“Mask up to keep it up”: Preliminary evidence of the association between erectile dysfunction and COVID-19
Funding information
This work was carried out within the framework of an Italian Ministry of University project and was partly supported by the PRIN 2017S9KTNE_002 grant by the Italian Ministry of Education, University and Research.
Abstract
Background
Erectile dysfunction (ED), as the hallmark of endothelial dysfunction, could be a short- or long-term complication of COVID-19. Additionally, being ED a clinical marker and predictor of non-communicable chronic diseases, particularly cardiovascular, subjects with ED could potentially have a higher risk of contracting COVID-19.
Objectives
To investigate the prevalence of ED among subjects with a reported diagnosis of COVID-19 and to measure the association of COVID-19 and ED.
Materials and methods
We reviewed data from the Sex@COVID online survey (performed between April 7 and May 4, 2020, in Italy) to retrieve a sample of Italian male sexually active subjects with reported SARS-CoV-2 infection. A matching sample of COVID-19-negative male sexually active subjects was also retrieved using propensity score matching in a 3:1 ratio. The survey used different standardized psychometric tools to measure effects of lockdown and social distancing on the intrapsychic, relational, and sexual health of Italian subjects.
Results
One hundred subjects were included in the analysis (25 COVID-positive; 75 COVID-negative). The prevalence of ED, measured with the Sexual Health Inventory for Men, was significantly higher in the COVID+ group (28% vs. 9.33%; p = 0.027). Logistic regression models confirmed a significant effect of COVID-19 on the development of ED, independently of other variables affecting erectile function, such as psychological status, age, and BMI [OR 5.66, 95% CI: 1.50–24.01]. Likewise, subjects with ED were more likely to have COVID-19, once corrected for age and BMI [OR 5.27, 95% CI: 1.49–20.09].
Discussion and conclusion
On top of well-described pathophysiological mechanisms, there is preliminary evidence in a real-life population of ED as a risk factor of developing COVID-19 and possibly occurring as a consequence of COVID-19. Universal vaccination against the COVID-19 and the personal protective equipment could possibly have the added benefit of preventing sexual dysfunctions.
1 INTRODUCTION
COVID-19, the coronavirus disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), features several clinical phenotypes, ranging from mild to severe forms: The “cytokine storm” 1, 2 may lead to the development of microvascular thrombotic and inflammatory processes, which in turn promote progression to possibly lethal pulmonary complications. Some evidence has also suggested that even “silent” asymptomatic forms of COVID-19 could have subclinical microvascular involvement3, 4 and that long-term cardiovascular sequelae could be expected in COVID-19 patients.5, 6 Endothelial dysfunction has been considered as the potential trigger for the onset of more severe forms, as well as the link between different comorbidities associated with COVID-197 : Indeed, COVID-19 is by all means an endothelial disease, in which systemic manifestations of the disease can potentially be due to tissue ischemia resulting from alterations in endothelial thrombotic/fibrinolytic balance.8 Additionally, endothelial cells express many of the co-factors used by the SARS-CoV-2 to invade host cells.9
Erectile dysfunction (ED) has been often considered a hallmark of endothelial dysfunction,10, 11 and as such, a potential association between ED and COVID-19 has also been postulated.12
Another interesting take on the association between ED and COVID-19 comes from the shared risk factors for the two conditions. Indeed, severity and prevalence of both ED and COVID-19 are higher among men suffering from hypertension, obesity, diabetes, and history of cardiovascular disease.13-16 Broadly speaking, ED is often considered a clinical marker of a “dysfunctional” phenotype, which often features cardiovascular events at an early age. This would possibly suggest that subjects with ED, due to the underlying conditions which impair erectile response, could also be more susceptible to contracting COVID-19.
The present study is, to our knowledge, the first one investigating the prevalence of ED and the possible association between ED and COVID-19 from real-life data in a large survey.
2 MATERIALS AND METHODS
2.1 Subjects
In order to retrieve an adequate sample for the present study, we reviewed data from the Sex@COVID study,17 a previous research project by our group. The Sex@COVID study was an anonymous web-based questionnaire investigating the psychological, relational, and sexual health of Italian subjects between April 7 and May 4, 2020.
Overall, 6821 subjects aged 18 years or older (females, 4177; males, 2644; mean age 32.83 ± 11.24 years) living in Italy, stratified according to marital status and sexual activity during lockdown, participated in the Sex@COVID study. All subjects provided informed consent to the study, which has been approved by our local Ethical Committee.
Nine hundred and eighty-five sexually active men were therefore identified in the Sex@COVID cohort, among which 25 (2.54%) reported being tested as positive for COVID-19.
2.2 Measures
Anxiety and depression were, respectively, measured by the GAD-718 (Generalized Anxiety Disorder Scale) and the PHQ-919 (Patient Health Questionnaire), two questionnaires validated for clinical use, which have already been used to assess psychological outcomes of COVID-19.20 For each test, scores ≥10 are considered suggestive of general anxiety disorder and depressive disorder, respectively. Erectile function was measured by the IIEF-5, or Sexual Health Inventory for Men (SHIM),21, 22 a shortened, five-item version of the International Index of Erectile Function23 often used in the clinical setting: Scores 21 or below are considered suggestive of ED, whereas scores 22–25 are considered normal.
2.3 Statistical analysis
Statistical analysis was performed with the statistical software R (version 3.6.2); statistical significance was set at p < 0.05. Propensity score matching was used in order to retrieve two matching samples, based on age, body mass index (BMI), and GAD-7 and PHQ-9 scores, using a 1:3 ratio to improve reliability of the results. Assessment of normality was performed using the Shapiro-Wilk test of normality. Wilcoxon rank sum test with continuity correction and Fisher's exact test (one-tailed) were used to assess differences in the numerical and categorical variables between study groups. Chi-squared goodness-of-fit test was used to measure differences in the prevalence of ED between study groups. Logistic regression models were fitted to assess to which extent age, GAD-7 and PHQ-9 scores, BMI, and history of COVID-19 affected erectile function and to measure the effect of age, BMI, and ED on the susceptibility to COVID-19.
2.4 Power analysis
Sample size was calculated based on a 28% prevalence of ED among the COVID+group, using a 0.8 effect size (accounting for a large effect size), with 1:3 ratio, α = 0.05, and power 0.95. According to these calculations, a total sample size of 87 (22 COVID+, 65 COVID−) was needed. By including 25 COVID+ and 75 COVID− subjects, the post-hoc analysis yielded a 0.97 statistical power.
3 RESULTS
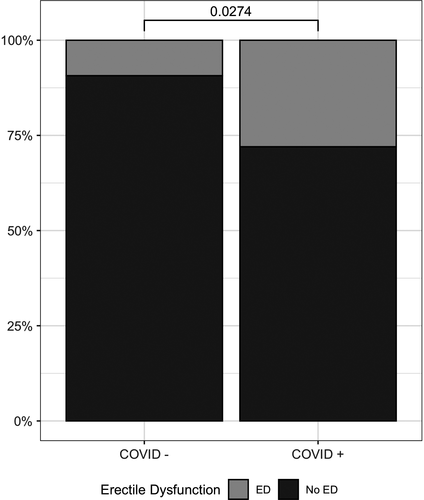
According to the suggested propensity score matching approach detailed above, 100 subjects from the 985 sexually active men belonging to the Sex@COVID cohort were retrieved: 25 subjects reported having contracted COVID-19 (COVID+), and a matching sample (based on age, BMI, and GAD-7 and PHQ-9 scores) of 75 subjects was recruited among those having no history of SARS-CoV-2 infection prior to filling the Sex@COVID survey (COVID-). Descriptive data of the study population are reported in Table 1. According to propensity score matching, no statistically significant difference was found for age, GAD-7 and PHQ-9 scores, and BMI between the two groups. The prevalence of ED was higher in the COVID+group (7/25, 28%) than in the COVID- group (7/75, 9.33%; p-value 0.0274) (Figure 1).
|
COVID+ (n = 25) |
COVID- (n = 75) |
p-value | |
|---|---|---|---|
| Age (years) | 39.00 [29.00, 45.00] | 42.00 [32.50, 49.00] | 0.142a |
| BMI (kg/m2) | 22.65 [20.83, 23.74] | 22.74 [20.98, 24.53] | 0.266a |
| GAD−7 score | 4.00 [2.00, 6.00] | 4.00 [2.00, 5.00] | 0.741a |
| PHQ−9 score | 5.00 [3.00, 6.00] | 4.00 [2.00, 5.00] | 0.873a |
| Erectile dysfunction | 7 (28%) | 7 (9.33%) | 0.027 b |
- Statistically significant values highlighted in bold.
- All data expressed as median [interquartile range] except prevalence of erectile dysfunction, expressed as n (%).
- Abbreviations: BMI, body mass index; GAD-7, Generalized Anxiety Disorder Scale;HQ-9, Patient Health Questionnaire.
- a Wilcoxon rank sum test with continuity correction
- b Fisher's exact test (one-tailed).
Logistic regression models confirmed the association of COVID-19 with ED of COVID-19 on the development of ED (Table 2): While age, BMI, and psychological health scores failed to reach statistical significance, history of COVID-19 was highly significant, resulting in a 5.66 odds ratio [95% confidence interval: 1.50–24.01] of having ED.
| Estimate | Std. error | p-value | Odds ratio [95% CI] | |
|---|---|---|---|---|
| Intercept | −9.607 | 3.216 | 0.003 | 0.00 [0.00–0.02] |
| Age (years) | 0.062 | 0.027 | 0.023 | 1.06 [1.01–1.13] |
| BMI | 0.143 | 0.106 | 0.178 | 1.15 [0.93–1.43] |
| GAD−7 score | 0.114 | 0.193 | 0.556 | 1.12 [0.77–1.66] |
| PHQ−9 score | 0.148 | 0.143 | 0.301 | 1.16 [0.88–1.55] |
| Previous COVID−19 | 1.734 | 0.694 | 0.013 | 5.66 [1.50–24.01] |
Since ED could be a valid clinical marker of several underlying and unaccounted conditions, such as diabetes and hypertension, we also measured in the same sample the likelihood of having a self-reported history of COVID-19 following a diagnosis of ED. Logistic regression models adjusted for age and BMI (Table 3) showed a significant association between ED and COVID-19, with a 5.27 odds ratio [95% CI: 1.49–20.09].
| Estimate | Std. error | p-value | Odds ratio [95% CI] | |
|---|---|---|---|---|
| Intercept | 1.910 | 2.194 | 0.384 | 6.75 [0.11–661.99] |
| Age (years) | −0.027 | 0.021 | 0.197 | 0.97 [0.93–1.01] |
| BMI | −0.096 | 0.089 | 0.281 | 0.91 [0.76–1.07] |
| Presence of ED | 1.662 | 0.652 | 0.011 | 5.27 [1.49–20.09] |
4 DISCUSSION
The large majority of the studies investigating the effects of COVID-19 on male sexual and reproductive health have focused on fertility and its preservation,24-30 and to the present date, there seems to be no definite evidence of the presence of SARS-CoV-2 in seminal fluid31, 32 ; however, there is reason to suspect that sexual quality of life and function might also be impaired as a consequence of COVID-19.12, 33, 34 While of course this is of relative importance to patients in intensive care units, the possible long-term effects on erectile function might be an additional cause of worry in COVID-19 patients. In the present study, to our best knowledge, we investigated for the first time the possible association between erectile function and COVID-19 in a real-life setting. Results of our study agree with the pathophysiological mechanisms linking ED, endothelial dysfunction, and COVID-19. By performing propensity score matching, we removed the possible bias resulting from age and BMI, factors which contribute to both increased prevalence of ED13, 14 and increased susceptibility to COVID-19.15, 16 Therefore, our results are highly suggestive of the role of the infection in the development of the sexual dysfunction and of the possible clinical relevance of COVID-19 as an additional risk factor for the development of ED. Additionally, considering the bidirectional interaction between sexual activity and psychological well-being,17 the removal of the possible influence of anxiety and depression confirmed that the increased prevalence of ED here found is not only a consequence of the psychological burden of lockdown, but also prominently due to other, bona fide organic factors, among which endothelial dysfunction is the most likely culprit. On the other hand, there is another plausible explanation: ED is a well-recognized surrogate marker of systemic health,11 and therefore, ED patients could already carry several underlying and unexplored risk factors, such as dyslipidemia, diabetes, and hypertension, which could increase the likelihood of contracting COVID-19. Hence, we also investigated whether subjects with ED, owing to the worse systemic health,11 could possibly be more at risk of developing COVID-19. Based on our preliminary results, ED and COVID-19 seem to be strongly associated, with COVID-19 increasing the chances of developing ED and ED being a marker of increased susceptibility to infection. While more adequately tailored studies are needed, we believe that our study highlights the possible relevance for sexual medicine and andrology of COVID-19 spectrum of disease and its short and long-term health consequences.
There is a solid pathogenetic background for the mechanisms through which COVID-19 could affect erectile function.12 Many machineries through which SARS-CoV-2 invades the host cells have been identified: The virus uses angiotensin-converting enzyme 2 (ACE2) as an entry point to the cells, and primes the spike protein, which facilitates viral entry into target cells, by employing the transmembrane protease, serine 2 TMPRSS2.35 The SARS-CoV-2 therefore shows some similarities with another coronavirus,36, 37 namely SARS-CoV, the virus strain responsible for the SARS outbreak occurring in 2003–2004.37-39 Since adult Leydig cells express ACE-2, testicular involvement due to COVID-19 has been hypothesized40 and confirmed in autopsy reports.41 Also, at least one case of acute orchitis following SARS-CoV-2 infection has been reported.42 The testicular injury reported in these studies could also be a consequence of alterations in the coagulative status, resulting in development of ischemia at a microvascular level.43 Independently of the etiology, testicular damage can possibly lead to the development of a form of hypergonadotropic hypogonadism44-48 : As testosterone modulates endothelial function,49 the possible effects of COVID-19 on erection could also be indirectly due to impaired testosterone secretion from the affected testis, besides the known direct effects of testosterone in male sexual response.50 Additionally, as higher testosterone levels are also associated with lower levels of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukins (IL-6 and IL-1β), and higher levels of anti-inflammatory cytokines (such as IL-10),51 the “immunothrombotic” mechanism described for COVID-1952 and involving the same cytokines5, 53 could be promoted by the hypogonadal state encountered in affected patients. The immunothrombosis could potentially affect the penile vessels, triggering endothelial dysfunction—therefore impairing vascular function and promoting progression to more severe forms of sexual dysfunction.54, 55 Other cardiovascular complications of COVID-19, such as cardiomyopathy and myocarditis,56, 57 could also be involved in the pathogenesis of ED even after the end of the acute phase, potentially becoming long-term cardiovascular sequelae.5, 6 Other potential factors could contribute to impaired erectile function in COVID-19 patients, such as pulmonary fibrosis58, 59 causing hypoxia in the penile vascular bed,60, 61 or anosmia and ageusia, both manifestations of COVID-1962, 63 with possible negative effects on sexual health.64-67 While these factors could possibly have a minor influence when considered individually, the likely presence of most, if not all, of them at the same time could easily allow the progression from a subclinical to an overt form of sexual dysfunction.68, 69 Considering the subclinical, yet non-negligible, cardiopulmonary damage reported in asymptomatic subjects,4 we speculate that the same progression from subclinical to an overt sexual dysfunction could also occur in subjects with “silent” forms of COVID-19 in the presence of additional risk factors—being the proverbial straw that breaks the camel's break.
Based on the presented evidence and on similarities to previous coronavirus diseases, ED could therefore be both a short-term and a long-term complication of COVID-19. The potential association between ED and COVID-19 might increase awareness of the importance of personal protective equipment, such as masks, and social distancing in a harm reduction perspective for long-term consequences.70 The use of masks as devices for the prevention of sexual dysfunctions is perhaps a bit stretched, but at present we believe that this could also possibly be an additional strategy to promote the use of personal protective equipment—as people have already posted on Twitter, using the hashtag “#MaskUpToKeepItUp.” Additionally, subjects with a sudden onset or worsening of ED might also consider precautionary quarantine or nasopharyngeal swab, as COVID-19 might act as a potential initiating trigger for the onset of erectile impairment, or an aggravating factor for its progression to more severe forms.
Likewise, subjects with ED should consider their erectile impairment as a sign of possible underlying conditions which could increase the likelihood of suffering from COVID-19. Therefore, the importance of investigating the possible causes of ED becomes of paramount importance in times like these: Identifying and treating potential comorbidities would have potentially beneficial effects on erectile function, while at the same time reducing the risk of contracting SARS-CoV-2 or developing more severe forms of COVID-19.
The present study has several limitations, including its retrospective nature, the recall bias associated with the use of online questionnaires, and the inclusion of diagnosis of COVID-19 based on the response to the survey, rather than on nasopharyngeal swabs. Additionally, as the survey did not investigate the potential comorbidities (such as diabetes, endocrine disorders, and hypertension 13, 50, 54, 71-73 ), treatments (such as anti-depressants 54, 71 ), and lifestyles (such as smoking 14, 54, 74 ) affecting sexual health, these findings only provide preliminary evidence of the association between COVID-19 and erectile dysfunction. However, at the time of the first Italian lockdown here explored, swabs were rarely available, and an online survey was the most reliable way to obtain information on a large cohort of patients without breaking restrictions. While our study could not include such data, based on the study design, more studies adequately tailored to investigate the endocrine function, most importantly testosterone, and penile vascular dynamics in patients with a history of COVID-19 are needed to provide definite evidence; however, at present our findings, no matter how limited, are highly suggestive of a potential long-term risk for male sexual function following COVID-19.
ACKNOWLEDGMENTS
None.
CONFLICTS OF INTEREST
The authors declare no competing interests for the present study.
AUTHOR'S CONTRIBUTIONS
Conceptualization, AS, DM and EAJ; data curation, AS and DM; formal analysis, AS and DM; investigation, AS, DM, GC, EL, EC; methodology, AS and DM.; project administration and supervision, EAJ; validation, EAJ; visualization, AS; writing—original draft, AS and DM; writing—review and editing, GC, EL, EC, GB and EAJ. We attest that all authors contributed significantly to the creation of this manuscript. We confirm that the order of authors listed in the manuscript has been approved by all named authors. All authors have read and agreed to the published version of the manuscript.




