Utility of exome sequencing in primary spermatogenic disorders: From research to diagnostics
Abstract
Background
Primary spermatogenic disorders represent a severe form of male infertility whereby sperm production is impaired due to testicular dysfunction, leading to reduced quality or quantity of spermatozoa. Gene-centered research has certainly demonstrated the importance of the genetic factor in the etiology of both poor sperm morphology or motility and reduced sperm count. In the last decade, next-generation sequencing has expanded the research to whole exome which has transformed our understanding of male infertility genetics, but uncertainty persists in its diagnostic yield, especially in large unrelated populations.
Objective
To evaluate the utility of exome sequencing in detecting genetic factors contributing to various traits of primary spermatogenic disorders, which is a crucial step before interpreting the diagnostic yield of the platform.
Materials and Methods
We manually curated 415 manuscripts and included 19 research studies that predominantly performed whole exome sequencing in cohorts of unrelated cases with primary spermatogenic defects.
Results
The detection rate, defined as the fraction of cases with an identifiable genetic cause, typically remained below 25% for quantitative defects of spermatozoa, whereas improved rates were observed for traits of abnormal sperm morphology/motility and in populations enriched with consanguineous families. Unlike the quantitative defects, the genetic architecture of the qualitative issues of spermatozoa featured a small number of recurrent genes describing a large fraction of studied cases. These observations were also in line with the lower biological complexity of the pathways affected by the reported genes.
Discussion and Conclusions
This review demonstrates the variability in detection rates of exome sequencing across semen phenotypes, which may have an impact on the expectations of the diagnostic yield in the clinical setting.
1 INTRODUCTION
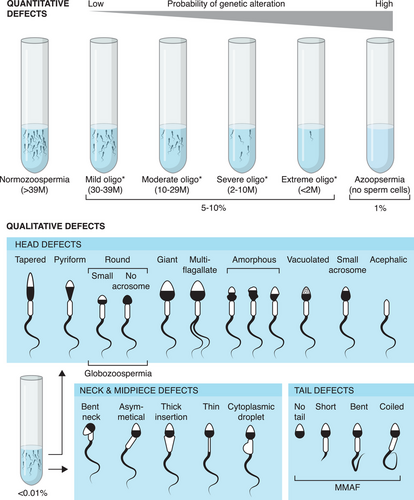
Infertility is a major health concern in the world affecting nearly 15% of couples attempting to conceive a child.1 Male factor infertility, accounting for approximately 50% of the cases, can be caused by hypothalamic-pituitary axis dysfunction, ductal obstruction or dysfunction, and quantitative or qualitative spermatogenic defects.2 Quantitative defects refer to reduced counts of spermatozoa in the ejaculate, whereas qualitative defects involve various morphological abnormalities of the spermatozoa (Figure 1). It has been shown that genetics plays a pivotal role in the etiology of male infertility and currently, the clinical diagnostic work-up includes i) analysis of karyotype and Y-Chromosome microdeletions (AZF regions) in patients with quantitative spermatogenic defects; ii) mutation screening of the CF transmembrane conductance regulator (CFTR) gene in patients with obstructive azoospermia; iii) sequencing of a gene panel involving a limited number of genes associated with hypogonadotropic hypogonadism in patients with hypogonadal-pituitary defects; iv) screening of mutations in dpy-19 like 2 (DPY19L2) and aurora kinase C (AURKC) genes in patients affected by globozoospermia and sperm macrocephaly, respectively.1 Nevertheless, many patients remain idiopathic, in particular those affected by quantitative defects of spermatogenesis, accounting for almost 50% of cases. During the last decade, next-generation sequencing, predominantly whole-exome sequencing (WES) which targets the coding sequences of the genome, has successfully been used for the identification of hundreds of genes involved in the etiology of spermatogenic defects.3 Application of WES can be equally informative when incorporated into routine diagnostic protocols, however, its usefulness is likely to vary across different phenotypes of spermatogenic disorders. In order to assess the diagnostic value of WES, it is crucial to ascertain its detection rate referring to the fraction of cases carrying rare variants predicted to be pathogenic by different in silico tools. Although hundreds of genes have been linked to the disorders of primary spermatogenic defects, the majority of the WES-based studies focus on singleton genes or cases, making it difficult to navigate the results and draw conclusions on the utility of WES as a diagnostic tool. In this review, we summarize the current advancements of WES in mapping the known and novel genetic etiologies linked to quantitative and qualitative defects of primary spermatogenic disorders based on large-scale WES studies. Specifically, we aim to assess the detection rate of genetic factors contributing to these traits.
2 MATERIALS AND METHODS
2.1 Literature search
This review aimed to identify studies that systematically explore the genetic basis of primary spermatogenic impairment using the next-generation sequencing (NGS) approach. A literature search for studies published before and including Dec 31, 2023, was performed based on the PubMed electronic database. The search terms included different phenotypic traits of quantitative (“azoospermia”, “cryptozoospermia”, “oligozoospermia”, “maturation arrest”, “hypospermatogenesis”, “sertoli cell only”) and qualitative (“teratozoospermia”, “macrozoospermia”, “globozoospermia”, “acephalic spermatozoa syndrome”, “asthenozoospermia”, “asthenoteratozoospermia”, “oligoasthenoteratozoospermia”, “MMAF”, or “multiple morphological abnormalities of the sperm flagella”) defects of spermatozoa but also “spermatogenic failure” in combination with various next-generation sequencing designs (“whole exome sequencing”, “whole genome sequencing” and “next generation sequencing”), and excluding review publications. The resulting list of publications was manually curated to only include studies performed in the cohorts of unrelated cases or large collections of families of primary spermatogenic disorders and excluding obstructive azoospermia or any syndromic phenotypes such as disorders of sex development, reproductive endocrine disorders, and primary ciliary dyskinesia (PCD). However, studies involving cases of asthenozoospermia with or without PCD-like features were included. Studies reporting single genes and/or cases, few families, or analyzing CNV or Y chromosome microdeletions were subject to exclusion. Additionally, reports on targeted gene sequencing were excluded although some gene panel screenings in primary spermatogenic impairment are discussed. In this review, we did not re-evaluate the pathogenicity of the reported variants nor the evidence on the reported gene-disease relationships.
2.2 Visualization of disease-linked pathways
The list of genes linked to azoospermia and multiple morphological abnormalities of the flagella (MMAF) in mice was extracted from the Mouse Genome Informatics database (MGI; https://www.informatics.jax.org/) on Dec 1, 2023. The search terms “MP:0005159” for azoospermia and “multiple AND morphological AND abnormalities AND flagella” for MMAF yielded 237 and 60 genes, respectively. As the search for “non-obstructive azoospermia” in mice yielded no results, the search term corresponding to ‘azoospermia’ was used instead which includes a few genes linked to the syndromic disorders of spermatogenic failure. The ‘GO Biological Processes’ terms and ‘KEGG pathways’ linked to azoospermia and MMAF genes were visualized using a Cytoscape plug-in ClueGO.4 Only terms with at least three genes per term and with an enrichment p-value ≤0.05 (two-sided hypergeometric test with Bonferroni step-down correction) were included.
3 MAIN TEXT
3.1 The architecture of spermatogenic disorders
The primary spermatogenic disorders can largely be categorized into defects affecting either the quantity or the quality of the spermatozoa.
3.1.1 Quantitative defects
According to the diagnostic criteria of the World Health Organization, the total sperm count in the ejaculate can be categorized into normal count (> 39 million sperm cells), reduced sperm count (oligozoospermia < 39 million sperm cells), or no spermatozoa in the ejaculate. In case the lack of spermatozoa is due to a molecular cause originating from the testis, (i.e., primary spermatogenic failure), it is defined as non-obstructive azoospermia (NOA).5 Reduced sperm count affects around 5% of men worldwide, whereas 1% of men present NOA. “Oligozoospermia” is a broad term, ranging from a few spermatozoa up to 39 million, and therefore the following subcategories have recently been proposed based on rounded 25% and 5% percentile thresholds: i) extreme oligozoospermia (from > 0 to < 2 million; including the diagnostic term “cryptozoospermia”); ii) severe oligozoospermia (from > 2 to < 10 million); iii) moderate oligozoospermia (from > 10 to < 29 million); iv) mild oligozoospermia (from > 30 to < 39 million sperm)6 (Figure 1). Similarly, NOA can be heterogeneous based on the histopathological findings of testes. NOA patients can present an absence of germ cells in the testis (sertoli cell only [SCO]), maturation arrest (MA) of germ cells at different cell developmental stages (Spermatogonia, spermatocyte or spermatid), or hypospermatogenesis.
The genetic causes of quantitative defects are highly heterogeneous with hundreds of genes reported to be mutated.3, 7-9 With the exception of a small number of genes, the mutated genes typically follow the autosomal recessive or X/Y linked inheritance mode.3, 10, 11 It has been suggested that for NOA, as the most severe phenotype of spermatogenic defects, the genetic component may play a larger role in the manifestation of the disease as compared to oligozoospermia.12 However, clear distinctions between the genetic causes of NOA and oligozoospermia, particularly extreme oligozoospermia, are challenging to make, as the same mutation or mutations in the same gene have been found in both phenotypes.13-18
3.1.2 Qualitative defects
An excess of various qualitative defects of spermatozoa (≥4% of sperm cells in the ejaculate) due to morphological abnormalities that can affect the sperm head, neck, midpiece, or endpiece is defined as teratozoospermia,5 which includes acephalic spermatozoa syndrome (headless flagella; ASS), macrozoospermia (big-headed spermatozoa), globozoospermia (round-headed spermatozoa without acrosome) and MMAF among others. The latter is characterized by a mix of spermatozoa with short, absent, irregularly shaped, or coiled flagella that severely impair sperm motility. The teratozoospermia disorders are all considered fairly rare (< 0.01% of the male population) and follow an autosomal recessive inheritance pattern, although cases of X-linked MMAF have been identified via WES studies as well.
In cases where teratozoospermia occurs uniformly across all spermatozoa, affected by the same type of defect, that is, monomorphic or pure forms, genetic causes are more likely to be identified with respect to mild forms in which only a fraction of sperm cells are affected. This suggests a systemic genetic factor disrupting spermiogenesis as opposed to environmental stress and/or epigenetic alterations. The genetic landscape of teratozoospermia is heterogeneous and often specific to the phenotype. For instance, mutations in the AURKC gene are identified in most cases of pure sperm macrocephaly, while pure forms of globozoospermia are commonly associated with, but not limited to, mutations in the DPY19L2 gene.19 Similarly, ASS is a rare type of teratozoospermia with 30%–50% of the cases attributed to mutations in Sad1 and UNC84 domain containing 5 (SUN5) gene.20, 21 MMAF is a relatively new category in which the number of mutated genes, often belonging to the Dynein Axonemal Heavy Chain (DNAH) and Cilia and Flagella associated protein (CFAP) gene families, have significantly increased in recent years.3 This phenotype is mostly considered to be independent of the other types of asthenoteratozoospermia, such as PCD-related defects, although the phenotypic and genetic distinction between the two may remain complicated at times.22, 23
3.2 Overview of WES studies on primary spermatogenic disorders
This study aimed to evaluate the utility of exome sequencing in identifying potential causal variations underlying primary spermatogenic disorders in the research context. We specifically set out to estimate the detection rate of deleterious variation in each study. To this end, 415 manuscripts were extracted for manual curation of which 19 were included in this review (Table 1, Table S1). These represent WES-based studies that reported disease-linked variation in all of the genes prioritized in their cohorts of cases. Manuscripts reporting a small number of cases or families (up to n = 5) or single case reports represent the main research strategies employed in studying the genetics of primary spermatogenic disorders but constituted one of the major categories of exclusion (20% and 12% of curated publications, respectively; Table S1). These studies often report a small collection of carriers that present disruptions in the same gene, thus preventing an unbiased calculation of the detection rate.
| Reference | Trait | Semen phenotype | Testicular phenotype | # of cases analyzed | # of carriers | # of genes | Detection rate | Consanguineous cases among carriers (%) |
|---|---|---|---|---|---|---|---|---|
| Liu et al., 2021 | Qual | ASS | NA | 12 | 7 | 2 | 58.3 | NA |
| Liu et al., 2020 | Qual | ASS | NA | 11 | 3 | 3 | 27.3 | NA |
| Liu et al., 2020 | Qual | ASZ | NA | Forty-five families | Seven families | 2 | 15.6 | Yes (42.9) |
| Oud et al., 2021 | Qual | ASZ | NA | 21 | 16 | 12 | 76.2 | Yes (NA) |
| Oud et al., 2020 | Qual | GLOB | NA | 15 | 8 | 7 | 53.3 | Yes (87.5) |
| Li et al., 2021 | Qual | MMAF | NA | 67 | 32 | 12 | 47.8 | NA |
| Qual | UASH | NA | 82 | 23 | 21 | 28.3 | NA | |
| Liu et al., 2019 | Qual | MMAF | NA | 42 | 24 | 5 | 57.1 | No |
| Tang et al., 2017 | Qual | MMAF | NA | 30 | 23 | 5 | 76.7 | Yes (13) |
| Sha et al., 2019 | Qual | MMAF | NA | 27 | 19 | 3 | 70.4 | Yes (26.3) |
| Quarantani et al., 2023 | Qual | ASZ | NA | 8 | 2 | 2 | 25.0 | No |
| Quant | Oligo | NA | 32 | 2 | 2 | 6.3 | No | |
| Quant | NOA | Diverse | 51 | 4 | 4 | 7.8 | No | |
| Chen et al., 2020 | Quant | NOA | SCO, MA | 291 | 35 | 27 | 12.0 | NA |
| Quant | EO | NA | 23 | 0 | 0 | 0 | NA | |
| Alhathal et al., 2020 | Quant | NOA | Diverse | 237 | 62 | 52 | 26.2 | NA |
| Quant | SO | Diverse | 48 | 7 | 7 | 14.6 | NA | |
| Krausz et al., 2020 | Quant | NOA | MA | 17 | 14 | 12 | 82.0 | Yes (NA) |
| Oud et al., 2022 (de novo variation) | Quant | NOA | Diverse | 111 trios | 15 | 15 | 13.5 | NA |
| Quant | EO | Diverse | 41 trios | 9 | 9 | 22.0 | NA | |
| Quant | SO | Diverse | 33 trios | 5 | 5 | 15.2 | NA | |
| Tang et al., 2022 | Quant | NOA | Diverse | 55 | 6 | 4 | 10.9 | NA |
| Ghieh et al., 2022 | Quant | NOA | MA | 26 | 15 | 14 | 57.6 | Yes (46.7) |
| Wu et al., 2022 | Quant | NOA | MA, NA | Twelve families | Seven families | 7 | 58.3 | Yes (100) |
| Hardy et al., 2022 | Quant | Oligo | NA | 17 | 2 | 2 | 11.8 | NA |
| Quant | SO | NA | 42 | 5 | 4 | 11.9 | NA | |
| Quant | NOA | NA | 35 | 2 | 3 | 5.7 | NA | |
| Nagirnaja et al., 2022 | Quant | NOA | Diverse | 915 | 180 | 221 | 19.7 | Yes (30.6) |
| Quant | EO | NA | 9 | 0 | 0 | 0 | No |
- Abbreviations: ASS, acephalic spermatozoa syndrome; ASZ, asthenozoospermia; EO, extreme oligozoospermia; GLOB, globozoospermia; MA, maturation arrest; MMAF, multiple morphological abnormalities of the sperm flagella; NA, information not available; NOA, mon-obstructive azoospermia; Oligo, oligozoospermia; Qual, qualitative; Quant, quantitative, SCO, Sertoli cell only; SO, severe oligozoospermia; UASH, unexplained abnormal sperm head.
A large fraction of excluded studies focuses on the function of singleton genes (23%) that are often originally prioritized with the WES platform in a larger collection of cases (Table S1). For example, the cohorts of MMAF from North Africa/Middle East/Europe (n = 167) and China (n = 90) have extensively been analyzed and re-analyzed using WES during the last 6−7 years (see Martinez et al.24 and Gao et al.,25 respectively, and the references within). Similarly, large cohorts of patients affected by non-obstructive azoospermia and extreme oligozoospermia from China (∼500 cases) and Germany (∼1500 cases) have given rise to single gene publications, but have also been used as replication cohorts.26-31 Although these authors have predominantly opted for a publication strategy of focused single-gene reports and their studies were subject to exclusion in this review for this reason, they have nevertheless significantly expanded our understanding of the genetic etiologies underlying the primary spermatogenic defects. Many of the single-gene reports have extensively explored the functional impact of the gene of interest on spermatogenesis using molecular biology techniques and animal models, including Trypanosoma brucei32 and non-human primate,33 in addition to the traditional mouse model. Finally, targeted WES sequencing of a pre-selected list of genes (i.e. gene panels) based on their location on X-chromosome34 or previous association with infertility35, 36 were excluded from this review (Table S1).
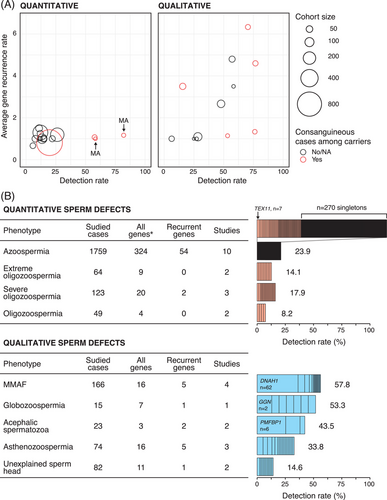
The final list of 19 publications that have used the WES platform and have reported all the results in a concise manner includes 10 studies on quantitative and nine on qualitative defects of spermatozoa (Table 1). As qualitative spermatogenic defects are generally rare in the population of men (Figure 1), the sample sizes in research studies have remained smaller as compared to the disorders of reduced sperm counts that affect more men. In the same line, sequencing of large cohorts of NOA and extreme oligozoospermia across different continents has allowed the identification of a large number of candidate genes as well as a few genes recurrently affected among cases (Table 1).37-39
All of the included studies utilized a WES-based approach followed by a fairly homogeneous analysis pipeline across the studies—filtering rare variation (typically MAF < 0.01 in public databases such as gnomAD) predicted to be deleterious by various public (e.g., CADD,40 MutationTaster,41 Polyphen,42 SIFT,43 REVEL,44 etc.) or in-house (PSAP) tools38 and factoring in the expression in testis when prioritizing genes. The studies have mainly addressed autosomal recessive and X/Y-linked variation with the exception of one study exploring de novo variation among NOA men.45 The fairly uniform approach to analyzing WES data and prioritizing variation allows us to draw comparative conclusions on the detection rates in different traits of spermatogenic disorders.
3.3 The detection rate in WES studies
In this review, the detection rate was defined as the fraction of studied cases in which a candidate genetic alteration has been identified. This definition differs from the more conservative estimate of the ‘diagnostic yield’, which represents the fraction of cases carrying deleterious disease variants defined as likely pathogenic or pathogenic according to the ACMG Interpreting Sequence Variant Guidelines.46 Comparing diagnostic yield across cohorts is challenging as the majority of research studies do not classify variants according to these guidelines and this is out of the scope of the current review. Of note, while the detection rate provides meaningful information from a research perspective, it is likely to be more optimistic as compared to the estimates of the diagnostic yield.
3.3.1 Quantitative defects of primary spermatogenic disorders
According to the current literature on the genetics of spermatogenic impairment, the etiology of most of the studied infertility traits is characterized by a monogenic inheritance pattern, whereby typically a single recessive or X-linked gene is implicated in each affected case or a family (reviewed in Kasak et al.8 and Vockel et al.7). The advantage of applying WES to cohorts of infertile men is the ability to screen all the coding variation in the genome at once ideally pinpointing a disease-causing gene in each case and providing a collection of disease-linked genes per phenotype. Nevertheless, even for large patient cohorts the fraction of cases with a detectable genetic lesion may remain low in some traits.
This phenomenon applies to the phenotypes of reduced sperm count, whereby, in the majority of reports, a disease-linked variation is typically identified in less than 25% of studied cases independent of the cohort size (range n = 8–924 cases) (Table 1 and Figure 2A). Interestingly, comparable detection rates are reported for both recessive or X-linked (average across cohorts, 21.7%) and de novo variation (average across phenotypes 16.9%) (Table 1). Oud et al.45 performed WES in a sample of 185 men with unexplained azoospermia or oligozoospermia and reported heterozygous or hemizygous de novo mutations in 29 possibly causative genes. Moreover, the authors identified significant enrichment of loss-of-function (LoF) and missense de novo mutations in genes that are intolerant to and functionally impacted by heterozygous deleterious variation in patients as compared to fertile controls. This is one of the rare examples suggesting the contribution of dominant variation in male infertility.45
It has been described for various human disease phenotypes that addressing homogeneous subtypes of a disease may be of benefit and yield improved detection rates in genome studies.47 In the context of NOA, patients can be subclassified according to their testicular histopathology as having SCO, MA, or hypospermatogenesis. Although the exact prevalence of each testicular phenotype in idiopathic NOA is not known, the majority of patients who undergo TESE present the testicular phenotype of SCO or hypospermatogenesis, whereas pure forms of idiopathic complete MA remain less common.48 Interestingly, the highest detection rates observed across WES studies of quantitative spermatogenic failure have been reported for men who present MA (Table 1 and Figure 2A). According to Krausz et al., as much as 82% (n = 14/17) of MA cases were attributed to a recessive genetic variation.18 The successful assignment of a potential genetic cause was achieved by a stringent prioritization of MA cases that additionally had consanguineous parents and/or family history of male or female infertility. Comparable success was reported by Ghieh et al., whereby recessive deleterious variants were identified in n = 15/26 (58%) men with MA at the spermatocyte stage, with a 100% detection rate observed among consanguineous (n = 7/7) and 42.1% (n = 8/19) among non-consanguineous MA cases.49 As the consequence of consanguinity, a recessive variation that is typically very rare or non-existent in a homozygous state in the general population is found in the background of large stretches of homozygosity in the genome and has commonly been reported in male infertility studies.8, 9 Similar tendencies were seen in the general NOA cohorts even without stratification by the testicular phenotype. In a WES study of 924 men with NOA, an improved detection rate of 76% was reported among 72 unrelated consanguineous cases as opposed to the 15% observed among the remainder of the men without any consanguineous background.38 In the same line, Wu et al. demonstrated based on a collection of 12 consanguineous families affected by NOA and premature ovarian insufficiency (POI) that WES analysis is able to identify recessive deleterious variation in known and novel genes linked to the disease in 58.3% of the families (n = 5/9 unrelated NOA men) (Table 1 and Figure 2A).50 An overlap in the genetic etiology between NOA and POI has previously been observed and suggests that a family history of POI may also be an indication of increased chances of heritable infertility among male members of the family, particularly in the presence of consanguinity.18, 51
3.3.2 Qualitative defects of primary spermatogenic disorders
Unlike quantitative sperm defects, the majority of the studies on the morphological abnormalities of the sperm report a disease-linked variation in over 25% of the cases. The phenotype of MMAF has benefited the most from WES analysis in recent years with four comprehensive studies included in this review (Table 1). According to these studies, a potential disease-linked genetic variation is found in over half of the cases, with up to 76.7% (n = 23/30) in Tang et al. (range across four studies 47.8%–76.7%) (Table 1 and Figure 2A).52 Numerous single-gene reports have been published based on the WES data from the two largest collections of MMAF cases from North Africa/Middle East/Europe (total up to 167 cases) and China (n = 90), however a comparable overall detection rate of 51.5% and 60.0% has been mentioned for their cohorts, respectively.24, 25 These results are largely powered by the consanguineous background of the cases based on these single-gene reports (see references within Martinez et al.24), as also observed in studies of men diagnosed with NOA (Figure 2A). A single comprehensive WES study by Oud et al. of patients with globozoospermia without mutations in DPY19L2 or spermatogenesis associated 16 (SPATA16), was similarly able to detect a candidate disease variant in 8/15 (53.3%) cases with 100% detection rate among seven men from consanguineous families and 12.5% (n = 1/8) among the remaining non-consanguineous cases.53
As acephalic spermatozoa syndrome is a rare teratozoospermia phenotype (< 0.01%), the case cohorts have remained fairly small (n = 11, 12) and are able to ascertain a recessive disease-linked variation to 27.3%–58.3% of the cases (Table 1).54, 55 Li et al. have analyzed a fairly large group of patients (n = 82) categorized as “unexplained abnormal sperm head phenotypes”, which includes abnormal spermatozoa with globozoospermia, small or deformed acrosome, pyriform-headed, amorphous-headed, small-headed and tapered-headed (Figure 1). The authors report a low detection rate of 14.6% (n = 12/82) based on the affected genes directly related to the phenotype, likely due to a short list of known genes linked to the underlying etiology.56 A varying success in the identification of a potential disease gene is evident in studies on asthenozoospermia, which features a severe reduction in sperm motility and may include a mosaic of cases that present MMAF and PCD-like symptoms, such as chronic sinus congestion or chronic respiratory disease (Table 1).57, 58
3.4 The comparative biological complexity of the spermatogenic disorders
The success of identifying a disease-linked variation is dependent on the intersection of the characteristics of the studied disease cohort (size, consanguinity, and homogeneity) and the genetic heterogeneity of the biological systems that grant functional spermatogenesis. Large heterogeneity leads to the necessity of larger sample sizes to reliably and recurrently discover novel genes leading to missing or dysfunctional spermatozoa that could ultimately be considered as being diagnostic.
3.4.1 Quantitative defects of primary spermatogenic disorders
The genetic causes of reduced sperm count form a complex list with each case typically presenting a unique disease-linked variation. For NOA and reduced sperm count combined, 443 out of 1995 cases carry variants in a total of 351 unique genes, of which the majority represent singleton observations (Figure 2B and Table S2). Among the genes affected in more than two carriers in the studies included in this review (AR, CCDC89, CEP250, DCAF12L1, DMRT1, FBXO15, KASH5, KLHL10, MAGEE2, MEI1, PNLDC1, SHOC1, SYCE1, TDRD9, TERB1, TEX11, TEX14, TTLL9, and ZNF711), most have been associated with NOA due to MA at the meiotic stage of spermatogenesis. The gene of most observations is X-linked testis expressed 11 (TEX11), a crucial factor in chromosomal synapsis, for which partial gene deletions were first linked to azoospermia due to meiotic arrest at pachytene stage by Yatsenko et al.59 Subsequently, multiple patients carrying variants in this gene have been reported, with seven of them included in this review (Figure 2B).18, 37, 39, 49, 60 Interestingly, 46/1995 patients with reduced sperm counts (n = 10 studies) carry variants in more than one gene potentially pointing to a di/oligogenic disease model. However, further functional and facility studies are warranted to validate this scenario. It is noteworthy that 62 patients (16%) carry variants in X-linked variants highlighting the relevance of genes located in this chromosome in the etiology of quantitative disturbances of spermatogenesis. This is in line with a targeted WES study of X-linked genes in 2354 patients with non-obstructive azoospermia or extreme oligozoospermia, where authors reported deleterious variants in 11.9% of cases, with RB binding protein 7, chromatin remodeling factor (RBBP7), which is important in chromatin regulation and metabolism, being the most frequently affected gene observed in 10 NOA men.34 This study underscored the significant role of the X chromosome in quantitative defects.34 Of note, although the findings reported here largely mirror what is known based on published literature, the spectrum of genes and the number of patients carrying variants in the same gene are likely underestimated, as many single-gene centered and family studies that have reported recurrent observations of an affected gene have been excluded from this review.
3.4.2 Qualitative defects of primary spermatogenic disorders
Contrary to the quantitative defects of spermatogenesis, the morphological abnormalities of the sperm predominantly feature a small number of genes responsible for a large fraction of the studied cases (Figure 2B and Table S2). On average, the genes are typically observed among cases recurrently resulting in a higher detection rate indicating that disruptions of a limited number of pathways lead to the morphological abnormalities of the spermatozoa (Figure 2). As the most striking example, DNAH1 alone is responsible for 37.3% (n = 62/166) of MMAF cases across four WES studies, followed by 12.7% of cases with deleterious variants in three CFAP family genes CFAP43, 44 and 65 (n = 10, 7 and 4 carriers, respectively). Members of both DNAH and CFAP families are essential for the axonemal organization of cilia. These findings are in line with the known genetic etiology of MMAF, whereby 28%−44% of MMAF cases are attributed to DNAH1.52 As an added value of WES, 11 novel case-specific genes, of which six belong to the DNAH family, have been implicated in MMAF in these studies. As an extreme manifestation of morphological sperm abnormalities, acephalic spermatozoa syndrome represents a disorder in which the majority of sperm cells in the ejaculate are headless due to defects of the head-tail coupling apparatus. Although SUN5 has been proposed as the main gene responsible for the phenotype (about 40% of the cases), only 1/10 cases carry SUN5 mutations across two WES studies incorporated in this review, compared to 6/10 cases with disruptions in polyamine modulated factor 1 binding protein 1 (PMFBP1) (Figure 2B and Table S2).21, 54, 55 For globozoospermia, recessive deletions in DPY19L2 describe most of the cases with pure forms of the disease, as extensively demonstrated previously.61 Nevertheless, WES could potentially provide a diagnosis in half of the cases without the DPY19L2 deletions, as reported for a cohort of 15 men (n = 7/15 with consanguinity) by Oud et al. (Table 1 and Figure 2B).53 Larger genetic complexity is observed for asthenozoospermia but also for the collection of cases with unexplained abnormal sperm head anomalies, partially due to the phenotypic heterogeneity of the cohorts.
Of note, 10 genes (CFAP44, DNAH1, DNAH5, DNAH6, FSIP2, MAGEA3, MDC1, PACRG, QRICH2, and TDRD9) reported in this review have been linked to both qualitative and quantitative spermatogenic defects. Interestingly, mRNA expression of all of these genes has been detected not only in late spermatids but also in early spermatogenic cells.62 In fact, a significant proportion of patients with qualitative defects also show reduced sperm counts, a semen phenotype called oligoasthenoteratozoospermia (OAT), indicating a detectable overlap between the genetic etiologies of the two phenotype categories of primary spermatogenic disorders.
3.4.3 Comparative view of biological pathways
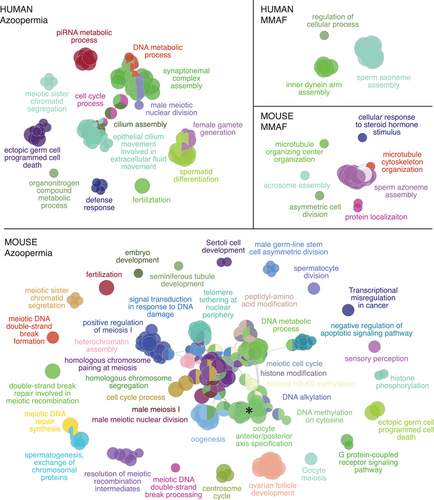
The observed differences in the genetic architecture of the quantitative and qualitative abnormalities of sperm point to the varying levels of heterogeneity in the biological pathways that are essential for the accurate completion of spermatogenesis. This is clearly highlighted when comparing NOA and MMAF, as the most studied disease categories in quantitative and qualitative sperm defects, respectively (Figure 3). The genes that were identified among NOA cases by the WES platform (n = 324) affect a wide array of biological processes most of which are known to be relevant for germ cell survival. These include, for example, pathways regulating meiotic division (e.g., synaptonemal complex assembly or meiotic sister chromatid segregation) and metabolism of piRNAs, disruption of which leads to the MA of spermatogenesis.63 On the other hand, only pathways related to cilium assembly dominate in MMAF, which is characterized by a high detection rate and a short list of recurrently affected genes (total, 16 genes; Figure 3). Although the progression of spermatogenesis partly differs in men and mice,64 the comparative view of azoospermia and MMAF in mice largely mirrors the differences in genetic complexity observed in men (Figures 2B and 3). These findings indicate that the larger list of genes linked to NOA, as opposed to MMAF (n = 324 and n = 16, respectively), across the WES studies in men is not purely due to differences in studied sample sizes (n = 1759 and n = 166 cases total, respectively).
4 DISCUSSION
The objective of this review was to explore the utility of WES-based tools in mapping the genetic variation in different subtypes of quantitative and qualitative defects of spermatogenesis in the context of research studies, which in turn is a precursor for estimating the diagnostic value of the method. For generating a reliable diagnostic output of WES studies, it would be imperative to systematically evaluate all described genes for their gene-disease relationships and classify the variants falling within these genes with at least moderate evidence for being causal in male infertility according to the ACMG guidelines.46 The most recent systematic review of the Gene-Disease Relationship includes data published until July 2020.3 Over the past 3 years, there has been a significant increase in publications reporting genetic alterations in infertile men. Notably, the majority of the systematic WES studies included here (n = 17/19) have been performed only within the last 3 years (Table 1) hinting that larger in-depth research on primary spermatogenic disorders is yet to come.
Recent studies exploring targeted sequencing of gene panels and whole exome sequencing highlight promising yet varied diagnostic outcomes in individuals with primary spermatogenic disorders. The diagnostic workup of the quantitative defects of spermatogenesis currently includes karyotype and AZF deletion analysis which only provides a genetic diagnosis in up to 15% of cases.1 A few studies have performed a targeted sequencing of gene panels providing first insights into the utility of the WES platform as another diagnostic tool. A study by Kherraf et al.35 performed WES in 96 NOA men to search for candidate variants in 151 pre-selected NOA candidate genes identifying the likely cause of the phenotype in 23% cases. Furthermore, Wyrwoll et al.36 sequenced a gene panel comprising 60 genes previously associated with quantitative defects in spermatogenesis in a cohort of 647 men. They reported variants classified as likely pathogenic or pathogenic according to ACMG guidelines in 5.4% of patients with spermatogenic failure, the closest representation of the diagnostic rate if targeted gene sequencing were to be applied as a diagnostic tool for NOA at this date. Although WES holds a significant promise for improving diagnostic outcomes in this group of patients, the low detection rate (< 20%) in the cohort of sporadic cases with unknown testis histology and without consanguinity or familiar history of male/female infertility is a point of concern. On the other hand, for the morphological abnormalities of the spermatozoa with a very specific biological cause and a restricted list of main causative genes, both a custom NGS panel and exome sequencing would prove informative, with the latter having an added value of identifying pathogenic variation in rare or novel disease genes. Particularly in the context of MMAF for which there are no guidelines for genetic testing in many countries to aid the counseling of the affected patients.57
Whole-genome sequencing (WGS) studies on large male infertility cohorts would soon enable us to expand beyond the coding variation and improve the detection of copy number variants that disrupt spermatogenesis. However, pinpointing the causative genetic lesion remains challenging even in exome-based studies. Most of the evaluated WES reports use testis-specific expression as one of the gene prioritization strategies, even though 85% of all human genes are known to be expressed in the testis.65 In addition to comprehensive functional studies on singleton genes, novel integrated catalogs of genome function, such as by Impact of Genomic Variation on Function (IGVF) Consortium,66 present promising tools for broadening our comprehension of the functional consequences of genome perturbations and ultimately improving the interpretation of the WES and WGS findings in the disease context.
While a precise characterization of disease phenotypes can notably enhance the diagnostic yield of a WES analysis, as demonstrated for the MA subtype of NOA patients, many infertile men seen in clinical settings exhibit a blend of morphological abnormalities and decreased sperm count or may transition between phenotypes over their lifetime. This phenotypic continuum extends into the genetic findings, whereby the same gene may be responsible for both the functional and structural integrity of the spermatozoa. Kherraf et al. identified homozygous mutations in the serine peptidase inhibitor Kazal type 2 (SPINK2) gene, vital for acrosome biogenesis, in two azoospermic siblings, with subsequent mouse studies also noting OAT in heterozygous carriers.67 In the same line, dual functions have been reported for the dynein gene axonemal dynein light chain domain containing 1 (AXDND1) gene, originally implicated in NOA in men.68 Disruption of AXDND1 causes issues with sperm tail development and function and ultimately leads to germ cell loss via an imbalance in spermatogonial commitment in mice.69 Although the progression of spermatogenesis differs between men and mice, the overlapping genetic space between various disease entities of primary spermatogenic disorders is likely to persist in men.69 Concurrently, based on the WES studies reviewed here, 10 genes were disrupted in both quantitative and qualitative defects of spermatogenesis. These include examples of genes essential for the structural organization of cilia (CFAP44, DNAH1, DNAH5, DNAH6, and FSIP2) but also genes determining the meiotic progression and survival of germ cells (MDC1, TDRD9, and MAGEA3).
In summary, the detection rate of exome sequencing varies among various semen phenotypes, with higher rates observed in qualitative compared to quantitative defects. This review represents an initial exploration into the application of exome sequencing in male infertility from a clinical standpoint. Future research should focus on assessing the diagnostic yield by determining the diagnostic value of the identified genes and variants.
AUTHOR CONTRIBUTIONS
Both authors contributed to the concept and design, research of the literature, analysis of the data, and writing of the manuscript.
ACKNOWLEDGMENTS
We are grateful to Dr. Katinka Vigh-Conrad for her assistance in substantially improving the design of the figure. ARE is supported by grants R01HD078641 and P50HD096723 from the National Institutes of Health of the United States of America.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the Supporting Information of this article.




