Spontaneous alterations in semen parameters are associated with age, accessory gland function and the FSHB c.-211G>T variant
Abstract
Background
There is a strong within-subject alteration of semen parameters in men with infertility. However, it remains unknown in which subgroup variations are likely to occur and which semen parameters are affected.
Objective
To evaluate parameters associated with spontaneous alterations in semen analysis.
Patients and methods
We retrospectively selected 3456 men with infertility without known causes affecting spermatogenesis or sperm output for analysis of repeated ejaculate samples. Exclusion criteria comprised sperm concentration <1 million/mL, abnormal follicle-stimulating hormone or low testosterone, and low bitesticular volume (<10 mL). Grouped linear two-level nested mixed-effect models were applied. The analyzed parameters included abstinence time, bitesticular volume, age, accessory gland markers, follicle-stimulating hormone, and FSHB c.-211 variants.
Results
Groups include A (n = 397): ≥1.0 to <5.0 million/mL, B (n = 708): ≥5.0 to <15.0 million/mL, and C (n = 2351): ≥15.0 million/mL. Groups A, B, and C: changes in ejaculate volume were associated with alterations in total sperm count and motility (p < 0.003). Changes were, controlled for abstinence time (p < 0.001), related to α-glucosidase, fructose, or zinc (p = 0.005–0.02). Group A + B: fluctuations in follicle-stimulating hormone level influenced sperm concentration/count (p = 0.004–0.02), albeit only in men with FSHB c.-211 GG (p = 0.007–0.02). T-allele carriers did not show changes in follicle-stimulating hormone levels (p > 0.1). Group B: age <50 years (p = 0.007–0.01) and normal bitesticular volume (p = 0.008–0.02) were associated with spontaneous increases in sperm concentration, count, and motility.
Conclusion
Semen parameters exhibit intra-individual alterations associated with organic, hormonal, and genetic variables. Changes are pronounced in younger men with normal bitesticular volume and oligozoospermia to almost normozoospermia. The effect is modulated by abstinence time, accessory gland function, and fluctuations in follicle-stimulating hormone level, which is bound to FSHB c.-211G>T variant. Judgment of semen analysis should be based on two semen samples, with abstinence times between 4 and 5 days. As a future perspective, it might be investigated whether younger men with normal bitesticular volume who are unable to elicit increases in serum follicle-stimulating hormone (FSHB c.-211 genotype of GT/TT) benefit from improving accessory gland function and increasing follicle-stimulating hormone.
1 INTRODUCTION
Infertility is a condition defined by not achieving a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse. Fertility problems affect about 10%−15% of couples of childbearing age. In about 30%−40% of infertile couples, the male partner is the major or sole cause of infertility.1 Partly, but not exclusively, male infertility is characterized by low sperm counts, reduced sperm motility, or a low percentage of spermatozoa with normal morphology. Men with infertility may suffer from genetic problems or hormone disorders, but a majority present as patients with so-called idiopathic infertility, which means that the cause is not known.2 About 7%−10% of men in their reproductive age suffer from reduced fecundity or infertility because of testicular, pre-testicular, or post-testicular conditions such as varicocoeles, cryptorchidism and hypogonadism as well as genetic issues.3 In addition, idiopathic infertility is found in more than one-third of infertile patients.4, 5 After careful diagnostic work-up, in 30% of men underlying and potentially treatable reasons for couple infertility may be elucidated.6 Couples with a male partner below reference values defined by the World Health Organization (WHO) are considered to have a male factor infertility. Other factors associated with male infertility are low ejaculate volume and decreased seminal markers of epididymal, prostatic, and seminal vesicle function, that is, α-glucosidase, zinc, and fructose.7
Recently, a single-nucleotide polymorphism (SNP; FSHB c.-280G>T, rs10835638, commonly denoted as FSHB -211G>T) in the promoter of the FSHB gene, coding for the β-subunit of follicle-stimulating hormone (FSH), has been observed to be associated with reduced serum FSH concentrations in men,8 and an increased prevalence of the −211 T allele of the FSH β-subunit promoter polymorphism connected to lower serum FSH concentrations in men with infertility has been described.9-12 Men with a GG genotype (wildtype) exhibit higher FSH concentrations than men with a GT or TT genotype.8, 13 It might be speculated that men with the GG wildtype are also able to elicit fluctuations of FSH concentrations that are more pronounced than those men with blunted FSH production due the GT or TT genotype.
Generally, a within-subject alteration of semen parameters is observable, occurring within a supposed intrinsic biological variation around a homeostatic setting point. Such a setting point might be defined by age, accessory gland function, testicular function, FSH, and related genetics. That means, different ejaculate samples from the same man may vary without therapy. Hence, the recommendation is to assess at least two semen samples because of this spontaneous variation of values and that such assessments should be performed with a time gap of 60–90 days to cover two different spermatogenic cycles.7 It is, however, not clear, in which potential subgroup of male patients with a factor affecting fertility the above-named variations are likely to occur and which semen parameters are likely to be affected or to be stable.14 Moreover, a recent guideline does not recommend a second semen analysis in males with normozoospermia and couple infertility, as normozoospermia is supposed to be a “normal” and stable condition,15 although evidence is weak.
The topic of ejaculate variability in men with infertility was recently analyzed with respect to within-subject and between-subject variability in 436 men with couple infertility.16 The within-subject variation was marked for all parameters (semen volume, sperm concentration, forward motility, and combined parameters). Nevertheless, such within-subject fluctuations were still smaller than the between-subject variability. This analysis was not stratified for markers of accessory gland function, hormones, testicular volume, or genetic setting points.
The study presented here aims at the elucidation of factors that influence such changes in semen parameters and might be associated with spontaneous improvements or deteriorations in standard ejaculate analysis without clinical intervention (i.e., medication). Such factors could be external, such as abstinence time and age, clinical nature (accessory gland function or testis volume, serum FSH concentrations), or defined by a genetic setting (e.g., the FSHB-211 promotor gene polymorphism).
This study includes men with unexplained infertility and does not include fertile men. Our intention was to restrict the information as a subject of research to men with factors related to fertility that are still normal and thus cannot explain the infertility as such.
Derived from this, a description of factors associated with an improvement in ejaculate parameters might identify potential treatment pathways, especially in subgroups of patients likely to benefit from interventions.
1.1 Hypothesis and aim
The WHO recommends two ejaculate analyses to obtain a comprehensive picture regarding the semen quality of men with fertility issues. We assume that in some men with idiopathic infertility, ejaculate parameters may spontaneously change in terms of sperm concentration, total sperm count, sperm motility, or sperm morphology without interventional medication apart from regression to the mean effects. The study aims to identify factors in conventional semen analysis based on the patients’ clinical data, genetic setting of the FSHB-211 promoter gene, and serum concentrations of FSH that might contribute to such variations.
Primary aim: To assess the factors that influence the results of a repeated conventional ejaculate analysis in subgroups of men in terms of clinically meaningful changes.
This was a longitudinal and retrospective approach, and each subject donated at least two semen samples, the first two of these were to analyzed. Sample data were acquired at the time of semen and blood donation. We did not use archived samples for later analysis.
2 METHODS
2.1 Study population
All patients who donated semen samples between September 1, 2010, and August 31, 2020, and had a sperm concentration of >1 million/mL were retrospectively identified among those who attended the Department of Clinical and Surgical Andrology, Centre of Reproductive Medicine and Andrology, University Clinic Münster (CeRA) by using our in-house database Androbase with complete documentation of clinical, laboratory, and genetic findings.17
All subjects provided written informed consent to the analysis of serum, ejaculate, and other material as approved by the Ethics Committee of the University and the State Medical Board (codes 2009-164-S; 2013-255-f-S). The study was performed according to the Declaration of Helsinki.
A total of 6153 patients with an initial sperm concentration >1 million/mL were identified, and further selection was performed according to the following criteria.
Exclusion criteria were as follows: serum concentration of total testosterone <12.0 nmol/L, FSH serum concentrations <1or >7 IU/L to exclude endocrinologically visible suspicion of impaired spermatogenesis, bitesticular volume (bTV) <10 mL to exclude men with a clear clinical suspicion of testicular impairment of spermatogenesis, history of unilateral orchiectomy, cryptorchidism, varicocoele testis of any degree, history of oncological disease, infections of the urogenital tract (see below), genetic findings (i.e., abnormal karyotype, deletions in the AZF-gene), diabetes mellitus of type 1 or type 2, men with gender dysphoria with or without any kind of treatment, current or previous treatment with finasteride, spironolactone, human chorion gonadotropin or other gonadotropins, ketoconazole, cimetidine, raloxifene, antiestrogens/selective estrogen receptor modulators, aromatase inhibitors, clomiphene citrate, testosterone preparations, dihydrotestosterone, estrogens, progesterone, synthetic progestins, luteinizing hormone (LH) releasing hormone analogs, gonadotropin releasing hormone (GnRH) antagonists.
Inclusion criteria were as follows: age ≥18 years, at least two ejaculate analyses (70–90 days between analyzed data sets) to account for two different cycles of spermatogenesis, sperm concentration in first analysis >1.0 million/mL (because ejaculate volume was a parameter of investigation, we abstained from using total sperm count), sperm motility (a + b) ≥1%, sperm morphology (normal spermatozoa) ≥1%, ejaculate volume ≥0.8 mL (half of lower limit of normal) to exclude severe obstructions or errors during donation procedure, no sign of infection (negative for bacteria, ureaplasma, mycoplasma, chlamydiae), leukocyte concentration <1 million/mL of seminal plasma, abstinence time 2−7 days, ultrasound investigation of the testes, hormone analysis including sex steroids and gonadotropins.
2.2 Hormone assessments
All venous blood samples were obtained between 08:00 am and 12:00 pm. Serum or plasma was separated at 800 g. Samples were analyzed after collection or snap frozen and stored at −20°C.
Serum testosterone concentrations were measured by a commercial ELISA kit (DRG Instruments GmbH, Marburg, Germany). This immunoassay for testosterone is calibrated quarterly against standards using liquid chromatography–mass spectrometry. The immunoassay regularly passes this quality check and reproduces the results of mass spectrometry with an imprecision of <10% in the range for serum testosterone concentrations between 5 and 20 nmol/L. Intra-assay coefficients of variation (CVs) were below 2%, and mean inter-assay CVs were below 5%.
Serum concentrations of FSH and LH were determined using highly specific time-resolved fluoro-immunoassays (Autodelfia, Freiburg, Germany). Mean intra-assay CVs were below 2%, and mean inter-assay CVs were below 5%. Proteo-hormone assays are under quarterly blinded external quality control and pass regularly.
2.3 Ejaculate analysis
Semen analysis was performed according to the 2010 WHO criteria.7 Semen analysis was subjected to rigorous internal and external quality control. The institution participates in an external quality control program of the German Society of Andrology (QuaDeGA) and passes regularly. Progressive sperm motility was defined as combined a- and b-form motility.
Neutral α-glucosidase, zinc, and fructose were measured by multiwell spectrophotometric assays.7 Samples were measured in duplicate, together with quality control pooled samples containing medium concentrations of markers that were included at the beginning and the end of the assay to monitor drift. Assay results were accepted when duplicates agreed within 10%, drift was <15%, and internal quality control samples fell within 2SD of the mean pre-assay values. Lower limits of reference in our laboratory are 13 μmol per ejaculate for fructose, 20 mU per ejaculate for α-glucosidase, and 2.4 μmol per ejaculate for zinc.7, 18
2.4 Genotyping
Genomic DNA was extracted from EDTA-preserved blood using the FlexiGene DNA extraction kit (QIAGEN, Düsseldorf, Germany). Genotyping was performed with the StepOnePlus Real-Time PCR-System, the TaqMan GTXpress Master Mix, and a TaqMan genotyping assay mix customized for the FSHB c. -280G>T SNPs (rs10835638, NC_000011.10:g.30230805G>T and NM_000510.2:c.-280G>T, most commonly denoted as FSHB -211G>T referring to the position upstream of the mRNA transcription start site, which is predicted 69 bp upstream of the ATG codon). The thermal cycling conditions were as follows: 2 min at 50°C followed by 15 min at 95°C and 35 cycles of 15 s at 95°C plus 1 min at 60°C. All genotyping results are documented in Androbase and have been submitted to dbSNP database.
2.5 Testicular ultrasound
Testicular volume was determined by ultrasonography (ultrasound scanner ProFocus; BK Medical, Gentofte, Denmark), and measurements of the testes were performed by applying a high-frequency 15-MHz linear array scanner. The volume was calculated by using the ellipsoid method19 and was in agreement with current standards.20
2.6 Statistics
- Group A: ≥1.0 to <5.0 million/mL
- Group B: ≥5.0 to <15.0 million/mL
- Group C: ≥15.0 million/mL
Parameters remained continuous within their groups.
The specific thresholds were selected according to the fifth WHO manual for semen analysis and further literature. A sperm concentration below 5 million/mL is named severe oligozoospermia. A sperm concentration between 5 and 15 million/mL is categorized as moderate to mild oligozoospermia. Values starting from 15 million/mL are categorized as normal.7, 21, 22
Power calculations were based on the known effects seen in repetitive ejaculate analyses (within-between interaction) in order to detect clinically meaningful changes in sperm concentration, total sperm count, sperm motility, and/or sperm morphology in comparison to the first investigation/baseline. We used an estimated effect size of 0.25 with a two-sided α-error = 0.01 and power = 0.95: a sample size of at least n = 54 was identified. Post hoc analysis using the same parameters on the study population yielded a power = 0.99 (calculated on PASS 14.0).
All other statistical analyses were performed using spss (Version 28.0; Chicago, IL, USA). The main type of analysis was as follows. The Shapiro–Wilk test was used for assessment of normal distribution. Variables that were not normally distributed were log-transformed or arcsin-transformed (percentage values) for parametrical analyses. Linear two-level nested mixed-effect models for repeated measurements were used. Individual differences were accounted for by using each individual patient code included in the analysis as random factor. The time between investigations was also defined as a random effect factor as well as an intercept (i.e., each patient and the time between the two investigations were included in the analysis acting as control).
- Delta ≥1.8 mL in ejaculate volume (more than 1SD) (decrease/no major change/increase)
- Delta ≥1.5 IU/L in FSH concentrations (more than 1SD) (decrease/no major change/increase)
- Age <50 years (threshold to recommend assisted reproduction according to father's age) (yes/no)
- bTV ≥20 mL (lower limit of normal) (yes/no)
These categorical parameters were created to investigate clinically relevant variables and are seen as of more practical value than a continuous parameter.
In addition, changes in abstinence time and changes in seminal glucosidase, fructose, and zinc content were evaluated as second-level nested continuous variables related to ejaculate volume if the latter exhibited a significant effect in either of its superposed categorical variables.
In the same manner, the FSHB-211 gene promotor polymorphism was included as second-level nested nominal variable (TT vs. GT vs. GG genotype) when changes in FSH concentrations had a significant effect in either of the ordinal categories.
Prior to this application of mixed linear models for repeated measurements, these possible factors related to sperm concentration were identified by an initial stepwise regression analysis of the primary ejaculate of the subjects. This analysis excluded testosterone, LH, and body mass index as effectors of sperm concentration in this cohort. Low testosterone and abnormal LH would not fit to the prerequisite that only men with unexplained infertility were to be examined. Those men would have primary or secondary hypogonadism and thus not fulfill the criterion of unexplained infertility.
Other tests where applicable and reported with data/tables: Kruskal–Wallis test, anova with Games–Howell test for unequal n and unequal variances (post hoc), Levene test for unequal variances, and Breusch–Pagan tests for heteroscedasticity.
3 RESULTS
3.1 Subgrouping of study cohort
According to the above-named selection criteria, the following groups of patients were included—initial sperm concentration ≥1.0 to <5.0 million/mL: Group A (n = 397), ≥5.0 to <15.0 million/mL: Group B (n = 708), ≥15.0 million/mL: Group C (n = 2351), summing to a total cohort of 3456 patients. For descriptive results and baseline comparisons of groups, see Table 1. The FSHB-211 TT genotype was present at a significantly higher amount within Group A, while the GG genotype was more present in Group C. Detailed data on FSH concentrations according to the FSHB-211 genotype are stated in Table 2.
| Parameter | Overall cohort (N = 3456), median, range | Group A: ≥1.0 to <5.0 million spermatozoa/mL (N = 397), median, range | Group B: ≥5.0 to <15.0 million spermatozoa/mL (N = 708), median, range | Group C: ≥15.0 million spermatozoa/mL (N = 2351), median, range | Kruskal–Wallis test (p) between groups |
|---|---|---|---|---|---|
| Age (years) | 35, 18−75a | 34, 18−62a | 34, 18−71a | 35, 18−75a | 0.039 |
| FSH (IU/L) | 3.5, 1.0−7.0b | 4.2, 1.0−7.0b | 3.8, 1.0−7.0b | 3.4, 1.0−7.0b | <0.001 |
| LH (IU/L) | 3.1, 0.9−14.5 | 3.4, 0.8−13.2 | 3.3, 0.9−14.5 | 3.0, 0.8−11.9 | <0.001 |
| Total testosterone (nmol/L) | 19.5, 12.0−36.6a | 19.4, 12.0−33.3a | 19.8, 12.0−34.3a | 19.5, 12.0−36.6a | 0.49 |
| Sperm count (million/ejaculate) | 160.6, 0.8−2863.4 | 10.3, 0.8−46.6 | 35.6, 4.6−116.4 | 172.2, 12.1−2863.4 | <0.001 |
| Sperm concentration (million/mL) | 40.2, 1.0−536b | 1.2, 1.0−4.9b | 2.8, 5.0−14.9b | 42.3, 15.0−536.6b | <0.001 |
| Progressive motility (%) | 45, 1−79a | 33, 1−70a | 42, 1−71a | 49, 1−79a | <0.001 |
| Normal morphology (%) | 4, 1−13a | 2, 1−9a | 3, 1−9a | 4, 1−13a | <0.001 |
| Ejaculate volume (mL) | 3.6, 0.8−14.0a | 3.6, 0.8−11.6a | 3.7, 0.9−14.0a | 3.5, 0.8−13.9a | 0.07 |
| α-Glucosidase (mU/ejaculate) | 90.5, 1.2−671.7 | 62.0, 1.2−337.4 | 71.5, 1.4−349.7 | 100.8, 1.3−671.7 | <0.001 |
| Fructose (μmol/ejaculate) | 58.9, 1.3−508.4 | 43.6, 1.4−272.4 | 45.1, 1.3−276.9 | 40.1, 1.5−508.4 | <0.001 |
| Zinc (μmol/ejaculate) | 7.2, 1.2−56.2 | 6.9, 1.2−34.6 | 7.0, 1.3−34.4 | 7.4, 1.3−56.2 | 0.15 |
| Bitesticular volume (mL) | 41, 10−81a | 36, 10−66a | 37, 10−72a | 44, 10−81a | <0.001 |
|
FSHB-211 genotype, N, % Hardy−Weinberg equilibrium (p) |
TT = 102, 3.0% GT = 922, 26.7% GG = 2432, 70.4% p = 0.16 |
TT = 17, 4.3% GT = 120, 30.2% GG = 260, 65.5% p = 0.44 |
TT = 19, 2.7% GT = 219, 30.9% GG = 470, 66.4% p = 0.12 |
TT = 66, 2.8% GT = 583, 24.8% GG = 1702, 72.4% p = 0.35 |
Chi-square test between groups: p < 0.001 |
- Note: Median and range, non-parametrical comparison of multiple groups according to the non-parametrical Kruskal–Wallis test. Normal ranges for α-glucosidase >20 mU/ejaculate, fructose >13 μmol/ejaculate, and zinc >2.4 μmol/ejaculate.
- Abbreviations: FSH, follicle-stimulating hormone; LH, luteinizing hormone.
- aLower limit because of selection process.
- bRange because of selection process.
| Group | FSHB-211 genotype | N | Mean | SD |
Post hoc test TT versus GT, p |
Post hoc test TT versus GG, p |
Post hoc test GT versus GG, p |
|---|---|---|---|---|---|---|---|
| A: low sperm count (≥1.0 to <5.0 million/mL) | TT | 17 | 3.5 | 1.5 | 0.15 | 0.08 | 0.60 |
| GT | 120 | 4.1 | 1.8 | ||||
| GG | 260 | 4.2 | 1.6 | ||||
| All | 397 | 4.1 | 1.6 | ||||
| B: low to normal sperm count (≥5.0 to < 15.0 million/mL) | TT | 19 | 3.2 | 1.5 | 0.18 | 0.03 | 0.01 |
| GT | 219 | 3.7 | 1.5 | ||||
| GG | 470 | 4.0 | 1.5 | ||||
| All | 708 | 3.9 | 1.5 | ||||
| C: normal sperm count (≥15.0 million/mL) | TT | 66 | 2.7 | 1.3 | 0.02 | <0.001 | <0.001 |
| GT | 583 | 3.1 | 1.3 | ||||
| GG | 1702 | 3.6 | 1.4 | ||||
| All | 2351 | 3.4 | 1.4 | ||||
| Total cohort | TT | 102 | 2.9 | 1.4 | <0.001 | <0.001 | <0.001 |
| GT | 922 | 3.4 | 1.4 | ||||
| GG | 2432 | 3.7 | 1.5 | ||||
| All | 3456 | 3.6 | 1.5 |
- Note: Detailed data on FSH concentrations according to the FSHB-211 genotype. Serum FSH concentrations (baseline) according to groups of sperm concentration and FSHB-211 genotype. Univariate anova, post hoc test: Games–Howell for unequal n and unequal variances (according to Levene test for unequal variances, Breusch–Pagan test for heteroscedasticity, both not significant).
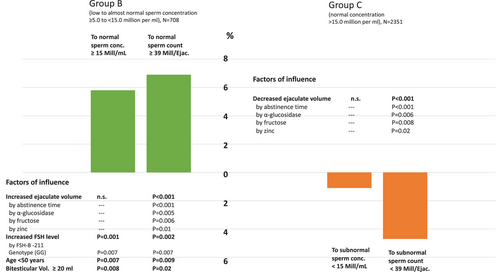
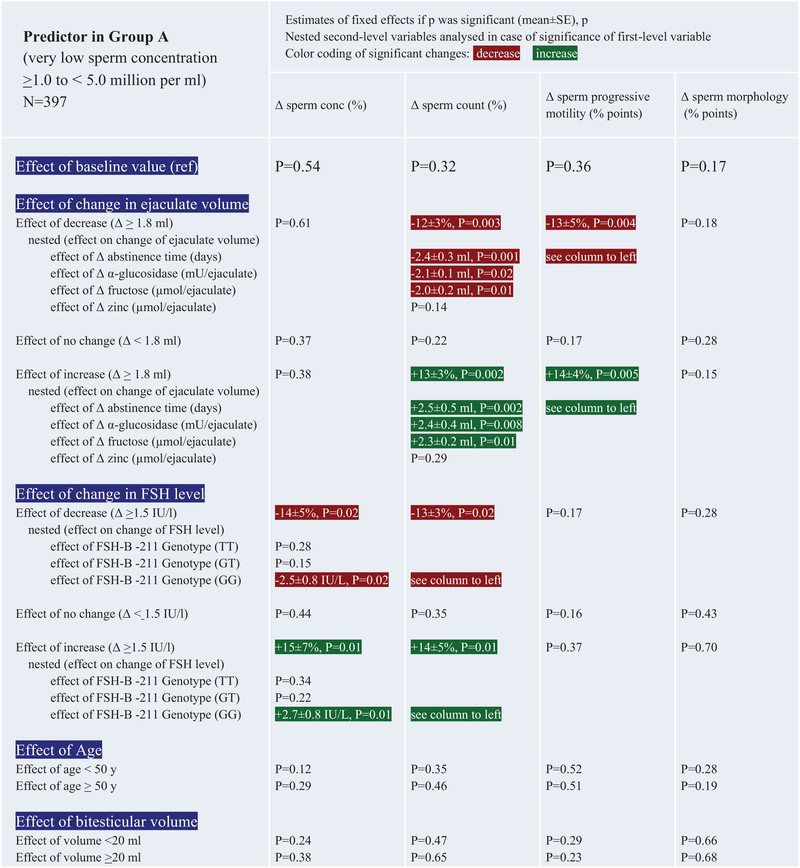
A certain proportion (percent) of patients spontaneously changed WHO criteria from mild oligozoospermia to normozoospermia and vice versa from the first to the second investigation. This is displayed in Figure 1 with associated parameters of linear two-level nested mixed-effect models; also see Table 3B,C for further details.
 |
 |
 |
- Note: Patient ID and time between investigations were used as random effect variables and were not significant in any model. Effects of second-level nested variables refer to changes in the first-level variable, for example, if changes in ejaculate volume had a significant effect on sperm count, the nested variable, for example, fructose content, is examined regarding its effect on changes on ejaculate volume and not on sperm count itself. As this result does not change from column to column because the change in ejaculate volume is the same, this is referred to as “see column to left” in case a change of the first-level variable was significant for more than one ejaculate parameter.
- Abbreviation: FSH, follicle-stimulating hormone.
3.2 Impact of ejaculate volume, FSH, age, and testicular volume on semen parameters in Groups A, B, and C
The following models (as detailed in Table 3A–C) report variables affecting changes in ejaculate parameters between the first and the second investigation and were obtained by linear two-level nested mixed-effect models. Variables were log-transformed or arcsin-transformed where required.
The effect of changes in ejaculate volume is observable in men with very low, low to almost normal, and normal sperm concentrations, having an impact on total sperm count rather than sperm concentration itself (Table 3A–C). Independent of the total sperm count, the percentage of progressively motile spermatozoa also increases or decreases with ejaculate volume.
Especially in men with sperm counts from ≥5.0 to <15.0 million/mL (Table 3B), spontaneous changes in ejaculate parameters are also dependent on younger age and normal testicular volume. The effect of age and testicular volume was not observable in men with severely decreased or normal sperm concentrations (Table 3A,C). Thus, a spontaneous improvement of sperm count or sperm concentration was possible in men with bTVs of >20 mL and/or age <50 years, albeit only in those patients with low to normal sperm concentrations.
3.3 Parameters affecting changes in ejaculate volume
As spontaneous changes in ejaculate volume had a marked effect on semen parameters in all groups, putative factors related to ejaculate volume were also assessed by stepwise backward multiple regression models in all subjects as whole group. Independent predictors for changes in ejaculate volume in multiple regression models over all groups were changes in abstinence time (p < 0.001), changes in seminal α-glucosidase (p = 0.01), changes in seminal fructose (p = 0.007), changes in seminal zinc (p = 0.01), and age <50 years (p = 0.008).
3.4 Parameters affecting fluctuations in serum concentration of FSH
Spontaneous fluctuations in FSH concentrations also seem to contribute to sperm output, mainly via changes in sperm concentration and thus total sperm count, at least in men with lower sperm concentrations than normal (see Table 3A–C). However, this is only the case in men with the FSHB-211 promotor gene wildtype (GG). This could also be seen in baseline values of this study, particularly in patients with low to normal or normal sperm concentrations (Table 2). Hence, the observed fluctuations in FSH concentrations, be it a decrease or an increase, are more likely to occur spontaneously in men with a GG genotype. Men carrying a TT or GT genotype in the FSHB-211 gene polymorphism presented lower FSH concentrations also in this study (Table 2). These patients are not as likely to exhibit fluctuations in FSH concentrations as do the carriers of the GG genotype and, thus, are not able to induce marked spontaneous changes in sperm concentrations facilitated via FSH release (Table 3A–C).
4 DISCUSSION
In this study, we included 3456 men with idiopathic infertility and compared changes in ejaculate/semen parameters between two consecutive visits without intermittent medical intervention. A limitation of our approach is the retrospective nature of the study, which cannot exclude the possibility of bias within the data set.
The patient selection process excluded identifiable underlying factors influencing spermatogenesis or ejaculation. However, the selection process included patients with pathological findings in terms of low sperm count/concentration, low sperm motility, low sperm morphology, and low ejaculate volume. The purpose of the analysis was to identify factors that might contribute to spontaneous changes in pathological findings in terms of sperm count or concentration.
In all three groups, separated by very low/low-normal/normal sperm count, we observed changes in ejaculate volume, which had an impact on total sperm count and sperm motility. Changes in ejaculate volume itself were dependent on abstinence time which is in agreement with a recently published study regarding longer abstinence time and related increases in sperm counts.23 Subsequently, markers of accessory gland function, such as α-glucosidase (epidydimal function), fructose (seminal vesical function), and zinc (prostate function), can indicate alterations in ejaculate volume. Especially in men with low to almost normal sperm counts, spontaneous changes in ejaculate parameters are also dependent on younger age and normal testicular volume.
Additionally, spontaneous fluctuations in FSH concentrations also seem to contribute to sperm output, mainly via changes in sperm concentration and thus total sperm count, at least in men with lower sperm concentrations than normal. However, this is only the case in men with the FSHB-211 promotor gene wildtype (GG).
Overall, ejaculate parameters such as total sperm count and sperm concentration and the amount of progressively motile spermatozoa may change in some individuals with idiopathic infertility up to 10%−15% over time without therapy while sperm morphology does not seem to be a subject of spontaneous alterations.
Apart from the description in either direction (deterioration or improvement of sperm parameters), a major clinical question is in which patients improvements of the ejaculate are possible or likely to occur in a spontaneous manner and to what extent such changes could be induced by therapeutical approaches. Regarding ejaculate volume and, hence, total sperm count this seems to be possible in all patient groups that are described here. A higher ejaculate volume at the second investigation is caused by longer abstinence time and, independently from this, a spontaneous improvement of urogenital accessory gland function, indicated by an increase in marker substances (glucosidase, fructose, zinc). Patients did not receive any antibiotic or anti-inflammatory treatment.
As a putative clinical consequence of the above-named results, one might speculate that especially in men with low-normal sperm parameters, normal testicular size, and age younger than 50 years a treatment attempt to increase ejaculate volume would be worthwhile. This could, for example, be an anti-inflammatory regimen. Cochrane authors conducted a review including 61 randomized controlled trials comparing 18 different antioxidants with placebo, no treatment or another antioxidant in a total population of 6264 subfertile men. It was calculated that out of 100 subfertile men not taking antioxidants, seven couples would have a clinical pregnancy, compared with between 12 and 26 couples per 100 who would have a clinical pregnancy if taking antioxidants.24 However, it is worth mentioning that some degree of oxidative stress is necessary for capacitation and acrosome reaction,25 suggesting that antioxidants should not be prescribed indiscriminately.
An evaluation of men with abnormal serum concentrations of FSH, low testosterone concentrations, low testicular volume or infections of the urogenital tract would also have been of interest. However, these men could not be given the attribute of “unexplained infertility,” as hypogonadism, abnormal FSH serum concentrations, low testicular volume or infections might point to a pathology of the testis, the pituitary or accessory glands. Thus, our results are restricted to men with unexplained infertility and cannot be generalized to all men with infertility, especially to those with putatively treatable and known causes for their infertility (possible treatment by gonadotropins, estrogen receptor modulators, aromatase inhibitors, antibiotics, for example).
In addition, an intact production of FSH, as seen in men with the FSHB-211 promotor gene polymorphism wildtype (GG) can contribute to spontaneous increases in FSH and, hence, higher sperm concentrations. These men have a reportedly higher pituitary production of FSH upon GnRH stimulation.26, 27 They present with higher FSH concentrations than men carrying the GT or TT genotype.13 It might be speculated that the external administration of recombinant FSH in men with the FSHB-211 promotor gene TT or GT variant might exhibit a similar effect,13 as these patients do not seem to be able to elicit spontaneous higher outputs of FSH. Such approaches have been proposed for placebo-controlled larger trials28 and positive results were seen in smaller studies, albeit without placebo-controlled arms.10, 29-31
Consequently, in normozoospermic men, significant fluctuations in semen quality can also be observed by variations in semen volume and biochemical markers. Thus, these men should have a second evaluation of their ejaculate in accordance with WHO recommendations, with an abstinence time of 4−5 days. Depending on the variables influencing the first semen analysis, semen characteristics may be over- or underestimated and thus decision making for further treatment options in couple infertility would not be set up with a fully valid result.
5 CONCLUSION
According to our study results, supporting World Health Organization recommendations, we suggest basing the judgment on semen quality on at least two semen samples, ideally with an abstinence time between 4 and 5 days. Overall, spontaneous changes in ejaculate parameters in men presenting with infertility are most pronounced in younger men with normal testicular volumes and low to almost normal sperm counts. The effect is modulated by abstinence time, accessory gland function and fluctuations in follicle-stimulating hormone concentrations, which, in turn, are bound to the FSHB-211 promotor gene polymorphism. For future clinical trials, these factors should be considered, and influenceable factors such as abstinence time should be kept constant/in a comparable fashion. Putatively and as a future perspective, especially a subgroup of patients might benefit most from therapeutical approaches improving accessory gland function by reducing inflammatory stress and increasing follicle-stimulating hormone concentrations (consider follicle-stimulating hormone treatment): men with a low to low-normal sperm count of younger age, impaired accessory gland function and/or a FSHB-211 promotor genotype of GT or TT. A set of parameters for the selection of a susceptible cohort of men with infertility for future interventional trials and also daily clinical assessment as well as treatment is thus presented here.
AUTHOR CONTRIBUTIONS
Study concept, cohort selection, data/statistical analysis, and writing manuscript: Michael Zitzmann. Writing manuscript and gave valuable comments during discussions of the outcome: Maria Schubert. Statistical analysis and valuable comments during discussions of the outcome: Andrea Sansone. Responsible for patient phenotyping and data acquisition, valuable comments during discussions of the outcome, and manuscript writing: Sabine Kliesch. All authors agree to publish the data of the study in this manuscript.
ACKNOWLEDGMENTS
We thank Jörg Gromoll and Lisa Lahrmann for the assessments of the FSHB c.-211 genotype. This study is supported by the German Research Foundation: Clinical Research Unit 326 (CRU)—Male Germ Cells from Genes to Function. Role: Clinical rotation position MS, genotyping of patient cohort.
CONFLICT OF INTEREST STATEMENT
Andrea Sansone was granted from Italian Ministry of University (PRIN Grant # 2017S9KTNE_002), and he was/is paid consultant for Menarini International and MySecretCase.com. Sabine Kliesch has been/is paid speaker and participates in data safety monitoring or advisory board for: Besins Health Care and Merck Serono; granted for participating in EDDIG Study (Kranus Health). Michael Zitzmann and Maria Schubert declare no conflicts of interest.
REFERENCES
Open Research
DATA AVAILABILITY STATEMENT
Data will be available upon request.