How exome sequencing improves the diagnostics and management of men with non-syndromic infertility
Abstract
Male infertility affects approximately 17% of all men and represents a complex disorder in which not only semen parameters such as sperm motility, morphology, and number of sperm are highly variable, but also testicular phenotypes range from normal spermatogenesis to complete absence of germ cells. Genetic factors significantly contribute to the disease but chromosomal aberrations, mostly Klinefelter syndrome, and microdeletions of the Y-chromosome have remained the only diagnostically and clinically considered genetic causes. Monogenic causes remain understudied and, thus, often unidentified, leaving the majority of the male factor couple infertility pathomechanistically unexplained. This has been changing mostly because of the introduction of exome sequencing that allows the analysis of multiple genes in large patient cohorts. As a result, pathogenic variants in single genes have been associated with non-syndromic forms of all aetiologic sub-categories in the last decade.
This review highlights the contribution of exome sequencing to the identification of novel disease genes for isolated (non-syndromic) male infertility by presenting the results of a comprehensive literature search. Both, reduced sperm count in azoospermic and oligozoospermic patients, and impaired sperm motility and/or morphology, in asthenozoospermic and/or teratozoospermic patients are highly heterogeneous diseases with well over 100 different candidate genes described for each entity. Applying the standardized evaluation criteria of the ClinGen gene curation working group, 70 genes with at least moderate evidence to contribute to the disease are highlighted. The implementation of these valid disease genes in clinical exome sequencing is important to increase the diagnostic yield in male infertility and, thus, improve clinical decision-making and appropriate genetic counseling.
Future advances in androgenetics will continue to depend on large-scale exome and genome sequencing studies of comprehensive international patient cohorts, which are the most promising approaches to identify additional disease genes and provide reliable data on the gene–disease relationship.
1 INTRODUCTION
More than 17% of people in the general population are affected by infertility, defined as the inability to conceive after 1 year of unprotected intercourse.1 Frequently, infertility is associated with serious consequences beyond the infertility itself. This includes psychological distress, social stigma, and economic deprivation.2 Further, an increased susceptibility to cancer in infertile men (and women) has been reported in a growing number of epidemiologic studies and genetics are a likely link.3, 4 Thus, understanding the causes of infertility is not only important to improve treatment but also to address accompanying risks.
It is estimated that male factors contribute to about 50% of the cases, either alone or in combination with female factors.5, 6 Male infertility can be part of syndromic disorders such as primary ciliary dyskinesia (PCD), Klinefelter syndrome, or cystic fibrosis. In addition, in disorders of the hypothalamic–pituitary–adrenal axis, it may be seen together with delayed or impaired sex development, ambiguous genitalia, hypogonadism, or androgen insensitivity. In contrast, male infertility can also present as isolated disease in otherwise healthy men without any further conditions. These non-syndromic forms refer to different phenotypic classifications,7 and are typically characterized by defects in spermatogenesis, that is, spermatogenic failure (SPGF), most often resulting in reduced sperm counts (oligozoospermia, human phenotype ontology, HPO: 0000798) or the complete absence of sperm from the ejaculate (azoospermia, HPO: 0000027). Further, disturbed spermiogenesis may lead to impaired sperm motility (asthenozoospermia, HPO: 0012207) and/or abnormal sperm morphology (teratozoospermia, HPO: 0012864), which is often combined with varying degrees of oligozoospermia.
It is assumed that in a striking proportion of infertile men, genetic factors contribute to the disease. 8 However, only in about 4% of all men in infertile couples a genetic cause can be identified.9 This fraction increases to ∼15% in azoospermic men, in whom the cause can be traced back to chromosomal aberrations, mostly Klinefelter syndrome (karyotype 47, XXY), and microdeletions of the Y-chromosomal azoospermia factor (AZF) region. Fittingly, these two are well established and routinely clinically analyzed causes of severe oligozoo-/azoospermia. In addition, since many years, also monogenic causes have been linked to all three aetiologic categories of reduced/impaired sperm count, motility, and morphology. However, for a long time, the diagnosis of monogenic causes of male infertility has remained limited to screening for variants in the CFTR gene in patients with azoospermia and suspected congenital bilateral absence of the vas deferens (CBAVD).10
With the development and availability of platforms for next-generation sequencing (NGS)-based technologies, the number of candidate genes for male infertility is steadily increasing, and it has become evident that genetically determined isolated male infertility is a highly heterogeneous disease.11 The era of NGS-based technologies in infertility assessment started with targeted gene panels, in which up to hundreds of candidate genes selected on the basis of known or suspected disease association were sequenced in parallel.12-14 With the decreasing cost of NGS chemistry, targeted methods have been replaced by exome sequencing, in which the coding regions as well as exon–intron boundaries of nearly all protein-encoding human genes are captured and sequenced. This enabled the identification of the causal homozygous genetic variant in consanguineous cases.15-17 and of potentially relevant de novo variants18, 19 in infertile men by sequencing them and their parents (trio exome sequencing). In addition, exome sequencing allows the simultaneous analysis of large cohorts of infertile patients, thus providing a powerful tool for the detection of extremely rare disease-causing genetic variants.
However, the implementation of NGS-based workflows for the detection of single/small nucleotide variants (SNVs) in routine male infertility diagnostic is still lagging behind other fields of medicine.20 One main reason is that for a striking number of the described candidate genes an evidence-based gene–disease association has not yet been determined and, thus, the identification of nucleotide variants in genes of uncertain evidence to contribute to the disease could lead to false positive results. To close this gap, this review provides not only an overview of exome-based studies for the identification of monogenic causes of non-syndromic male infertility but also highlights those genes with a valid gene–disease relationship (GDR). Based on these data, the utility of implementing exome sequencing in the diagnosis and management of infertile men is demonstrated.
2 Increasing knowledge of isolated male infertility from exome-based genetic studies
To identify studies using exome sequencing to discover genetic variants associated with male infertility, a PubMed search was performed by combining the terms whole-exome, exome, sequencing, next-generation sequencing, male infertility, azoospermia, oligozoospermia, asthenozoospermia, teratozoospermia, multiple anomalies of the sperm flagella, acephalic sperm, and spermatogenesis in various combinations (Figure 1). This approach resulted in the identification of 335 studies published between 2014 and 2023 (Table S1), with a peak of 79 studies in 2023 (Figure 2). In more than 90% of publications, single or few functionally related genes were identified through family-based exome sequencing.21-24 or by screening for variants in specific genes in larger patient cohorts.25-27

With the increasing availability of exome sequencing and also the development of bioinformatics pipelines that allow for data analysis in batch formats, also larger cohorts of infertile men have been systematically evaluated for putative harmful nucleotide variants in broad sets of candidate genes (Figure 2). These comprehensive studies account for less than 10% of the publications and differ in the phenotypic spectrum of the patients involved, that is, male infertility,28, 29 oligozoo- and azoospermia,30-32 and impaired sperm motility or morphology33-35; they differ in the list of genes investigated, that is, only X-chromosomal genes36, 37 or genes with previously evaluated GDR,20, 38 or they focus on a specific inheritance mode, that is, de novo variants.18, 19
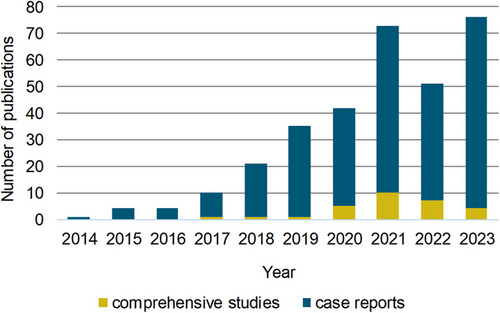
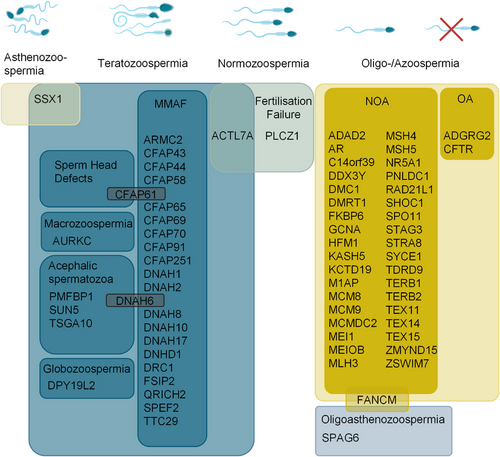
In total, the literature screening identified 313 different genes that were described in the context of exome sequencing-related genetic male infertility studies between 2014 and 2023 (Figure 1, Table S1). This highlights the enormous heterogeneity of the disease, and also demonstrates how challenging it is to separate these genes into (1) validated disease genes, (2) candidate genes with promising evidence for a contribution to the disease, that is, the knockout of the mouse ortholog leads to male infertility, and (3) candidate genes with limited evidence where the association with the disease is, for example, solely based on a testis-specific expression profile of the respective gene. To address this issue, we performed a structured gene–GDR analysis based on the guidelines of the ClinGen gene curation working group, considering only the 106 genes that were highlighted in at least two independent publications, as this is an important requirement in the ClinGen evaluation process to identify valid disease genes (Figure 1, Table S2). Based on the collection of genetic and functional data, we then identified 70 distinct genes with at least moderate evidence to contribute to male infertility (Table 1, Figure 3). Only two of these genes follow an autosomal dominant mode of inheritance, six genes are located on the sex chromosomes, and accordingly hemizygous variants contribute to the disease, and 62 disease genes follow an autosomal recessive inheritance pattern (Table 2), which is in line with the reported significant increase of male factor fertility in consanguineous couples39—similar to other disease entities.
| Gene | Phenotype | HPO Phenotype ID | OMIM Phenotype ID | Gene–disease relationship ClinGen | Mode of inheritance |
|---|---|---|---|---|---|
| Phenotype: Azoospermia | |||||
| ADGRG2 | OA | 0011962 | 300985 | Definitive | XL |
| CFTR | 277180 | Definitive | AR | ||
| ADAD2 | NOA | 0011961 | NA | Strong | AR |
| AR | NA | Definitive | XL | ||
| C14orf39 | 619202 | Strong | AR | ||
| DMC1 | NA | Moderate | AR | ||
| DDX3Y | NA | Moderate | YL | ||
| DMRT1 | NA | Moderate | AD | ||
| FKBP6 | 620103 | Strong | AR | ||
| GCNA | 301077 | Moderate | XL | ||
| HFM1 | NA | Strong | AR | ||
| KASH5 | 620547 | Strong | AR | ||
| KCTD19 | NA | Strong | AR | ||
| M1AP | 619108 | Definitive | AR | ||
| MCM8 | NA | Moderate | AR | ||
| MCM9 | NA | Moderate | AR | ||
| MCMDC2 | NA | Moderate | AR | ||
| MEI1 | NA | Definitive | AR | ||
| MEIOB | 617706 | Definitive | AR | ||
| MLH3 | NA | Moderate | AR | ||
| MSH4 | 108420 | Definitive | AR | ||
| MSH5 | 619937 | Strong | AR | ||
| NR5A1 | 613957 | Definitive | AD | ||
| PNLDC1 | 619528 | Strong | AR | ||
| RAD21L1 | NA | Moderate | AR | ||
| SHOC1 | 619949 | Definitive | AR | ||
| SPO11 | NA | Moderate | AR | ||
| STAG3 | 619672 | Strong | AR | ||
| STRA8 | NA | Strong | AR | ||
| SYCE1 | 616950 | Definitive | AR | ||
| TDRD9 | 618110 | Moderate | AR | ||
| TERB1 | 619646 | Moderate | AR | ||
| TERB2 | 619645 | Moderate | AR | ||
| TEX11 | 309120 | Definitive | XL | ||
| TEX14 | 617707 | Strong | AR | ||
| TEX15 | 617960 | Strong | AR | ||
| ZMYND15 | 615842 | Strong | AR | ||
| ZSWIM7 | 619831 | Moderate | AR | ||
| Phenotype: Astheno-, teratozoospermia | |||||
| ARMC2 | MMAF |
0012207 0012868 0033393 |
618433 | Definitive | AR |
| CFAP43 | 617592 | Definitive | AR | ||
| CFAP44 | 617593 | Definitive | AR | ||
| CFAP58 | 619129 | Strong | AR | ||
| CFAP65 | 618664 | Strong | AR | ||
| CFAP69 | 617959 | Strong | AR | ||
| CFAP70 | 618670 | Moderate | AR | ||
| CFAP91 | 619177 | Moderate | AR | ||
| CFAP251 | 618152 | Strong | AR | ||
| DNAH1 | 617576 | Definitive | AR | ||
| DNAH2 | 619094 | Moderate | AR | ||
| DNAH8 | 619095 | Strong | AR | ||
| DNAH10 | 619515 | Strong | AR | ||
| DNAH17 | 618643 | Definitive | AR | ||
| DNHD1 | 619712 | Strong | AR | ||
| DRC1 | 620222 | Strong | AR | ||
| FSIP2 | 618153 | Definitive | AR | ||
| QRICH2 | 618341 | Strong | AR | ||
| SPEF2 | 618751 | Strong | AR | ||
| TTC29 | 618745 | Strong | AR | ||
| AURKC | Macrozoospermia | 0025437 | 243060 | Strong | AR |
| DPY19L2 | Globozoospermia | 0033393 | 613958 | Definitive | AR |
| PMFBP1 | Acephalic sperm with oligozoospermia | 0012869, 0000798 | 618112 | Strong | AR |
| SUN5 | Acephalic sperm | 0012869 | 617187 | Strong | AR |
| TSGA10 | Acephalic sperm | 0012869 | 617961 | Moderate | AR |
| Phenotype: others, mixed phenotypes | |||||
| ACTL7A | Fertilisation failure, teratozoospermia | 0034812, 0012864 | 620499 | STRONG | AR |
| CFAP61 | MMAF, sperm head defects | 0012207, 0033393 | 620409 | Strong | AR |
| DNAH6 | MMAF, acephalic sperm | 0012207, 0033393 | NA | Moderate | AR |
| FANCM | NOA, Oligoasthenozoospermia | 0011961, 0000798, 0012207 | 618086 | Moderate | AR |
| PLCZ1 | Fertilisation failure; acrosomal defects | 0012865 | 617214 | Definitive | AR |
| SPAG6 | Oligozoo-, azoo-, asthenozoospermia | 0000798, 0000027, 0012207 | NA | Moderate | AR |
| SSX1 | Asthenoteratozoospermia | 0012207, 0012864 | 301099 | Moderate | XL |
- Abbreviations: AD, Autosomal dominant; AR, autosomal recessive; HPO, human phenotype ontology; MMAF, multiple abnormalities of the sperm flagella; NA, not annotated; OMIM, online Mendelian inheritance in man; XL, X-linked; YL, Y-linked.
| Phenotype | Mode of Inheritance | ||||||
|---|---|---|---|---|---|---|---|
| (semen analysis/testicular histology) | AR N = 62 | AD N = 2 | XL N = 5 | YL N = 1 | Total N = 70 | ||
| Teratozoospermia | Globozoospermia | 1 | 1 | ||||
| Acephalic sperm | 3 | 3 | |||||
| Macrozoospermia | 1 | 1 | |||||
| Asthenozoo-spermia | MMAF | 20 | 20 | ||||
| Diverse semen phenotypes | 3 | 1 | 4 | ||||
| Azoospermia | Non-obstructive Azoospermia/oligozoospermia | 31 | 2 | 3 | 1 | 37 | |
| Obstructive azoospermia | 1 | 1 | 2 | ||||
| Normal semen phenotype | Fertilisation failure | 2 | 2 | ||||
- Abbreviations: AD, Autosomal dominant; AR, autosomal recessive; MMAF, multiple abnormalities of the sperm flagella; XL, X-linked; YL, Y-linked.
3 Monogenic forms of male infertility: different phenotypic groups account for different sets of disease genes
Among the total number of 313 genes identified in the context of isolated male infertility, the highest number of genes (n = 196) has been reported in men with a reduced sperm count in the ejaculate (Table S1). Within this group of patients, azoospermia, which is defined as the total absence of sperm, represents the most severe form. It can be categorized into two entities: obstructive azoospermia (OA, HPO: 0011962), which is characterized by the inability to release sperm after passing through the epididymis due to the absence or structural anomalies of the vas deferens, and non-obstructive azoospermia (NOA, HPO: 0011961), which is caused by impaired or abolished spermatogenesis.
The main genetic causes of OA can be linked to biallelic pathogenic variants in the CFTR gene, resulting in CBAVD and this genetic diagnosis accounts for 80% of men with CBAVD and 47% of men with OA.40 Accordingly, sequencing of the definitive disease gene CFTR in patients with azoospermia and suspected CBAVD has been already routinely performed in the pre-NGS era of genetic diagnostics. With the increasing number of exome-related genetic studies, OA due to CBAVD in the absence of associated unilateral renal agenesis has also been clearly linked to hemizygous variants in the X-linked gene ADGRG241, 42 representing a second disease gene with definitive evidence.20
NOA and oligozoospermia are conditions that refer to the inability of the testes to produce mature sperm in normal amounts due to impaired spermatogenesis. These disorders together account for more than 50% of infertile men and also for this condition a predominant genetic cause of the disease is suspected.43 The only two widely recognized genetic causes for NOA or severe oligozoospermia are chromosomal aberrations, mostly Klinefelter syndrome, and microdeletions of the Y-chromosomal AZF regions.
In recent years, it has become apparent that also monogenic causes significantly contribute to the disease. According to our literature review, 194 human genes have been associated with NOA and/or (severe) oligozoospermia based on NGS techniques. Of these genes, 54 were highlighted in more than one publication with 37 genes achieving at least moderate evidence according to ClinGen gene curation criteria (Table 2). The enormous heterogeneity of NOA was already suspected based on expression profile data from human testis, where more than 1,992 genes show elevated expression and 582 genes are specifically expressed.44
Not unexpected, several of the NOA disease genes encode proteins with a crucial function in meiosis, the key molecular process during spermatogenesis. Within this group, C14ORF3945, 47–HFM1,47-49 KASH5,50, 51 M1AP,27, 52 MEI1,53, 54 MSH426, 31 MSH5,26, 47 MEIOB,47, 55, 56 SHOC1,31, 57 STAG3,13, 58 and TEX1130, 59, 60 represent disease genes with an already strong or definitive GDR. Other meiosis-related genes, such as MCMDC247, 54 and DMC1,47, 61 represent disease genes for NOA with moderate evidence through the reporting of homozygous loss-of-function (LoF) variants in more than one case. Furthermore, genes of the piRNA pathway such as PNLDC1,62, 63 PIWIL2,29, 64 FKBP6,25, 28 TEX15,38, 65 and ADAD231, 66, 67 represent valid disease genes for NOA or impaired spermatogenesis, in line with the well-established link between piRNA pathway genes and spermatogenesis in mice.68 It is noteworthy that additional genes with a reported function in meiosis and piRNA biogenesis have only been described as candidate disease genes in a single publication. Here, C11ORF80,54 IHO1,54 HENMT1,47 and TDRD1263 are promising candidates, because for all these genes the knockout of their respective orthologous gene in mice resulted in male infertility. Thus, it can be confidently expected that the list of valid disease genes encoding meiosis- or piRNA-related proteins will expand as more variants in these genes might be identified and reported in the coming years. Besides, multiple exome-based studies provide evidence that isolated NOA can also result from variants in genes that can also cause more severe phenotypes within the spectrum of 46, XY differences of sexual development (DSD), including AR,69-71 DMRT1,38, 71, 72 and NR5A1.71, 73, 74 Finally, for other genes with evidence to contribute to NOA, such as DDX3Y75 and KCTD19,76, 77 the pathomechanism by which impaired protein function affects spermatogenesis still needs to be unraveled.
In the context of asthenozoospermia and/or teratozoospermia, the literature search identified 122 different candidate genes (Table S1) described in exome-based studies, again highlighting the heterogeneous nature also of congenital qualitatively impaired sperm. Strengthening their genetic evidence, 47 of these genes have been highlighted as candidate genes for the disease in at least two separate publications and of these genes, 29 reach an at least moderate GDR (Table 1, Figure 3).
Not surprisingly, several of the genes have been described in association with both impaired sperm motility and morphology (Figure 3). Particularly those genes in which variants have been reported as causes for the relatively recently coined term “morphological defects of the sperm flagellum” (MMAF) contribute to both entities. Several of these genes encode proteins important for assembly of the axoneme (CFAP43,78-80 CFAP44,79, 81 CFAP69,82, 83 CFAP65,84, 85 TTC2986, 87) and/or the mitochondrial sheet (CFAP5888, 89), the axonemal fibrous sheet (FSIP290-92), the axonemal radial spoke (CFAP61,93, 94 CFAP25195, 96), or the axonemal central pair complex, (ARMC297, 98). Other MMAF genes encode axonemal dyneins (DNAH1,22, 99 DNAH8,100, 101 DNAH10,102, 103 DNAH17,104, 105 DNHD1106, 107), regulate expression of sperm tail structural proteins (QRICH2108, 109), or are involved in intra-flagellar transport processes known to be essential for cilia development and maintenance (SPEF2110-112). Of note, several of the genes described in patients affected by asthenozoospermia, for example, DNAH1, DNAH8, CCDC103, and DRC1 represent disease genes for PCD,113 highlighting the wide phenotypic variability of genetic variants in ciliopathy-related genes.
The group of genes associated with teratozoospermia also comprises some that are related to defective sperm head morphology accompanied by impaired acrosome shape and function (Table 1, Figure 3). One extreme and highly specific form of this condition is globozoospermia (HPO: 0012205), which is characterized by universal round-headed sperm without acrosome that are unable to adhere to or penetrate the zona pellucida. Here, DPY19L2,114, 115 involved in acrosome formation,116 represents the only definitive disease genes for this type of morphologically abnormal sperm. Acephalic sperm (HPO: 0012869) is a further severe type of teratozoospermia, defined as semen composed of mostly headless sperm. Exome sequencing has helped to independently link pathogenic variants in SUN5,117, 118 PMFBP1,119, 120 and TSGA10121, 122 to this condition. Furthermore, macrozoospermia (HPO: 0025437) due to pathogenic variants in the only definitive disease gene, AURKC,123, 124 a component of the chromosomal passenger complex that is involved in meiotic segregation,125 is characterized by aneuploid sperm with large sperm heads and/or multiple tails.
Finally, monogenic forms of male infertility have been described as cause of fertilization failure. In this condition, the number and morphology of the sperm is not affected, but the sperm are still unable to fertilize the oocyte. Here, acrosomal defects that do not result in morphological abnormalities, may also be involved and valid disease genes related to this disorder include PLCZ1126-128 being crucial for Ca2+ oscillations important for egg activation during fertilization129 and ACTL7A128, 130 encoding an actin-like protein essential for acrosome biogenesis.131 Recently, copy number variation (CNV) analysis have identified single gene deletions of CATSPER2, encoding a subunit of the sperm specific CatSper ion channel linking also this gene to non-syndromic fertilization failure.132-134 A contiguous gene deletion syndrome including both CATSPER2 and the neighboring STRC causes both infertility and sensorineural hearing loss and is termed deafness–infertility syndrome (DIS, OMIM: 611102).
While the overlap between genes contributing to asthenozoospermia and teratozoospermia is obvious, some genes also cause a reduced sperm count in combination with impaired sperm motility and/or sperm morphology.77, 135, 136 In contrast, only few genes such as PNLDC162, 136 or TDRD916, 35 have been reported to cause a phenotypic spectrum that encompasses isolated forms of all three entities. Only the systematic analysis of multiple carriers affected by variants in the same gene will shed light on the question which disease genes show a clear genotype–phenotype correlation and for which genes the reproductive phenotype is more variable than previously assumed.
4 Calling copy number variation from exome sequencing data in male infertility
Exome sequencing not only allows for the identification of single nucleotide variants and small deletions and insertions, but also provides the basis to identify CNVs that may contribute to the disease. However, analysis of these data is still challenging and reliability strongly depends on the individual setup/workflows, for example, the exome sequencing depth/coverage. Therefore, additional approaches including qPCR, digital droplet PCR and/or single nucleotide polymorphism (SNP) array analysis are advised to confirm the called CNVs.
Consistent with these challenges, only a few studies using NGS approaches in the context of non-syndromic male infertility have reported on CNVs31, 50, 132, 137 and only single studies have focused on this kind of genetic alterations and systematically screened for CNVs in larger cohorts of infertile men.138, 139 Based on SNP arrays and array comparative genomic hybridization (CGH) analysis, it has been suggested that also CNVs contribute substantially to the genetic causes of SPGF.69, 140, 141 However, at least for CNVs affecting autosomes, this has not yet been verified by exome sequencing data54, 138, 142 and only systematic calling of CNVs from exome data of infertile men will show whether this observation can be confirmed or not.
Larger deletions affecting the sex chromosomes, such as microdeletions within the Y-chromosomal AZF region, and also exon-/gene-deletions affecting the coding sequence of the established disease gene TEX1120, 31, 59 are well-known causes of male infertility. For the time being, the EAA/EMQN best practice guidelines still recommend sequence-tagged site multiplex PCR (STS-PCR).143 as the gold standard to screen for AZF microdeletions, but exome-based studies have already demonstrated that this technique is equally suitable for detecting Y-chromosomal microdeletions. In addition, it will allow for the identification of SNVs in DDX3Y, a valid disease gene within the AZFa region in which LoF variants were shown to cause the same phenotype as complete deletions of this region.75 The benefit of exome sequencing in the context of AZF region analysis lies therefore in the broader sensitivity because it also detects partial AZF deletions, single gene and exon deletions, and SNVs in one single test.144, 145
5 Translating research to the clinic: strategies for advancing the standard of care
Recommendations for routine clinical genetic diagnostics in infertile men have remained unchanged for decades and still rely on chromosomal analysis (karyotyping), PCR-based screening for AZF microdeletions, and sequencing of the CFTR gene in patients affected by azoospermia or severe oligozoospermia. Gene panel-based screening for monogenic causes not only in azoospermic patients but also in patients with terato- and asthenozoospermia is rarely performed. One major prerequisite for the implementation of NGS-based techniques in the diagnostic workflow is the description of phenotype-specific gene sets. To this end, only genes with sufficient evidence to contribute to the disease should be included, to avoid false-positive reports. Some studies have focused on this problem and aimed to categorize male infertility associated genes (Table S2).11, 20 However, these studies differ in the validation criteria that were applied to assess the gene–disease association. In order to compare the assessment results, a subsequent implementation of standardized evaluation criteria as provided by the Clinical Genome Resource (ClinGen) consortium is necessary. Based on these criteria, a first panel for azoospermia was suggested including 21 genes with at least moderate evidence, in which likely pathogenic variants had been identified in a large cohort of almost 650 infertile men.20 Compared to that study, this review increased the number of valid disease genes for azoospermia and oligozoospermia to 39 by including more recent publications and also data on a comprehensive candidate gene analysis (Figure 3, Table 1, Table S2). Additionally, a curated gene panel is also proposed for other semen phenotypes, including a further 31 genes. To continuously improve the quality of gene–disease evaluation and increase the number of genes in the context of male infertility, a ClinGen Gene Curation Expert panel should be implemented.
Among the studies reporting the results of exome sequencing in large cohorts of infertile men, the diagnostic yield is also highly variable, ranging from 8.5% for azoospermia when only validated disease genes and (likely) pathogenic variants based on the ACMG criteria are considered20 to more than 20% in studies that not only consider a broader spectrum of phenotypes but also include variants of uncertain significance identified in candidate genes.29, 47 Therefore, the implementation of standardized evaluation criteria is also a prerequisite for calculating the diagnostic yield in the different aetiologies of male infertility. In the coming years, it can be expected that the diagnostic yield for monogenic causes of male infertility will continue to increase rapidly, while new disease genes are identified and data supporting their clinical validity accumulate. In this regard, another advantage of exome sequencing should be noted: once these data are available, they can be reanalyzed in the future when novel valid disease genes are described.
Men with isolated infertility of suspected genetic origin also benefit from a causal diagnosis from a therapeutic point of view, because the prognosis for successful medically assisted reproduction (MAR) such as in vitro fertilization (IVF) and testicular sperm extraction (TESE) for intracytoplasmic sperm injection (ICSI), which for many couples represent the only option for parenthood, differ in terms of phenotype/genotype. Couples in which the man is affected by macrozoospermia have for example a high incidence of aneuploidy and, therefore, low fertilization and pregnancy rates after ICSI.146 In addition, the success of TESE is largely dependent on the testicular phenotype, which depends on the pathomechanism relating to the underlying genetic cause. Thus, genetic diagnosis prior to TESE helps to identify individuals with virtually zero success rate for sperm retrieval and avoid surgeries doomed to fail.
Indeed, while TESE is successful in more than 95% of men with CBAVD due to biallelic variant in CFTR,147 success rates are significantly lower with 40%−50% in cases of NOA.148 In the latter, TESE is not advised in men with complete microdeletions of the AZFa, AZFb, or AZFbc regions, because these concordantly result in complete loss of germ cells in the testis (Sertoli cell-only phenotype) or arrested spermatogenesis.149 In contrast, men with AZFc deletions have TESE success rates of around 50%.150 Detailed data on TESE outcome for monogenic causes of NOA have so far only been shown for a few validated disease genes such as TEX11, TEX14, and MSH4.20 Comprehensive studies of exome-based genetic diagnostics in large cohorts of men with NOA, which include also detailed data on TESE outcome, will therefore help to provide sound recommendations for more disease genes,20, 47, 54 while still allowing the couple to make their own reproductive choices.
In addition to the great advantages of exome sequencing for genetic diagnostics, there are also some challenges of this technique that need to be addressed. The bottleneck is no longer in the primary wet lab process and the high costs incurred, but in interpreting the genetic data obtained. This interpretation requires sufficient expert knowledge not only in genetics, but also in andrology, to filter out the most relevant disease-causing variant from a patient's exome and to assess whether the identified disease gene is consistent with the patient's reproductive phenotype. In addition, as exome sequencing is incorporated into the diagnostic workflow of infertile men, the parallel identification of actionable secondary genetic findings is increasing, including gene variants identified in disease genes for familial cancer or late-onset congenital heart diseases. A recent study on the amount of actionable secondary findings in patients with NOA highlights a striking contribution of genes essential for maintaining genome integrity in both mitosis and meiosis.151 This is not unexpected, as cancer genes such as BRCA2, MLH1, and PMS2 have already been associated with impaired spermatogenesis and male infertility152-154 indicating a phenotypic overlap between both entities. Addressing the issue of how to deal with secondary findings in the diagnostic workup of infertile men will, therefore, become increasingly important, and structured communication guidelines are needed to inform about future health concerns for patients consulting for infertility, their families, and potential offspring.
6 OUTLOOK
Beyond exome sequencing, genome sequencing has also been used since a few years to discover variants in the entire human genome.52, 155 In addition to the advantage of covering intergenic and non-coding regions, a further benefit of this technique is that it is not based on the enrichment of target sequences by PCR, so that the resulting sequence reads are evenly distributed across the genome and no differences in the mean coverages per exon are observed.156 Consequently, and in contrast to exome sequencing, nucleotide variants can be reliably called at an average sequencing depth of only 30x.157 In addition, genome-wide structural variations can not only be more reliably called compared to exome sequencing, the technique also allows for the identification of genomic breakpoints in case of translocations, a benefit that is especially obtained from long-read genome sequencing approaches. Therefore, given the number of male infertility-associated diseases related to structural variations, such as microdeletions of the Y-chromosomal AZF regions and whole gene or whole exon deletions in TEX11 or CATPER2, a single test can be performed to identify nucleotide variants as well as CNVs, significantly increasing the diagnostic yield in patients.
In recent years, the identification of novel disease genes for male infertility has mainly focused on recessive genes and accordingly within the group of genes with at least moderate GDR > 95% of genes reveal an autosomal recessive mode of inheritance (Table 2). Because men with infertility are less likely to have children and pass on a pathogenic variant, pedigree data are only rarely available to investigate putative autosomal dominant and X-linked diseases. To address the extent to which autosomal dominant traits contribute to the disease, screening for de novo variants19 and also statistical burden analysis,37 in which the amount of predicted pathogenic variants in cohorts of infertile men is compared with the occurrence of such variants in fertile male control populations, are valuable tools. However, also these approaches depend on the availability of large patient cohorts. In this regard, the value of international, large-scale collaboration as fostered by the (IMIGC, http://www.imigc.org/) cannot be underestimated. Recruiting, phenotyping, and exome/genome sequencing of infertile men, thus, remain an essential task to increase the amount of clinically relevant disease genes in male infertility and to provide genotype-specific therapy options for affected men.
AUTHOR CONTRIBUTIONS
All authors revised and approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Clara Barkhaus, MD, and Johanna Kuss, PhD for their support in the ClinGen assessments. This study was carried out within the frame of the German Research Foundation sponsored Clinical Research Unit ‘Male Germ Cells’ (DFG CRU326, project number 329621271), the IZKF Münster (Tüt4/011/23), and the German Federal Ministry for Education and Research (BMBF) as part of the Junior Scientist Research Centre ‘ReproTrack.MS’ (grant 01GR2303).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the Supporting information of this review.




