Hemoglobin decline as a signal for hyperprolactinemia onset prior to prolactinoma diagnosis in hypogonadal men
Abstract
Background
Men harboring prolactinomas frequently suffer from central hypogonadism with secondary anemia. They present insidious and nonspecific symptoms of hypogonadism, making it difficult to diagnose the disease and determine its duration. The result is a delay in diagnosis, which may have harmful hormonal and metabolic consequences. We hypothesized that a decrease in hemoglobin (HB) levels prior to prolactinoma diagnosis, may signal hyperprolactinemia onset and estimate disease duration.
Methods
We retrospectively evaluated the prediagnosis temporal trends in HB levels of 70 males with prolactinoma, diagnosed from January 2010 to July 2022. Men without hypogonadism, patients that received testosterone, and those with unrelated anemia were excluded.
Results
Sixty-one of seventy men (87%) with prolactinoma presented with hypogonadism, and forty men (57%) had HB levels ≤13.5 g/dL at diagnosis. We identified 25 patients with “informative” HB curves (mean age, 46.1±14.9 years; median prolactin, 952 ng/mL; median follow-up, 14.0 years), demonstrating an obvious prediagnosis HB decrease (greater than 1.0 g/dL), from a prediagnosis baseline HB of 14.4 ± 0.3 to 12.9 ± 0.5 g/dL at diagnosis. The median “low-HB duration” (from the first low HB measurement to hyperprolactinemia diagnosis) was 6.1 years (IQR, 3.3–8.8 years). In symptomatic patients, we identified a correlation between “low-HB duration” and patient-reported sexual dysfunction duration (n = 17, R = 0.502, p = 0.04). The “low-HB duration” was significantly longer than the reported sexual dysfunction duration (7.0 ± 4.5 vs. 2.9 ± 2.5 years, p = 0.01).
Conclusions
In our cohort of men with prolactinomas and hypogonadism, we found a marked decrease in HB levels that preceded prolactinoma diagnosis by a median of 6.1 years, with a mean delay of 4.1 years between HB decrease and hypogonadal symptoms appearance. These results suggest that HB decline prior to prolactinoma diagnosis may serve as a marker for hyperprolactinemia onset in a subset of hypogonadal men and allow a more accurate assessment of disease duration.
1 INTRODUCTION
Prolactinomas are uncommon among men, and present differently in males and females.1 Male prolactinomas are usually larger and more invasive.2 As hyperprolactinemia causes hypogonadotropic hypogonadism, women are aware of symptoms onset (as amenorrhea).3 On the other hand, male patients present with nonspecific symptoms of hypogonadism, such as decreased libido, erectile dysfunction, and weakness, usually with a slow and subtle onset.4 Visual symptoms, derived from tumor mass effect, are more common in male patients with macroprolactinoma and develop at a slow pace and aggravate unnoticed over long periods of time.5
As with other secreting pituitary tumors, patients harboring prolactinomas suffer from a significant delay in diagnosis.6 Many studies have estimated the duration of the disease according to symptoms onset2, 3, 5, 7-11; however, a latent period of asymptomatic (or unnoticed) hyperprolactinemia may be reasonably assumed. Moreover, the estimated duration of symptoms, reported by the patient, provides an inaccurate assessment, subject to recall bias. Relevant data were accumulated in the 1980s, when erectile problems lasted for many years until diagnosis.7 Patients were reluctant to share sexual difficulties, which were not an accepted part of public discourse during those years, or because there were no effective treatments for erectile dysfunction at the time. More recent studies show a significant diagnostic delay in male prolactinoma, with a median delay of 1−4 years.8, 9
This diagnostic delay may cause detrimental consequences. Untreated prolactinomas are associated with an increased risk of bone fractures.12 A longer duration of prolactinoma symptoms in men was found to be associated with lower bone mineral density (BMD), and higher prevalence of vertebral fractures.9, 10
Hyperprolactinemia is also associated with obesity and the metabolic syndrome, mostly based on data originating from mouse models.13 However, clinical studies have shown improvement in metabolic parameters (body mass index [BMI], insulin resistance [HOMA-IR], low-density lipoprotein [LDL] cholesterol levels) in hyperprolactinemic patients treated with dopamine agonists.14, 15
In 1950, Vahlquist noted that hemoglobin (HB) concentrations are higher in males, and postulated that this variation is the result of different sex hormone profiles (or “endocrine nature”).16 We now know that testosterone induces erythropoiesis in men, in a dose-dependent manner.17 Ellegala et al. showed that hematopoiesis is impaired in men with low testosterone levels in cases of hypogonadism secondary to pituitary adenoma.18 It was discovered that anemia is a common finding in men with macroprolactinoma, associated with hypogonadism and large tumor size.19, 20 Following treatment with dopamine agonists, achievement of prolactin normalization and an increase in testosterone levels, rising HB levels were observed.19
As estimation of disease duration based on symptom onset does not work as well in men as it does in women, we searched for a more accurate estimation method in male prolactinoma.
We hypothesized that a decrease in HB levels (detected on routine blood counts) in otherwise healthy men, later diagnosed with prolactinomas and central hypogonadism, may signal the onset of clinically significant hyperprolactinemia and provide an objective estimate of disease duration, independent of patient-reported symptoms.
The aim of this study was to evaluate the duration of prolactinoma habitation in hypogonadal men with prolactinomas through the examination of temporal trends in HB levels prior to prolactinoma diagnosis.
1.1 Study design and methods
This is a single-center, retrospective cohort study in male prolactinoma patients treated at the Pituitary Clinic of the Institute of Endocrinology, Rabin Medical Center (RMC), Israel. The study was approved by the RMC Ethics Review Board (approval number RMC-0516-16) with waiver of consent. The study was carried out according to the Declaration of Helsinki.
1.2 Patients
Patients were identified by review of the prolactinoma registry at the pituitary clinic at RMC. All patients with hyperprolactinemia and a pituitary adenoma depicted by magnetic resonance imaging (MRI), diagnosed from January 2010 to July 2022, were included in our registry. All patients were diagnosed at our pituitary clinic or referred immediately after diagnosis. Patients that received antipsychotic drugs, and those with baseline prolactin levels <95 ng/mL (2000 mIU/L) were excluded (to avoid “stalk effect” in men with large nonfunctioning tumors).21
Medical treatment with cabergoline was initiated in all patients immediately after diagnosis.
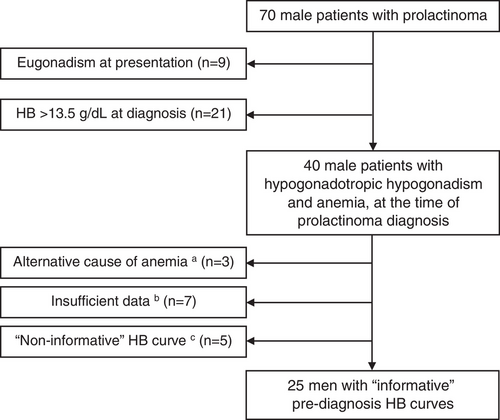
Men without hypogonadism, patients with normal HB levels (above 13.5 g/dL) at the time of prolactinoma diagnosis, patients with other causes of anemia, patients that received testosterone prior to diagnosis, and those with insufficient data (less than 4 pre-diagnosis HB measurements) were not included in the study cohort (Figure 1).
1.3 Data collection
Patient's baseline demographic, clinical and biochemical parameters, sellar MRI and visual field tests were obtained. All available laboratory tests (including routine laboratory tests, performed prior to prolactinoma diagnosis) were collected for each patient, including prolactin, testosterone, LH, FSH, morning cortisol, TSH and FT4 measurements, complete blood count, ferritin, iron, transferrin, vitamin B12, folic acid, and C-reactive protein. Data regarding cabergoline treatment, testosterone replacement, surgical therapy, and radiotherapy were also collected.
During the index clinic visit, patients were specifically asked about the presence of decreased sexual desire, erectile dysfunction, and headaches, and their respective durations. Hypogonadism symptoms were defined as any complaint of decreased libido or erectile dysfunction, or both.
Hypogonadism was defined as total testosterone level below the lower limit of the normal reference range of 2.8 ng/mL.
Anemia in men was defined as HB concentration of 13.5 g/dL or less, according to the definition of the American Society of Hematology.22
Prediagnosis temporal trends in HB were evaluated for all patients included in the registry. An HB curve (HB levels over time) was regarded “informative” if the patient had two consecutive prediagnosis measurements at least 1.0 g/dL above the HB nadir at diagnosis (“pre-diagnosis baseline HB”, Figure 2). Noteworthy, we assumed a decrement of 1 g/dL based on a previous study that showed an increase in HB levels of 0.7 g/dL (from 13.2 to 13.9 g/dL) after prolactin suppression with cabergoline treatment,18 added to 0.3 g/dL, equating a 2.5% Hb measurement error, as reported by our laboratory.
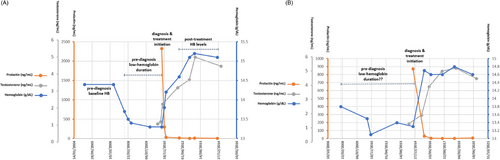
The prediagnosis “low-HB duration” was defined as the time from the first below-baseline HB measurement to hyperprolactinemia diagnosis (Figure 2).
“Posttreatment HB levels” were defined as the calculated average of all HB measurements performed later than 3 months from treatment initiation (Figure 2).
Patients with fewer than four prediagnosis HB measurements were labeled “insufficient data” and were excluded from the study.
If a patient's HB curve had no “prediagnosis baseline HB” (despite > four prediagnosis HB measurements), his HB curve was labeled as “noninformative” (Figure 2). However, data regarding those patients are reported in the results section.
Patients with iron deficiency anemia (mean corpuscular volume < 80 fl, ferritin < 20 ng/mL, or transferrin saturation < 20%), folic acid deficiency (<2.7 ng/mL), vitamin B12 deficiency (<160 pg/mL), or those with elevated TSH levels, were excluded from analysis. Patients with chronic inflammatory diseases (e.g., inflammatory bowel disease or rheumatoid arthritis), chronic infections, or continuously elevated inflammation markers (C-reactive protein or erythrocyte sedimentation rate), active malignancy or an active gastrointestinal bleeding during the follow-up period were not included.
1.3.1 Biochemical evaluation
Serum prolactin levels were measured by immunometric assay (Immulite 2000; Siemens), which has a sensitivity of 0.15 ng/mL. The intra-assay coefficients of variation (CVs) for prolactin concentrations of 22 and 164 ng/mL were 2.3% and 3.8%, respectively; the corresponding interassay CV was 6%. Reference levels for men in our laboratory are 2−20 ng/mL. Levels ≥200 ng/mL were calculated after appropriate serum dilutions.
Total testosterone levels were measured by electrochemiluminescence assay (Cobas e601; Roche Diagnostics, Indianapolis, IN, USA). The total testosterone reference values for adult men in our laboratory are 2.8–9.6 ng/mL.
HB levels were measured using an automatic ABX Micros CRP 200 analyzer (Clinical Laboratory International, Brussels, Belgium), with a laboratory reported HB measurement error of up to 2.5% (for example, for a baseline HB measurement of 16.0 g/dL, a decrease > 0.4 g/dL was considered significant).
The reference levels for all other laboratory tests were determined according to each kit manufacturer's instructions.
1.3.2 Statistical analysis
Study sample size was calculated using the WinPEPI software (Version 11.65, Brixton Health), with a 5% significance level, 80% power, and standard deviations of 1.2 g/dL in the prediagnosis and at-diagnosis groups.18, 19 A clinically significant difference of 1.0 g/dL between HB levels in the two groups was assumed. A minimum of 23 men was required.
Statistical analysis was performed using IBM SPSS version 27.0 (IBM Corp., Armonk, NY). Continuous variables were presented using mean ± SD, or median (IQR), as appropriate. Dichotomous variables were presented by N (%). Differences between study groups were assessed using the t-test, the Mann–Whitney test, and the chi-square test for normally distributed continuous, non-normally distributed continuous, and categorical variables, respectively. Correlations were assessed using Pearson's r and Spearman's r for normal and non-normal continuous variables, respectively. Two-sided p-values less than 0.05 were considered statistically significant.
2 RESULTS
We identified a total of 70 male patients with prolactinoma, diagnosed between January 2010 and July 2022 (Figure 1). Of these, nine men (12.9%) presented with testosterone levels within the normal reference range. Twenty-one men (30%) presented with hypogonadism and normal HB levels (above 13.5 g/dL), and 40 hypogonadal men (57.1%) met the criteria for anemia (HB ≤13.5 g/dL) at diagnosis (Figure 1).
2.1 Comparison of patients with and without anemia at presentation
After the exclusion of 3 patients with other cause of anemia, we compared the baseline characteristics of patients with and without normocytic anemia (HB levels were 12.7 ± 0.6 and 14.4 ± 0.7 g/dL, respectively, p < 0.01, Table 1). At the time of prolactinoma diagnosis, the two groups did not differ in prolactin levels, adenoma diameter, or LH levels (Table 1). However, prolactinoma patients that presented with anemia had lower baseline testosterone levels (1.3 ± 0.8 vs. 2.6 ± 1.1 ng/mL, p < 0.01) and higher rates of sexual dysfunction (81% vs. 53%, p < 0.01, Table 1).
| Group | Anemia at presentation | No anemia at presentation | p-Value |
|---|---|---|---|
| N | 37 | 30 | |
| Hemoglobin (g/dL), mean (SD) | 12.7 (0.6) | 14.4 (0.7) | <0.01 |
| Age at diagnosis (years), mean (SD) | 46.2 (13.8) | 46.4 (15.5) | 0.97 |
| Prolactin (ng/mL), median (IQR) | 1374 (508–3630) | 747 (297–2189) | 0.25 |
| Adenoma maximal diameter (mm), mean (SD) | 25.6 (11.0) | 24.5 (13.8) | 0.71 |
| Testosterone (ng/mL), mean (SD) | 1.3 (0.8) | 2.6 (1.1) | <0.01 |
| Luteinizing hormone (mIU/mL), mean (SD) | 1.7 (1.7) | 1.9 (1.5) | 0.64 |
| Follicle-stimulating hormone (mIU/mL), mean (SD) | 3.3 (3.8) | 2.6 (2.1) | 0.40 |
| Sexual dysfunction, n (%) | 30 (81%) | 16 (53%) | 0.01 |
| Headaches, n (%) | 17 (46%) | 15 (50%) | 0.80 |
- Note: Men with other cause of anemia were excluded (n = 3).
2.2 Patients’ characteristics at prolactinoma diagnosis
Out of 40 hypogonadal men with anemia at diagnosis we excluded three patients with microcytic anemia (i.e., iron deficiency anemia, unrelated to prolactinoma diagnosis), seven patients with insufficient prediagnosis laboratory data, and five patients with “noninformative” HB curves (Figure 1).
The study cohort included 25 male patients that were diagnosed with prolactinoma and concomitant anemia (Figure 1). Mean age at diagnosis was 46.1 ± 14.9 years (Table 2). Median prolactin level at diagnosis was 952 ng/mL (IQR, 374−2612 ng/mL), and mean baseline (maximal) adenoma diameter was 23.8 ± 10.3 mm, including three patients with giant prolactinomas (diameter > 40 mm). All patients had hypogonadotropic hypogonadism at presentation, with mean testosterone levels of 1.3 ± 0.8 ng/mL, and LH and FSH levels of 1.9 ± 1.8 and 3.4 ± 3.7 mIU/mL (n = 24), respectively (Table 2).
| Variable | Cohort |
|---|---|
| N | 25 |
| Age at diagnosis (years), mean (SD) | 46.1 (14.9) |
| Prolactin (ng/mL), median (IQR) | 952 (374–2612) |
| Adenoma maximal diameter (mm), mean (SD) | 23.8 (10.3) |
| Testosterone (ng/mL), mean (SD) | 1.3 (0.8) |
| Luteinizing hormone (mIU/mL), mean (SD) | 1.9 (1.8) |
| Follicle-stimulating hormone (mIU/mL), mean (SD) | 3.4 (3.7) |
| Hemoglobin (g/dL), mean (SD) | 12.9 (0.5) |
| Sexual dysfunction, n (%) | 17 (68%) |
| Headaches, n (%) | 8 (32%) |
2.3 Symptoms duration
At diagnosis, 17 of 25 men (68.0%) complained of sexual dysfunction (decreased libido and/or erectile dysfunction), and eight men (32.0%) suffered from headaches (five patients reported both symptoms). The mean reported duration of hypogonadal symptoms before diagnosis was 2.7 ± 2.4 years, and the duration of headaches was 2.1 ± 3.2 years. Five men had no sexual dysfunction or headaches at presentation: four presented with gynecomastia, and one asymptomatic patient was incidentally diagnosed after performing brain imaging.
2.4 Anemia duration and correlation with clinical manifestations
All 25 patients in our cohort had normocytic anemia at the time of diagnosis, without alternative etiology for anemia other than hypogonadism. All had “informative” prediagnosis HB curves (Figure 2), with a median follow-up (from first baseline HB measurement to last clinic visit) of 14.0 years (IQR, 11.4–15.5 years). The mean prediagnosis baseline HB was 14.4 ± 0.3 g/dL. The first measurement to exhibit HB decline was made (after the last baseline measurement) at an average interval of 19.4 months, with a decrease to a mean HB of 13.5 ± 0.3 g/dL. The mean HB level at prolactinoma diagnosis was 12.9 ± 0.5 g/dL (Table 2, Figure 3A). The median “low-HB duration” (used to estimate hyperprolactinemia duration), from the first measurement that demonstrated HB decline to hyperprolactinemia diagnosis, was 6.1 years (IQR, 3.3–8.8 years, Figure 3B).
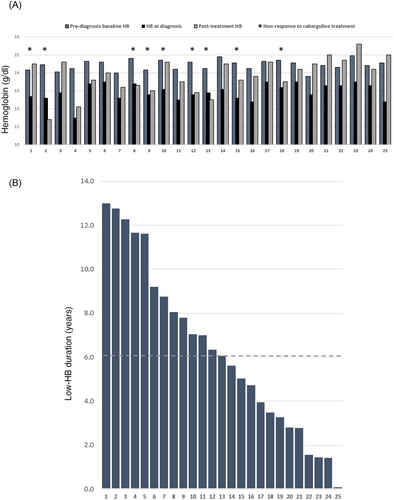
We discovered a positive correlation between the “low-HB duration” and the patient-reported sexual dysfunction duration (n = 17, Pearson's R = 0.502, p = 0.04, Figure 4A). And yet, the “Low-HB duration” was significantly longer than sexual dysfunction duration (n = 17, 7.0 ± 4.5 vs. 2.9 ± 2.5 years, respectively, p = 0.01), with a mean difference of 4.1 years.
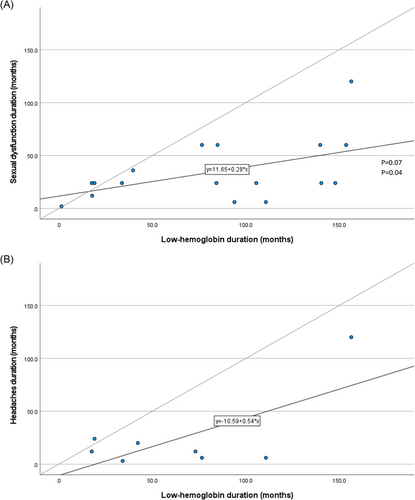
Correlation between the duration of “low-HB” and headaches was statistically insignificant (n = 8, R = 0.677, p = 0.07). Prediagnosis “low-HB duration” was longer than headaches duration (n = 8, 5.5 ± 4.0 vs. 2.1 ± 3.2 years, respectively, p = 0.02), with a mean difference of 3.4 years (Figure 4B).
We found no correlation between “low-HB duration” and baseline prolactin levels (R = 0.100, p = 0.63), testosterone levels (R = 0.164, p = 0.43) or adenoma size (R = 0.035, p = 0.87).
2.5 Posttreatment HB levels and anemia duration, and their association with response to medical treatment
After cabergoline treatment initiation, mean HB levels rose from 12.9 ± 0.5 g/dL at diagnosis to 13.9 ± 0.9 g/dL, while median prolactin levels decreased from 952 ng/mL to 12 ng/mL and mean testosterone levels increased from 1.3 ± 0.8 ng/mL to 3.2 ± 2.0 ng/mL.
We divided our cohort into two groups: men that normalized their prolactin levels (responders), and men that did not achieve prolactin normalization at the end of follow-up (non responders). In patients who responded to treatment (n = 16), mean HB levels increased from 13.0 ± 0.6 g/dL at diagnosis to 14.3 ± 0.8 g/dL (p < 0.01), prolactin dropped from a median of 785 ng/mL to 8 ng/mL (p < 0.01), and testosterone increased from 1.4 ± 0.7 ng/mL to 4.0±1.9 ng/mL (p < 0.01, Figure 5). In those who did not respond to cabergoline (n = 9), HB was 12.8 ± 0.3 g/dL at diagnosis and 13.1 ± 0.9 g/dL at the end of follow-up (p = 0.33), median prolactin was 4039 ng/mL at diagnosis and 51 ng/mL after treatment (p < 0.01), and testosterone was 1.1 ± 0.9 ng/mL and 1.7 ± 1.3 ng/mL (p = 0.15), respectively (Figure 5).
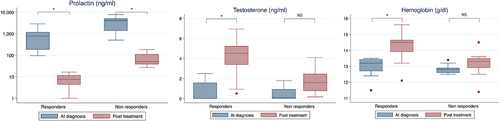
Mean “low-HB duration” was 5.5 ± 4.1 years in men with good response to medical treatment and 7.8 ± 3.2 years in nonresponders (p = 0.18).
2.6 Patients with “noninformative” HB curves
Five men with hypogonadism and normocytic anemia had “noninformative” HB curves (Figure 2) and were therefore excluded from the main cohort (at diagnosis: prolactin range, 1380−4819 ng/mL; mean testosterone, 1.2 ± 1.0 ng/mL). Nonetheless, their mean HB at diagnosis was 12.7 ± 0.5 g/dL, with a median anemia duration of 6.6 years (underestimated, as no normal prediagnosis baseline HB levels were available). After cabergoline treatment, mean HB rose to 13.6 ± 0.9 g/dL, prolactin decreased to 13−125 ng/mL and mean testosterone increased to 3.3 ± 1.9 ng/mL.
3 DISCUSSION
In our registry of male patients with prolactinoma 87% of patients presented with hypogonadotropic hypogonadism and 70% had anemia at diagnosis.
The study cohort included 25 otherwise healthy men that were diagnosed with prolactinoma and concomitant anemia. In this cohort, we identified a marked decrease in HB levels that preceded prolactinoma diagnosis by a median of 6.1 years. We found that the prediagnosis anemia duration correlated well with patient-reported sexual dysfunction duration, and yet, a mean delay of 4.1 years between HB decrease and hypogonadal symptoms appearance was demonstrated. These results support our hypothesis that in men with prolactinoma the hyperprolactinemia precedes the “subjective” hypogonadal symptoms, sometimes by many years. The “low-HB duration” may be used as a more accurate marker of disease duration in a subset of patients (35.7% percent of total male prolactinoma cases, and 62.5% percent of those presented with hypogonadism and anemia, in our cohort).
After review of the literature, we found only two reports concerning the duration of anemia in prolactinoma patients. In 2010, Maclean and Hanley23 described a case of a 70-year-old male evaluated for normocytic anemia (HB of 10.9 g/dL) with no identifiable cause. Six years later, he complained of low libido and long-standing erectile dysfunction, and hyperprolactinemic hypogonadism was demonstrated, together with a pituitary macroadenoma. Noteworthy, BMD established a concomitant diagnosis of osteoporosis. In another case, reported by Iglesias and Dies,24 a 58-year-old male presented with anemia (HB of 11.3 g/dL) and 10 years of erectile dysfunction. The patient's laboratory revealed that he suffered from normocytic anemia 6 years prior to prolactinoma diagnosis. The patient received cabergoline and achieved prolactin normalization, but still suffered from hypogonadism persistence. His anemia resolved only following testosterone replacement treatment.
Previous studies evaluated the presence of anemia in men harboring prolactinoma. Shimon et al.19 evaluated 36 male patients with macroprolactinoma and noticed that 44% percent of the cohort presented with HB below 13.0 g/dL. Mean HB levels increased from 13.2 to 13.9 g/dL following cabergoline treatment. In men who presented with testosterone below 1 ng/mL, baseline HB levels were significantly lower compared to men with higher testosterone levels (12.6 vs. 13.5 g/dL). Iglesias et al.20 investigated the clinical significance of anemia in 26 men with prolactinoma (four patients with microprolactinoma, and 22 with macroprolactinoma). Nine men (35%) presented with HB below 13.0 g/dL. At diagnosis, HB levels were significantly lower in men with hypogonadism compared to eugonadal men. Our study shows that prolactinoma patients who presented with anemia had lower testosterone levels and higher rates of sexual dysfunction, even though prolactin levels were not significantly different between the groups (with and without anemia, Table 1). This finding highlights the variable individual sensitivity to high prolactin levels between different patients.
It should be noted that the high prevalence of anemia in our cohort (70%) stems from the selection of patients with higher prolactin levels (prolactin IQR, 274−2612 ng/mL) and a higher HB cutoff for defining anemia in males (according to the American Society of Hematology definition for anemia, with HB cutoff of 13.5 g/dL). In our cohort, mean HB levels rose from 12.9 ± 0.5 g/dL at diagnosis to 13.9 ± 0.9 g/dL following treatment. This increase in HB levels, of 1.0 g/dL on average, is slightly higher than that described in previous studies, but is appropriate for the study cohort, in whom prediagnosis baseline HB was at least 1.0 g/dL above the HB nadir at diagnosis.
The clinical implications of accurate evaluation of hyperprolactinemia duration are many and of significance for patients' health. Greenspan et al.25 showed that men with prolactinoma and hypogonadism had cortical osteopenia, related to hyperprolactinemia duration. Ensuing studies demonstrated that long-term hyperprolactinemia increases the risk of fractures, and that longer duration of hyperprolactinemic symptoms is associated with lower BMD and higher rate of vertebral fractures.9, 10, 12 There are limited data regarding the effect of hyperprolactinemia (and its duration) on various metabolic parameters, including BMI, HOMA-IR and LDL cholesterol levels.14, 15, 26
The duration of hypogonadal symptoms in our study was 2.7 ± 2.4 years on average. Older studies found longer duration of symptoms in male prolactinoma of 4−5 years.2, 5 However, more recent studies reported shorter symptoms duration.8, 11 These differences may be attributed to improvements in imaging studies and hormonal measurement techniques.
In this study, we identified a more accurate and objective method for the assessment of hyperprolactinemia duration in men with prolactinomas, by retrospectively examining the patients' blood counts and HB levels (Figure 2). As we hypothesized, the anemia duration is significantly longer than the subjective patient-reported symptoms duration. Although this finding has clinical importance, the approach we used has several limitations. It relies on the presence of many routine blood tests in young and healthy men (average age at diagnosis of 46 years), which is often not available. The reliability of this method requires that no external factors will affect the patient's HB level over time, but this is not always the case as any event of dehydration, infection or bleeding may alter HB levels. In addition, while monitoring an informative HB curve allows a better assessment of hyperprolactinemia duration compared to symptoms duration, it still reflects an underestimation. In some cases, there is a long time-interval between the last test that exhibited a normal HB as baseline, and the next test, that exhibited a drop in HB level, from which the anemia interval counts, as it is impossible to determine exactly when, within that interval, anemia appeared. Finally, this method has been evaluated only in a subset of hypogonadal men with significant hyperprolactinemia and anemia.
Our study has limitations. Due to its retrospective nature, it was impossible to assess symptoms severity, and patient-reported measures may have been subject to recall bias. We had no information regarding smoking habits or alcohol consumption. We did not exclude overweight patients, or patients with disorders of glucose metabolism, despite the possibility that these disorders may indirectly influence HB levels.27, 28 The total testosterone reference values for men in our laboratory (2.8–9.6 ng/mL) may slightly differ from those generally used in recent guidelines.29 Finally, we used a single reference range for total testosterone measurement for all patients, although there is a modest age-related decline in normal testosterone levels in men over 50 years of age.30
In conclusion, over 50% of male patients with macroprolactinoma exhibit anemia at diagnosis. In our cohort of otherwise healthy men with prolactinoma and hypogonadism, we found a marked decrease in HB levels that preceded prolactinoma diagnosis by a median of 6.1 years. We found that prediagnosis “low-HB duration” correlated well with patient-reported sexual dysfunction duration, and yet, a mean delay of 4.1 years between HB decrease and symptoms appearance was demonstrated. These results support our hypothesis that HB decline prior to prolactinoma diagnosis may serve as a marker for hyperprolactinemia onset in a subset of hypogonadal men and allow a more accurate assessment of disease duration.
AUTHOR CONTRIBUTIONS
All authors contributed to study conception and design. YR and IS performed a literature search. YR, HDB, GT, HMI, AA, and IS contributed to data collection. YR, IR, and IS contributed to data analysis and synthesis. YR and IS drafted the first version of the manuscript. All authors contributed to writing and critically reviewing the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
FUNDING INFORMATION
The authors received no external funding for this study.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.