.0 | 0.6 | 1.1 | |
| SD | 0.8 | 0.8 | 0.7 | 0.8 | ||
| Prior treatment** | Yes | N | 286 | 135 | 80 | 501 |
| % | 90.8% | 58.2% | 68.4% | 75.5% | ||
| Indication*** | A | N | 202 | NA | NA | 202 |
| % | 64.1% | 64.1% | ||||
| B | N | 113 | NA | NA | 113 | |
| % | 35.9% | 35.9% | ||||
| Follow-up in months | Mean | 11.6 | 11.4 | 11.5 | 11.5 | |
| SD | 1.0 | 1.0 | 1.0 | 1.0 | ||
| Estimated propensity score* | Mean | 0.630 | 0.446 | 0.298 | 0.507 | |
| SD | 0.195 | 0.176 | 0.151 | 0.221 | ||
- Abbreviations: ARR, annualized relapse rate; DMF, dimethyl fumarate; EDSS, Expanded Disability Status Scale; NA, not applicable; SD, standard deviation.
- * Comparison using Kruskal-Wallis test revealed P-value < 0.05.
- ** Comparison using Chi-Quadrat test revealed P-value < 0.05.
- *** Indication A = at least one relapse in the prior 12 mo despite treatment with either interferon beta or glatiramer acetate; indication B = at least two severe relapses in the prior 12 mo in treatment-naive patients.
Estimated mean annualized relapse rates (ARR) over 12 months from the GLM were 0.21 (95% CI: 0.13-0.28) for fingolimod, 0.20 (95% CI: 0.10-0.31) for DMF and 0.19 (95% CI: 0.08-0.30) for teriflunomide treatment, leading to an incidence rate ratio (IRR) of 1.01 for fingolimod vs DMF (95% CI: 0.53-1.94, P = 0.967) and an IRR of 0.92 for teriflunomide vs DMF (95% CI: 0.42-2.03, P = 0.844).
Estimated mean relapse counts from the GLM after the first 3 months were 0.03 (95% CI: 0.02-0.51) for fingolimod, 0.08 (95% CI: 0.02-0.15) for DMF and 0.01 (95% CI: 0.00-0.01) for teriflunomide, leading to an IRR of 0.41 for fingolimod vs DMF (95% CI: 0.16-1.05, P = 0.063) and an IRR of 0.06 for teriflunomide vs DMF (95% CI: 0.01-0.46, P = 0.008), showing already a treatment effect within the first three months of treatment for all drugs, but more pronounced for teriflunomide and fingolimod.
62 patients treated with fingolimod (19.7%) experienced a relapse in the 12 months period, and respective frequencies were 33 for those treated with DMF (14.2%) and 15 for teriflunomide (12.8%), with a HR = 1.20 for fingolimod vs DMF (95% CI: 0.67-2.15, P = 0.540) and 0.95 for teriflunomide vs DMF (95% CI: 0.47-1.91, P = 0.876) (Figure 1).
Mean EDSS change in the fingolimod group was −0.05 (worsening) (95% CI: −0.16 to 0.07) vs 0.08 (improvement) (95% CI: 0.01-0.15) for DMF with an average treatment effect (ATE) (fingolimod vs DMF) of −0.13 (95% CI: −0.26 to 0.01, P = 0.064) and 0.03 (improvement) for teriflunomide (95% CI: −0.07 to 0.13) vs 0.08 (improvement) (95% CI: 0.01-0.15) for DMF with an ATE (teriflunomide vs DMF) of −0.05 (95% CI: −0.17 to 0.08, P = 0.447).
A significant difference was found analysing sustained EDSS progression for 12 weeks concerning fingolimod vs DMF (HR: 2.94, 95% CI: 1.12-7.72; P = 0.028), and the same trend was observed analysing sustained EDSS progression for 24 weeks fingolimod vs DMF (HR: 3.31, 95% CI: 0.85-12.99; P = 0.085). No significant difference was observed regarding sustained EDSS progression for 12 weeks and 24 weeks between teriflunomide and DMF (HR: 2.16, 95% CI: 0.64-7.28; P = 0.213 and HR: 3.29, 95% CI: 0.69-15.78; P = 0.136) (Figure 2A,B), also seeing a trend towards a reduced EDSS progression with DMF.
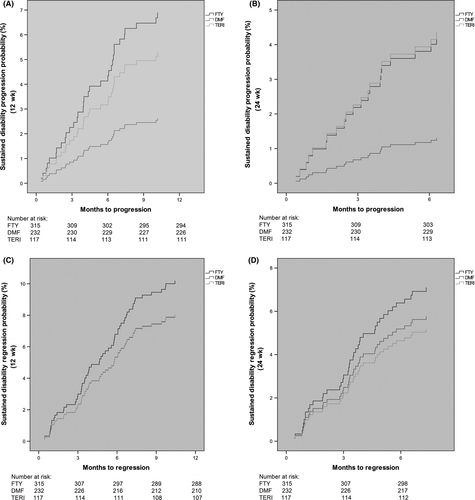
No significant differences were seen analysing sustained EDSS regression for 12 and 24 weeks comparing fingolimod vs DMF (HR: 1.05, 95% CI: 0.53-2.07; P = 0.897 and HR: 1.12, 95% CI: 0.49-2.59; P = 0.784) and teriflunomide vs DMF (HR: 1.01, 95% CI: 0.45-2.25; P = 0.981 and HR: 0.96, 95% CI: 0.36-2.52; P = 0.931) (Figure 2C,D).
For the total cohort, 1165 RRMS patients were included. The baseline data of these 1165 patients (517 with fingolimod, 426 with DMF and 222 with teriflunomide) are summarized in Table 2 and again show a certain imbalance for some baseline variables.
| Baseline therapy | ||||||
|---|---|---|---|---|---|---|
|
Fingolimod N = 517 |
DMF N = 426 |
Teriflunomide N = 222 |
All N = 1165 |
|||
| Female | N | 351 | 291 | 139 | 781 | |
| % | 67.9% | 68.3% | 62.6% | 67.0% | ||
| Age* | Mean | 39.3 | 37.3 | 42.6 | 39.2 | |
| SD | 10.5 | 10.7 | 10.5 | 10.7 | ||
| Duration of MS at baseline (y)* | Mean | 9.4 | 6.8 | 8.3 | 8.3 | |
| SD | 7.6 | 7.8 | 7.7 | 7.8 | ||
| EDSS* at baseline* | Mean | 2.4 | 1.7 | 2.0 | 2.1 | |
| SD | 1.6 | 1.3 | 1.4 | 1.5 | ||
| Relapse rate within 12 mo prior treatment start* | Mean | 1.35 | 0.99 | 0.67 | 1.09 | |
| SD | 0.87 | 0.81 | 0.71 | 0.86 | ||
| Prior treatment** | yes | N | 451 | 236 | 149 | 836 |
| % | 87.2% | 55.4% | 67.1% | 71.8% | ||
| Indication*** | A | N | 323 | NA | NA | 323 |
| % | 62.5% | 62.5% | ||||
| B | N | 194 | NA | NA | 194 | |
| % | 37.5% | 37.5% | ||||
| Follow-up in months* | Mean | 21.71 | 16.18 | 19.16 | 19.20 | |
| SD | 12.06 | 9.81 | 11.17 | 11.38 | ||
| Estimated propensity score* | Mean | 0.5831 | 0.4613 | 0.2921 | 0.4831 | |
| SD | 0.1946 | 0.1786 | 0.1399 | 0.2091 | ||
- Abbreviations: ARR, annualized relapse rate; DMF, dimethyl fumarate; EDSS, Expanded Disability Status Scale; NA, not applicable; SD, standard deviation.
- * Comparison using Kruskal-Wallis test revealed P-value < 0.05.
- ** Comparison using Chi-Quadrat test revealed P-value < 0.05.
- *** Indication A = at least one relapse in the prior 12 mo despite treatment with either interferon beta or glatiramer acetate; indication B = at least two severe relapses in the prior 12 mo in treatment-naive patients.
107 (20.7%) patients interrupted fingolimod treatment (55 stopped, 15 paused and 37 switched), 67 (15.7%) patients DMF (43 stopped, 4 paused and 20 switched) and 42 (18.9%) patients teriflunomide (17 stopped, 2 paused and 23 switched).
The hazard ratios for treatment interruption comparing fingolimod vs DMF were 1.03 (95% CI: 0.72-1.48; P = 0.866) and 1.07 comparing teriflunomide vs DMF (95% CI: 0.69-1.66), P = 0.770 (Figure 3).
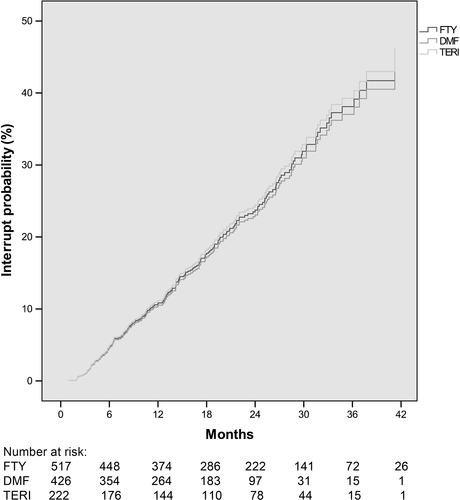
Mean time period until treatment interruption was 14.2 months (SD 9.4) for fingolimod, 14.7 months (SD 8.2) for DMF and 13.4 months (SD 9.8) for teriflunomide.
Thirty-seven patients switched from fingolimod (7.2%) to natalizumab (n = 18), to DMF (n = 10), to alemtuzumab (n = 7) or to teriflunomide (n = 2). Twenty patients switched from DMF (4.7%) to fingolimod (n = 8), to natalizumab (n = 8), to alemtuzumab (n = 2) or to teriflunomide (n = 2) and 23 patients from teriflunomide (10.4%) to fingolimod (n = 10), to natalizumab (n = 7) or to DMF (n = 6).
The reasons for interrupting fingolimod were mainly adverse events (AEs) (n = 51), patient's wishes (patient's decision) (n = 48) and disease progression (clinical and/or radiological activity) (n = 40), for DMF patients’ wishes (n = 37), AEs (n = 22) and disease progression (n = 18). The main reasons for interrupting teriflunomide were patients’ wishes (n = 20), followed by disease progression (n = 19) and AEs (n = 15). Pregnancy or the wishes to conceive were documented in either nine patients in the fingolimod and DMF group and in one patient in the teriflunomide cohort. The treating neurologist could name several reasons per patient.
The ARR for patients staying on treatment over the whole observation period (19.2 months, SD 11.4) was 0.19 (SD 0.46) for fingolimod, 0.14 (SD 0.48) for DMF and 0.15 (SD 0.45) for teriflunomide and for patients with treatment interruption 0.41 (SD 0.8) under fingolimod, 0.61 (SD 1.06) under DMF and 0.60 (SD 1.04) under teriflunomide until interruption.
The ARR after switching to another treatment or restarting after a treatment interruption stayed low in all treatment groups (fingolimod 0.17 (SD 0.46), DMF 0.09 (SD 0.40) and teriflunomide 0.12 (SD 0.33). Mean washout period or treatment pause were 3.4 (SD 3.7) months for fingolimod, 3.9 (SD 5.7) months for DMF and 2.7 (SD 2.6) months for teriflunomide. Mean observation period after treatment switch or restart was 10.6 (SD 9.9) months for fingolimod, 8.4 (SD 7.8) months for DMF and 12 (SD 9.5) months for teriflunomide.
4 DISCUSSION
In this observational study with prospectively collected data, we compared the efficacy of fingolimod, DMF and teriflunomide in 664 patients. All treatments were associated with a lower ARR in comparison with the year prior to either therapy. Fingolimod reduced ARR from 1.3 to 0.21, DMF from 1.0 to 0.2 and teriflunomide from 0.6 to 0.19.
We found no significant difference analysing the estimated mean ARR over 12 months from the GLM. In contrast, estimated mean ARR after the first 3 months from the GLM were 0.03 for fingolimod, 0.08 for DMF and 0.01 for teriflunomide leading to an IRR of 0.41 for fingolimod vs DMF (P = 0.063) and of 0.06 for teriflunomide vs DMF (P = 0.008), showing already an early treatment effect for all therapies, but more pronounced for teriflunomide and fingolimod. This early treatment effect under fingolimod was already shown in other studies,18, 19 and the low ARR under teriflunomide in the first three months of treatment is mainly attributed to the lower ARR of the teriflunomide cohort at baseline.
The hazard ratio for relapse probability in the 12 months continuous treatment period was 1.20 for fingolimod vs DMF and 0.95 for teriflunomide vs DMF, without significant differences (P = 0.540 and P = 0.876). In addition, we found no difference analysing EDSS change, EDSS progression or regression, except regarding reduced sustained EDSS progression for 12 weeks concerning DMF vs fingolimod (P = 0.028).
The different indications resulted in differences in the cohorts at baseline. In particular, the teriflunomide group was older and less likely to have had a relapse in the prior 12 months. Over 90% of the fingolimod patients had received prior treatment as compared to only 58% of the DMF and 68% of the teriflunomide cohort. In contrast, DMF patients were younger and less disabled with a shorter disease duration. Being fully aware of the documented differences, we used inverse probability weighting to control these differences.
A further limitation concerns the observation period over 12 months in terms of efficacy analysis. In comparison with two studies with an observation period of 24 months and another study with an observation period of 12 months, we found similar relapse rates.10-12 There were no substantial differences regarding the 12 and 24 months analyses. The mean time to discontinuation ranged from 4 to 10 months indicating highest disease activity within the first 12 months.
Our results are within the range of the findings of other observational studies using propensity scores for matching baselines covariates.10-12 Vollmer et al10 compared 271 fingolimod and 342 DMF patients over 2 years and found no significant difference in efficacy, including relapses, contrast enhancement or new T2 lesions on brain MRI. Hersh et al12 also showed in 395 DMF and 264 fingolimod patients comparable clinical efficacy, overall brain MRI activity but increased Gadolinium-enhancing lesions after treatment initiation with DMF. Boster et al20 analysed a large health insurance claims database (n = 6372) and observed similar effectiveness between DMF- and fingolimod-treated patients.
NNT for preventing one relapse within a 2-year treatment period was similar for DMF and teriflunomide, with marginally lower NNT observed with fingolimod. By contrast, for relapses requiring hospitalization, NNT was substantially lower for teriflunomide compared with DMF. For fingolimod, there were inconsistent outcomes between the two pivotal studies for relapses requiring hospitalization; thus, comparative conclusions against DMF or teriflunomide could not be clearly established. NNTs to prevent one patient from experiencing disability worsening were similar in DEFINE, FREEDOMS, TEMSO and TOWER but were higher in CONFIRM and FREEDOMS II.9
In addition, the hazard ratio for treatment interruption comparing fingolimod vs DMF was 1.03 (P = 0.866) and 1.07 comparing teriflunomide vs DMF (P < 0.770). 107 (20.7%) patients interrupted treatment from fingolimod, 67 (15.7%) patients from DMF and 42 (18.9%) patients from teriflunomide.
The main reason for interrupting fingolimod and DMF were adverse events, but for teriflunomide disease progression, resulting in a higher switching rate in the teriflunomide cohort as compared to fingolimod- and DMF-treated patients.
These results are in contrast to Vollmer et al,10 who found a lower discontinuation rate for fingolimod (34.3%) vs DMF (47.1%), which was driven by adverse events. Hersh et al12 also reported a higher likelihood of early discontinuation of DMF (41.3% vs 35.6%), mostly due to again adverse events. The lower discontinuation rate in our study is, first, caused by the shorter observation period (19 vs 24 months) and, secondly, attributed to an overall increased adherence in the Austrian MS centres.
The strengths of our study are that this work represents data from a nationwide observational study comprising patients in Austria who have been treated with fingolimod, DMF and teriflunomide since 2014. The AMSTR is a secure web-based platform, which enables treating neurologists in all Austrian MS centres to perform immediate online documentation during patient visits. To ensure high documentation and data quality in terms of completeness and plausibility, the AMSTR is monitored by an external and independent clinical research organization. These real-world data show a low ARR, progression rate and discontinuation rate for all three oral drugs reflecting a high-quality maintenance of multiple sclerosis patients in Austria.
Observational studies, like our study, miss the two most important parts of randomized clinical trials, that is randomization and blinding. However, it is meanwhile to some extent agreed that propensity scoring can compensate to a certain degree for the lack of randomization and may control for known and recorded confounding covariates.21 In everyday clinical practice, however, patients’ and neurologists’ attitudes towards the choice of treatment may be influenced by different non-recordable clinical or subclinical conditions that also may influence future disease activity.
The lack of blinding here may not have the same effect as in placebo-controlled trials, because our study was a post hoc study, which was not planned at the time of the prospective data collection, and therefore, patients and treating neurologists could not be aware that their medical recordings would be used for analysis.
The most important limitation of our study is the missing MRI data during the observational period. MRI data were only available at baseline before starting treatment with fingolimod, DMF and teriflunomide and were included as an independent variable for propensity score matching.
In conclusion, we found no difference analysing ARR, probability for experiencing a relapse, EDSS change, treatment interruption, EDSS progression and EDSS regression, except regarding reduced sustained EDSS progression for 12 weeks concerning DMF vs fingolimod (P = 0.028).
ACKNOWLEDGEMENTS
The Steering Group wishes to thank all Austrian MS centres for contributing data to the registry and to the patients for providing written informed consent.
CONFLICTS OF INTEREST
Michael Guger received support and honoraria for research, consultation, lectures and education from Almirall, Bayer, Biogen, Celgene, Genzyme, MedDay, Merck, Novartis, Octapharma, Roche, Sanofi Aventis, Shire and TEVA ratiopharm. Christian Enzinger received funding for travel and speaker honoraria from Biogen, Bayer Schering, Merck Serono, Novartis, Roche, Shire, Genzyme and Teva Pharmaceutical Industries Ltd./Sanofi Aventis, research support from Merck Serono, Biogen and Teva Pharmaceutical Industries Ltd./Sanofi Aventis and serving on scientific advisory boards for Bayer Schering, Biogen, Merck Serono, Novartis, Roche and Teva Pharmaceutical Industries Ltd./Sanofi Aventis. Fritz Leutmezer has received funding for travel and speaker honoraria from Biogen, Bayer Schering Pharma, Merck Serono, Novartis, Genzyme, Santhera and Teva Pharmaceutical Industries Ltd./Sanofi Aventis. Jörg Kraus received consulting and/or research funding and/or educational support from Almirall, Bayer, Biogen, Celgene, MedDay, Medtronic, Merck, Novartis, Roche, Sanofi Aventis, Shire and TEVA ratiopharm. Stefan Kalcher declares that there is no conflict of interest. Erich Kvas declares that there is no conflict of interest. Thomas Berger has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for multiple sclerosis: Almirall, Bayer, Biogen, Biologix, Bionorica, Genzyme, MedDay, Merck, Novartis, Octapharma, Roche, Sanofi/Genzyme, TG Pharmaceuticals, TEVA ratiopharm and UCB. His institution has received financial support in the last 12 months by unrestricted research grants (Biogen, Bayer, Merck, Novartis, Sanofi/Genzyme and TEVA ratiopharm) and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, Merck, Novartis, Octapharma, Roche, Sanofi/Genzyme and TEVA.




