Li-ESWT treatment reduces inflammation, oxidative stress, and pain via the PI3K/AKT/FOXO1 pathway in autoimmune prostatitis rat models
Bin Feng and Zhilong Dong contributed equally to this work and co-first authors.
Abstract
Background
Due to limited data on the pathogenesis of chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) and the suboptimal therapeutic effect, the development of new and effective treatment modalities was needed urgently. Low-intensity extracorporeal shock wave therapy (Li-ESWT) has been reported for the treatment of CP/CPPS. However, the underlying mechanism remains to be elucidated.
Objective
To interrogated the efficacy and the mechanism of Li-ESWT in the treatment of CP/CPPS.
Materials and Methods
According to different treatments, RWPE-1 cells (human prostate epithelial cells) were randomly divided into three groups: control group, LPS (lipopolysaccharide) group, or Li-ESWT group (LPS-induced RWPE-1 managed by Li-ESWT). Following the Li-ESWT treatment, the levels of oxidative stress were assayed. We then established a rat model of experimental autoimmune prostatitis (EAP) by injecting prostatic protein homogenate mixed with complete Freund's adjuvant. The Sprague-Dawley rats were randomly divided into the control group, EAP group, or Li-ESWT group. Von Frey Filament was used to quantify pelvic hyperalgesia in the rats. Prostates tissues from each group were collected for immunohistochemistry, oxidation stress, and Western blot analysis.
Results
Histological analysis showed reduced inflammation and expression of cytokines (TNF-α, IL-1β, IL-6, COX-2, SP) in prostate tissues from the Li-ESWT group compared with those from the EAP group (all p < 0.05). Similarly, there was reduced pelvic pain and allergic symptoms in the Li-ESWT group compared with the EAP group (all p < 0.05). Besides, Li-ESWT treatment could decrease oxidative stress in the prostate and in RWPE-1 cells, respectively (both p < 0.05). Moreover, the Li-ESWT upregulated the expression of CAT through the inhibition of phosphorylation of AKT/FOXO1 signaling pathway.
Discussion and Conclusions
Li-ESWT may reduce inflammation, oxidative stress, and pain in rats with autoimmunity-induced prostatitis via the PI3 K/AKT/FOXO1 pathway. It implies that Li-ESWT can present a potential therapeutic option for the treatment of CP/CPPS.
1 INTRODUCTION
Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is a syndrome defined by diverse etiologies. CP/CPPS is characterized by urinary urgency, frequency, and odynuria associated with pain and/or discomfort in the prostate, perineum, groin, scrotum, and suprapubic region.1, 2 The global prevalence of CP/CPPS is approximately 2.2%–16.0%.3, 4 Although CP/CPPS is not life-threatening, the long-term and constant pain can seriously affect the quality of life of patients.5 Several major hypotheses have been proposed for the pathogenesis of CP/CPPS, such as chronic inflammation/autoimmunity,6 pelvic floor dysfunction,6 and cryptic infections.7 However, there still have limited data on the molecular mechanisms of CP/CPPS-related pathologies.
Evidence suggests that CP/CPPS may be the result of dysregulated inflammation in the form of autoimmunity against prostate antigens.6 Chronic inflammation may be an important prerequisite for CP/CPPS. Characterization of these infiltrates in expressed prostatic secretion (EPS), ejaculates as well as in prostate tissue samples showed an increase in granulocytes, macrophages, and activated T and B lymphocytes.8 Besides, high levels of inflammatory cytokines, chemokines, and mast cells degranulation products such as IL-1β, TNF-α, IFN-γ, IL-6, MCP-1/CCL2, and MIP-1α/CCL3 have also been detected in clinical samples from CP/CPPS patients.8-11 Thus, there is an active inflammatory process in uninfected male genital tract. On the other hand, an imbalance between peroxidation and antioxidation in the prostate plays an important role in the development of CP/CPPS.12 Because the activation of inflammatory cells within the prostate, chronic inflammation increases the production of reactive oxygen species (ROS), which disrupts sperm proteins, DNA, and membranes integrity.13 Moreover, our previous findings demonstrated that, after anti-inflammatory treatment in CP/CPPS patients, there was an upregulation of antioxidant enzymes and a decline of oxidation products in prostatic fluid.14
To date, effective treatment of the CP/CPPS remains a global challenge. The widely used treatments for CP/CPPS include alpha-blockers, antibiotics, anti-inflammatory, analgesics, and antioxidants, either as monotherapy or in combination.15, 16 In addition to these conventional medications, there are some alternative treatment options, such as acupuncture, acupoint and rectal massage, physical therapy, and lifestyle intervention.17, 18 All these treatment modalities have, however, shown limited efficacy on the treatment of CP/CPPS.19 Therefore, it is necessary to explore more effective treatments for CP/CPPS.
Extracorporeal shock wave therapy has been widely adopted in the treatment of urolithiasis.20 Besides, it can be used for chronic diseases such as arthritis, tendinitis, plantar fasciitis, and diabetic wounds.21 Clinical studies have also demonstrated the positive effect of Li-ESWT on pelvic pain and urinary dysfunction in CP/CPPS patients.22 However, the mechanisms underlying its beneficial effects are still undefined. To interrogate the effect of Li-ESWT on the prostate microenvironment of CP/CPPS and the potential therapeutic mechanisms, we observed the behavioral response of CP/CPPS rats, assayed and reported perturbations in related indicators which contain inflammation, oxidative stress, and pain.
2 MATERIALS AND METHODS
2.1 In vitro study
Human RWPE-1 cells (ATCC, VA), which are human prostate epithelial cells, were cultured in dulbecco's modified eagle's medium (DMEM) with 10% fetal bovine serum (FBS). LPS (Solarbio) was employed to induce inflammation in RWPE-1 cells.23 To determine the effect of Li-ESWT on inflammatory response, the LPS-induced RWPE-1 cells were treated with a focused extracorporeal shock wave medical device. Cells were randomly divided into three groups: control group, LPS group, and Li-ESWT group. Specifically, the Li-ESWT probe was kept in contact with the bottom of the culture flask containing adherent cells. Li-ESWT was performed after the cells adherence of greater than 95%, and only one treatment per generation. On the other hand, the cells in the control group received a sham treatment. The Li-ESWT group of cells [200 pulses (0.09 mJ/mm2)] was cultured in DMEM-high glucose 10% FBS culture medium for 3 h, followed by co-cultured with LPS for 6 h. After 12 h, the cells were harvested and counted, and then stored at −80℃.
2.2 Animal experiments
All animal experimental procedures were approved by the Institute of the Animal Experiment Ethics Committee at Lanzhou University Second Hospital (Approval No. D2019-177) and were performed following the Guidelines for the Care and Use of Laboratory Animals (NIH publication No. 85–23, National Academy Press, Washington, DC, USA, revised 1996). Fifty-two-month-old male Sprague-Dawley (SD) rats (180–220 g) were obtained from Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences. All the animals were raised under the specific pathogen-free (SPF) environmental conditions at 23 ± 2°C and 55 ± 5% humidity. Besides, the rats had free access to food and water and were given one week to adapt to the new environment before the experiment.
2.3 Establishment of the animal model
Prostate extracts were obtained from prostate tissue of twenty SD rats and were homogenized in a glass homogenizer with TritonX-100 (Solarbio). The homogenate was centrifuged at 10,000 g for 30 min at 4°C, and then, the supernatant was collected as prostate antigens (PAgs) and stored at −80℃.
Twenty-seven rats were randomly divided into the control group, EAP group, or Li-ESWT group (9 rats each). The rat model of EAP was established as previously described.24 Equal volume of PAgs was emulsified with complete Freund's adjuvant (CFA), followed by multipoint intradermal injection into the EAP group and Li-ESWT group rats. Each rat was intradermally immunized with 1.0 ml of an isovolumetric mixture at different locations: abdomen, bilateral shoulder area, and bilateral hip area. Each rat in the control group was injected with isovolumetric saline. The immunization was given four times, at days 0, 7, 14, and 28. On day 30, rats in the Li-ESWT group were treated with Li-ESWT, whereas rats in the control or EAP groups were treated with sham operation only.
2.4 Von Frey filament in behavioral testing
Referred hyperalgesia and tactile allodynia were quantified using the Von Frey filaments (North Coast Medical) applied to the pubic region or the scrotal base. Six sizes of Von Frey filaments were used on days 0, 10, 20, 30, 40, and 50 according to the protocol.25 Stimulation was performed when the rats remained quiet with the abdomen and scrotum resting on the bottom of the cage. Since the animal model cannot express its discomfort directly, this experiment assesses the experimental results through a series of behaviors such as sudden abdominal contraction, immediate licking or grasping the stimulation site, and jumping, all of which were defined as positive responses.25, 26
2.5 The test of Li-ESWT on animal models
Rats were anesthetized with 1% sodium pentobarbital, exposed in a supine position and the abdominal hair was shaved. The shock wave applicator was placed on the pubic region. Rats in the Li-ESWT group received transcutaneous Li-ESWT therapy with an energy level of 0.20 mJ/mm2 and a frequency of 120 pulses/min for a total of 200 pulses. The Li-ESWT was administered every two days for 4 weeks. After treatment, the rats were anesthetized and sacrificed to collect their prostate tissues in each group. The tissues were stored at −80°C for subsequent experiments.
2.6 Histology and immunohistochemistry
The collected prostates from each group were stored in 4% paraformaldehyde/0.1 mol/L PBS (pH 7.2) fixative, washed with water and dehydrated in gradient ethanol, xylene transparent, wax immersion, and embedding treatment at room temperature. The prostate tissues were then sliced for later use. Pathological sections were stained using hematoxylin and eosin (H&E) staining, and local pathological changes of the prostate were observed under the microscope.
The prostate tissue was treated with 1% H2O2 to block endogenous peroxidase and then heated to 97°C for 15 min in the antigen recovery solution. The samples were incubated with rat anti-rabbit TNF-α (bs-2081R; Bioss), anti-rabbit IL-1β antibody (bs-0812R; Bioss), anti-rabbit IL-6 antibody (bs-0782R; Bioss), anti-rabbit substance P (SP) antibody (bs-0065R, Bioss), or anti-rabbit COX-2 antibody (ab182422, Bio-Rad) at room temperature for 60 min, and then washed with 1× phosphate-buffered solution (PBS). The sections were then incubated with secondary antibody (Sigma), and then, the samples were developed using 3,3-Diaminobenzidine (DAB) (Sigma) to stain the sections with hematoxylin solution Counterstain.
2.7 Evaluation of oxidative stress
The intracellular ROS levels of the control group, LPS group, and the Li-ESWT group were determined by fluorescent dichlorofluorescein (DCF) assay with ROS Assay Kit (Jiancheng Bio). ROS generation was measured using 2,7-dichlorofluorescin diacetate (DCFH-DA) as described by Wang and Joseph with some modifications.27 About 1 × 104 RWPE-1 cells were inoculated in a culture flask. After being harvested, the cells were treated with DCFH-DA (10 μmol/L) for 20 min at 37℃ in the dark, and then washed twice with PBS. Intracellular ROS, as indicated by the fluorescence intensity of DCF was observed using a fluorescence microscope.
The prepared prostate tissue and RWPE-1 cells were homogenized for protein extraction, and then, the protein concentration was determined. We then used assay kits to detect superoxide dismutase (SOD) activity, malondialdehyde (MDA), glutathione (GSH), glutathione disulfide (GSSG), NADPH, or NADP+ in the prostate or cell samples. All operations were performed via following the manufacturer's instructions (Comin Bio).
2.8 Western blotting assay
The RIPA lysis buffer was used to lyse prostate tissue, followed by quantification of protein content using a BCA kit. The protein samples were then loaded and separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to a polyvinylidene fluoride (PVDF) membrane (Bio-Rad). The blot was blocked in 5% skimmed milk powder in Tris hydrochloride buffer and incubated with monoclonal anti-AKT (GeneTex), anti-phospho AKT (GeneTex), anti-FOXO1 (Abcam), anti-phospho FOXO1 (Abcam), anti-CAT (GeneTex), anti-phospholipid or anti-GAPDH (Abcam) antibody, overnight at 4°C. The membranes were washed with TBST three times and were incubated with secondary goat anti-rabbit antibodies for 1 h at room temperature. After washing, the blots were observed by using the ECL chemiluminescence method for protein expression, and ImageJ software was used to analyze the optical density of the protein bands.
2.9 Statistical analysis
All data were collected and analyzed by SPSS v.25.0 software (IBM Corp). The differences among the treatments were evaluated using the Student's t test in behavioral testing. The rest data were subjected to ANOVA analysis. All the data are presented as mean ± SD, and a p-value <0.05 was considered statistically significant. Data descriptions were performed using GraphPad Prism v.5.0 (GraphPad Software Inc)™.
3 RESULTS
3.1 Li-ESWT ameliorated the autoimmune-induced inflammation in the prostates
The morphological structure of prostate tissue is shown in Figure 1A. In the EAP group, a large number of inflammatory cells infiltrated in the prostate, and edema, hyperemia, fibrosis, and destruction of glandular epithelial cells were observed in the stroma. Compared with the Li-ESWT group, inflammatory cells infiltration in the prostate was significantly reduced, acini were relatively normal, and glandular epithelial cells were mildly hyperplastic, without injury or atrophy.
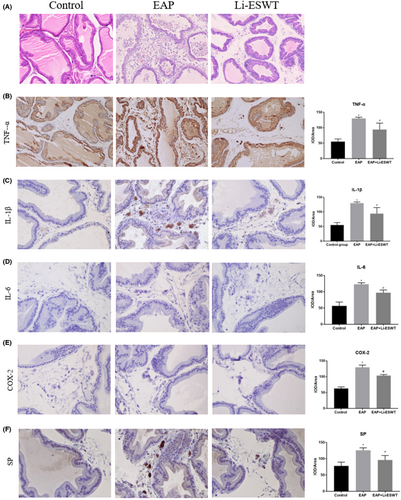
3.2 Li-ESWT ameliorated inflammation in EAP rats
Compared with the control group, the levels of TNF-α, IL-6, and IL-1β were significantly increased in the EAP group. However, after Li-ESWT treatment, the expression of these measures was significantly reduced (Figure 1B-D). Thus, Li-ESWT may reduce the prostate inflammation induced by autoimmunity.
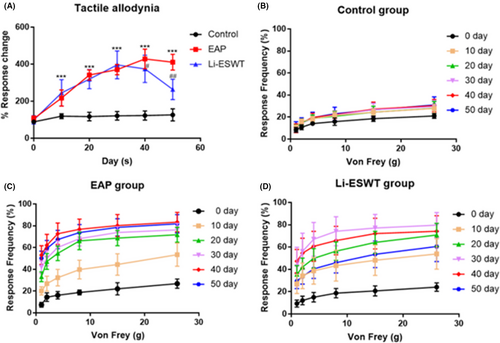
3.3 Li-ESWT inhibits the decrease of pain threshold and hypersensitivity in the pelvic area
Tactile allodynic behavioral responses were measured at 0, 10, 20, 30, 40, and 50 days for rats in each group. During day 10 to 50, significant allodynic responses were detected in rats in the EAP and Li-ESWT groups. However, from day 40 onwards, the tactile allodynia responses in the Li-ESWT group were significantly improved compared with that in the EAP group (Figure 2A-D). Furthermore, while COX-2 and SP positive inflammatory cells were significantly increased in tissues suffering from prostatitis, their expression was significantly reduced by Li-ESWT treatment (Figure 1E-F).
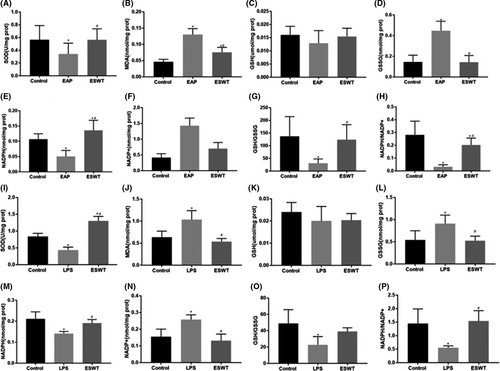
3.4 Li-ESWT improves the oxidative stress in CP/CPPS rat prostate and LPS-induced RWPE-1 cells
We evaluated the antioxidant activity via using antioxidants markers (such as SOD, GSH, and NAPDH). Oxidation products (such as MDA, GSSG, and NADP+) play a key role in monitoring and indicating the degree of peroxidation damage. As shown in Figure 3, there was a decreased level of SOD, GSH, NAPDH, GSH/GSSG, and NAPDH/NADP+ in the model group both prostate tissues (Figure 3A-H) and cells (Figure 3I-P). However, the activities of MDA, GSSG, and NADP+ were instead significantly increased. Compared with the model group, SOD, GSH, GSH/GSSG, and NAPDH/NADP+ were increased in the Li-ESWT group. In contrast, the levels of MDA, GSSG, and NADP+ were significantly decreased.
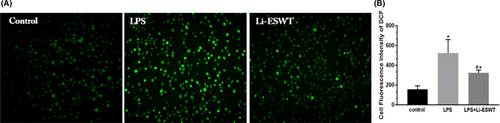
3.5 Li-ESWT improved LPS-induced intracellular ROS generation
The ability of LPS to induce oxidative stress was determined by examining the level of ROS in RWPE-1 cells. Fluorescence microscope images (Figure 4A) and quantitative data (Figure 4B) showed that the Li-ESWT group had less production of intracellular ROS than the LPS group.
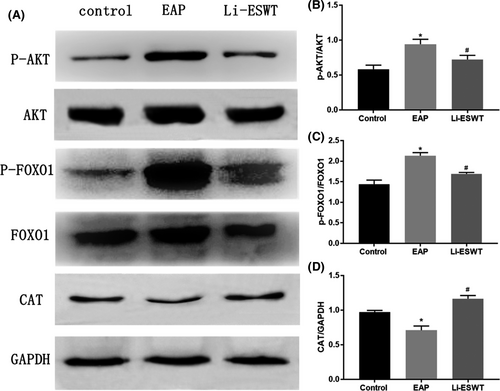
3.6 Li-ESWT ameliorated autoimmune-induced prostatitis in rats through the AKT/FOXO1 signaling pathway
The dysregulation of AKT/FOXO1 signaling is often correlated with the production of oxidative stress and inflammation. The immunoblot assay showed an upregulation of p-AKT and p-FOXO1, while a downregulation of CAT expression in the EAP group compared with the control group (Figure 5A-D). Interestingly, Li-ESWT treatment could significantly reduce the expression of p-AKT and p-FOXO1, whereas increase of CAT expression. Therefore, the effect of Li-ESWT on autoimmune-induced prostatitis in rats might be related to the PI3 K/Akt/FoxO1 signaling pathway.
4 DISCUSSION
In the present study, we found that Li-ESWT could reduce inflammatory infiltration and improve pain and hypersensitivity symptoms for autoimmunity-induced prostatitis in EAP rats. Mechanistically, Li-ESWT treatment downregulated inflammatory factors, such as TNF-α, IL-1β, and IL-6. Meanwhile, it inhibited the expression of pain-related factors, COX-2, and SP. Also, Li-ESWT improved oxidative stress state both in prostate tissue and in vitro prostate epithelial cells. Regarding signaling modulation, Li-ESWT may exert anti-inflammatory effect through PI3 K/AKT/FOXO1 pathway.
Recent data have indicated that CP/CPPS is closely related to autoimmune inflammation.6 The important role of inflammatory factors in the pathogenesis of CP/CPPS has been documented.28 Here, we employed an EAP model to not only reflect many key characteristics of CP/CPPS but improve the understanding of the possible pathophysiological immune mechanisms.29, 30
TNF-α, IL-1β, and IL-6 are considered to be important pro-inflammatory factors in the development of CP/CPPS.31-33 For the treatment, Li-ESWT has an anti-inflammatory effect against various diseases.34, 35 Li-ESWT can significantly decreased IL-6, TNF-α, and IL-1β but increased IL-10 (an anti-inflammatory mediator), which may contribute to the preservation of the functions of the pancreatic beta cells.36 Zhao et al12 showed that lycopene, a natural antioxidant for the treatment of CP/CPPS rats, can reduce the expression of inflammatory factors such as TNF-α, IL-1β, and IL-6.
The pain symptoms in patients with CP/CPPS are long-term, recurrent, and mechanistically unelucidated, which makes treatment difficulty. Evidence has demonstrated that tissue inflammatory injury may cause chronic pain.37 Cytokines, such as SP, TNF, and ILs, released after inflammatory injury can increase the sensitivity of peripheral afferent nerves to stimulation.38, 39 The reduction of COX-2 slows down the production of prostaglandin E2 and relieves CP/CPPS-related pain.23 Inflammatory stimuli are known to induce the secretion of SP, calcitonin gene-related peptide, and nerve growth factor (NGF) from nerve terminals, resulting in plasma extravasation, edema, and hyperalgesia, commonly referred to as neurogenic inflammation.40 Our findings showed decreased expression of COX-2 and SP after Li-ESWT treatment, perhaps responsible for the improved pain and hypersensitivity symptoms in EAP rats. The ability of Li-ESWT to relieve prostate pain was also found in a capsaicin-induced rat model.41 In addition,Li-ESWT might inhibit inflammation by polarizing pro-inflammatory M1 macrophages to anti-inflammatory M2 macrophages.33 Compared with M0 and M1 cells, M2 macrophages released higher amounts of opioid peptides, including Met-enkephalin, dynorphin A (1–17), and β-endorphin and correlated with amelioration of tactile hypersensitivity.42 Furthermore, Li-ESWT may affect CPPS through reduction of passive muscle tone, overstimulation of pain receptors, interruption of nerve impulse flow, or affecting the neuroplasticity of pain memory.43
Recent studies have shown that oxidative stress occurs in CP/CPPS, and oxidative damage can be detected in prostate tissue, prostatic fluid, and systemic blood circulation.12, 14 It may be due to the damage of prostate epithelial cells, which causes the aggregation of inflammatory cells and the release of ROS, which in turn leads to changes in protein structure and function.44 SOD and catalase (CAT) are essential for maintaining redox balance in the body and are used as biomarkers for the production of ROS.45 SOD is a naturally occurring superoxide radical scavenger in the body. Through the above reaction, harmful superoxide radicals can be converted into hydrogen peroxide (H2O2), which is then decomposed into harmless H2O by the body's CAT.13 MDA is a lipid peroxidation product, which damages cell function by changing the activity of biological membranes and is an important marker for oxidative damage.46 Intracellular redox homeostasis is regulated by coupling reactions (including conversion of H2O2 and GSH to H2O and GSSG by glutathione peroxidase; Glutathione reductase reduces GSSG and NADPH to GSH and NADP+).47 The ratio of GSH to GSSG and NADPH to NADP+ can also reflect the oxidative stress state of tissues and cells.48, 49 This study shown that oxidative stress evoked by inflammatory stimuli both in prostate tissue and in vitro prostate epithelial cells, which was alleviated by Li-ESWT treatment. These results indicate that Li-ESWT may have potential antioxidant effects.
Jeon et al23 showed that ESWT inhibits the production of inflammatory factors by inhibiting the NF-κB and MAPK signaling pathways. In this study, we demonstrated that the therapeutic effects of Li-ESWT were associated with inhibition of the PI3 K/AKT/FOXO1 pathway, which is crucial signaling in cellular anti-oxidative stress as well as anti-inflammation. Phosphatidylinositol-3-kinase (PI3 K) is an intracellular phosphatidylinositol kinase, and Akt is the direct downstream effector of PI3 K. The PI3 K/AKT pathway is an important intracellular signaling that regulates the cell cycle by transmitting various upstream signals.50 The FOXO1 transcription factor, which is a downstream target protein for AKT, is phosphorylated by activated AKT, resulting in inhibition of nuclear membrane transport and transcription.51 FOXO1 regulates the expression of CAT and SOD proteins in the nucleus by promoting the transcription of downstream genes.52 CAT and SOD synergistically convert ROS into H2O to resist oxidative stress. The production of ROS is the key to the progression of many inflammatory diseases, and it is both a signal molecule and a mediator of inflammation.53 Therefore, we consider PI3 K/AKT/FOXO1 as a possible signaling pathway that affects the development of inflammation and oxidative stress in CP/CPPS.
Despite these strengths, several limitations need to be acknowledged. The Li-ESWT management was conducted in the early stage of CP/CPPS, and its efficacy in late-stage CP/CPPS needs to be further explored. Besides, EAP rats in this study can only be treated for one month due to the effective time limit. Therefore, further studies are still required to estimate the long-term safety and efficacy of this treatment option. Lastly, although Li-ESWT treatment for CP/CPPS has been reported in several studies,54 there still do not have unified shock wave generator, undefined optimal dosages for its operation, and the underlying mechanism needs more in-depth study.
5 CONCLUSIONS
Li-ESWT can reduce inflammatory infiltration, and ameliorate pain, and hypersensitivity symptoms in autoimmunity-induced prostatitis rats. Mechanistically, Li-ESWT treatment can downregulate inflammatory factors, improve oxidative stress state in prostate, and inhibit the expression of pain-related factors. Li-ESWT may exert anti-inflammatory effect through PI3 K/AKT/FOXO1 signaling. Li-ESWT is a potential therapeutic option for the treatment of CP/CPPS.
ACKNOWLEDGEMENTS
This study was financially supported by the National Natural Science Foundation of China (Project number: 81874088).
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS CONTRIBUTION
B. F., Z. D., Z. W., Z. Y., and C. L. conceived and designed the research. B. F., Y. W., and H. C. performed experiments. G. Y. and Z. H. collected and analyzed the data. X. Z. and Z. S. interpreted the data. B. F. and E. Y drafted and edited the manuscript. Z. J and M. C provided the LI-ESWT device. All of the authors approved the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data may be shared on request from the corresponding author.