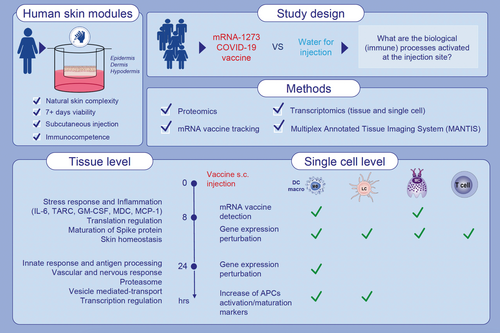
Multimodal profiling of biostabilized human skin modules reveals a coordinated ecosystem response to injected mRNA-1273 COVID-19 vaccine
Emeline Pagès and Nicolas Gaudenzio are co-senior authors.
Abstract
Background
The field of drug development is witnessing a remarkable surge in the development of innovative strategies. There is a need to develop technological platforms capable of generating human data prior to progressing to clinical trials.
Methods
Here we introduce a new flexible solution designed for the comprehensive monitoring of the natural human skin ecosystem's response to immunogenic drugs over time. Based on unique bioengineering to preserve surgical resections in a long survival state, it allows for the first time a comprehensive analysis of resident immune cells response at both organ and single-cell levels.
Results
Upon injection of the mRNA-1273 COVID-19 vaccine, we characterized precise sequential molecular events triggered upon detection of the exogenous substance. The vaccine consistently targets DC/macrophages and mast cells, regardless of the administration route, while promoting specific cell–cell communications in surrounding immune cell subsets.
Conclusion
Given its direct translational relevance, this approach provides a multiscale vision of genuine human tissue immunity that could pave the way toward the development of new vaccination and drug development strategies.
Graphical Abstract
Biostabilized human skin modules are preserved immunocompetent over 10 days and allow the monitoring of the immune response to mRNA vaccine at the injection site. Single-cell RNA sequencing reveals that mRNA-1273 COVID-19 vaccine is preferentially incorporated into DC/macrophages and mast cells in human skin modules 8 h after injection. Upon vaccine injection, skin-resident dendritic cells and Langerhans cells acquire biomarkers of mature antigen-presenting cells and of homing to lymph nodes. Abbreviations: APC, antigen presenting cell; COVID-19, coronavirus disease 2019;DC, dendritic cell; GM-CSF, granulocyte-macrophage colony-stimulating factor;IL-6, interleukin 6; LC, Langerhans cell; MC, mast cell; MCP-1, macrophage/monocyte chemotactic protein-1; MDC, macrophage-derived chemokine; TARC, thymusand activation-regulated chemokine.
1 INTRODUCTION
The human immune system is composed of a plethora of specialized cell types distributed across organs and modulated throughout a person's life upon exposure to environmental triggers.1 The skin is composed of a wide variety of myeloid cells and lymphoid cells that interact with an organized network of structural elements to form a tightly regulated ecosystem.2 Such unique immune features of the skin make it a promising anatomical site for studying the human immune response ex vivo, while preserving the natural complexity at the organ level. Recent advances in mRNA vaccine technology have led to the development of highly effective vaccines, which utilize lipid nanoparticles (LNP) to deliver the mRNA into cells. These vaccines have demonstrated remarkable efficacy in clinical trials3, 4 and have been authorized for emergency use worldwide during the COVID-19 pandemic.5 With the increasing number of academic laboratories and pharmaceutical companies developing new mRNA-based vaccines and exploring alternative routes of administration through the skin,6, 7 there is a critical need for scalable technological platforms to evaluate the immunogenicity and safety of vaccines before advancing to clinical trials. Notably, how human tissue-resident structural and immune cells interact with, and are modulated by, mRNA-loaded LNPs at the site of injection remains a promising area of investigation.8
Current in vitro technologies have made significant strides in replicating selected aspects of the human skin as a tissue; however, they remain unable to capture all aspects of the native organ organization, the genetic diversity found in the human population and the inherent complexity of the human immune system.9-11 The accessibility to large quantities of donated human skin leftover from surgical resections has prompted scientists to use cultured skin punch biopsies to better understand the response to vaccines.12, 13 The existing limitations of current in vitro culture protocols make it challenging to preserve the viability of skin explants for extended periods of time, in particular the subcutaneous adipose tissue, which is an essential component for subcutaneous administration.14, 15 Here we describe a general framework primarily designed for the longitudinal profiling of the human skin ecosystem in response to vaccines at the site of injection. The method is scalable enough to conduct multiple parallel experiments, including transcriptomics, 3D imaging, and secretomics in one donor and then to extend to selectable cohorts based on age, gender, and ethnicity. We designed an adaptable analytical pipeline composed of integrated multiparametric analyses to extract complementary immune datasets from each individual module, including an in silico approach to selectively track and quantify mRNA vaccines incorporation at the single cell level.
2 RESULTS
2.1 Biostabilized human skin modules are structurally stable and injectable subcutaneously and intradermally
The preservation of skin viability is one of the major challenges in developing ex vivo models that accurately mimic the human organ outside of the body. To circumvent this limitation, we developed a series of standardized engineering steps to quickly biostabilize human skin biopsies, while preserving an air–liquid interface and the appropriate biomechanical properties of the tissue. We obtain skin resections from abdominoplasties with an average size of 499 cm2 per donor (calculated with 500 surgical procedures), from which up to 50 individual 23 mm diameter explants can be punched out using a mechanical precision press. Subsequently, we encased each explant into a solid gel matrix and a silicone ring was placed on the top to emulate natural tension and maintain the biomechanical property found in the skin. These individual human skin “modules” were then carefully positioned in a transwell system and bathed in a chemically defined culture medium devoid of animal product, which was replaced on a daily basis (Figure 1A).
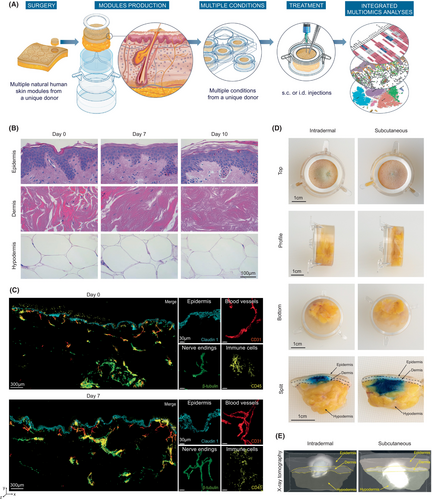
To evaluate potential donor-intrinsic changes in global tissue architecture and cellular gene expression profiles over the culture period, we performed a combination of histochemical and single-cell RNA sequencing (scRNA-Seq) longitudinal analyses, within the same individual donor (Figure S1). We first used hematoxylin and eosin (H&E) staining, a commonly used method in histology to visualize most tissue structures. No apparent changes were observed in the integrity or overall cellular structure of the skin over 7 days. The epidermal and dermal layers remained visually unaltered, with preserved keratinocytes and collagen fibers, respectively. We could detect some discrete morphological changes in the epidermis–dermis junction and a tendency for dermal fibroblast to decrease in number when the skin modules were analyzed at Day 10. Interestingly, the subcutaneous adipocyte layers remained morphologically unaltered over the 10-day culture period (Figure 1B). Using fluorescence microscopy, we analyzed the expression of active caspase-3, as a readout of tissue apoptosis, and the distribution of filaggrin and keratin 14 to assess changes in the distribution of key epidermal structural proteins.16 The active caspase-3 was detected in few cells at Day 0 and its expression did not increase over the culture period (Figure S2A). The keratin 14 was expressed in the basal layers during the entire culture period while filaggrin initially localized in the stratum corneum for the first 5 days, followed by a slight downregulation after 7 days of culture (Figure S2B,C). We next used two-photon imaging to assess the stability of the following additional skin components at the protein level in a large field of view and in 3D: claudin 1 (a key component of the epidermal barrier), CD45 (an immune cell marker), CD31 (a blood vessel marker), and β3-tubulin (that identifies peripheral nerve endings). In line with our previous observations (Figure 1B; Figure S2), all analyzed fluorescent markers were detected in the skin modules following 7 days of culture, without noticeable changes in either intensity or anatomical distribution (Figure 1C).
To explore the suitability of the human skin modules as a model for studying injectable drugs, we conducted subcutaneous (s.c.) and intradermal (i.d.) infusion experiments using a clinical-grade syringe. We introduced a colored aqueous solution directly into the adipose layer (s.c.) or the dermis (i.d.) of the skin within the modules. This allowed us to examine the distribution and diffusion patterns of the injected solutions, providing insights into the potential leakage of injectable drugs out of the tissue. We found that 60 min after a single s.c. injection, the colored solution remained within the subcutaneous compartment, and started to slowly diffuse in the upper layers of the skin, including the dermis and epidermis (Figure 1D, right panel). Conversely, after a single i.d. injection, the colored solution remained within the dermal compartment, and started to diffuse slightly into the epidermis and the subcutaneous space (Figure 1D, left panel). These data were also confirmed using 3D x-ray tomography analysis after the injection of a radiocontrast agent (Figure 1E; Videos S1 and S2). Importantly, there was no detectable leakage of the solution outside of the skin explant, indicating a well-contained distribution of the injected substance within the module environment.
2.2 Longitudinal assessment of the transcriptomic program of structural and immune compartments at the single cell level
We next investigated potential subtle transcriptomic changes in phenotype and/or activation status of both structural and immune cells over time. We produced human skin modules from the same individual and performed 10× Genomics scRNA-Seq analysis of the dissociated skin at Days 0 (i.e., within 24 h after surgery), 3, 5, and 10 (Figure S1) to generate a longitudinal transcriptomic profile for each individual cell (all quality controls are described in the Methods section). Using the t-distributed stochastic neighbor embedding (t-SNE) approach, we analyzed the expression patterns of 36,601 genes in 26,553 individual cells from the aggregated dataset of all time points and could unambiguously identify the presence of nine global clusters (Figure 2A). All populations were annotated based on their combined expression of cardinal marker genes (Figure 2B, Figure S3A). We next analyzed the distribution of all cells on the aggregated t-SNE graph based on their time point of analysis. We observed an overall homogeneous distribution of the cells on the aggregated t-SNE based on their original phenotype and biomarker, but not based on the time point of analysis (Figure 2C, Figure S3B). In order to objectively evaluate any transcriptomic variations at the tissue level, we applied a pseudo-bulk transformation in the global single-cell dataset of each time point.17 By calculating a Pearson correlation heatmap, we assessed the correlation between the transcriptomic profiles of Days 0, 3, 5, and 10. We observed a consistently high correlation exceeding 0.995 across all time points, indicating that the overall transcriptomic program of the skin remained stable over time (Figure 2D). In line with these findings, we found that all identified clusters harbored similar proportions of cells in either G1, S, or G2M phases, suggesting a conserved cell cycling capability and/or viability in each population (Figure S3C). When analyzed in terms of relative proportions of each identified cluster, we observed a progressive decrease in the proportion of fibroblasts and endothelial cells over the 10 days period, an increase in keratinocytes differentiation program mainly at Days 3 and 5, while the immune lymphoid and myeloid compartments stayed relatively stable over time (Figure 2E).
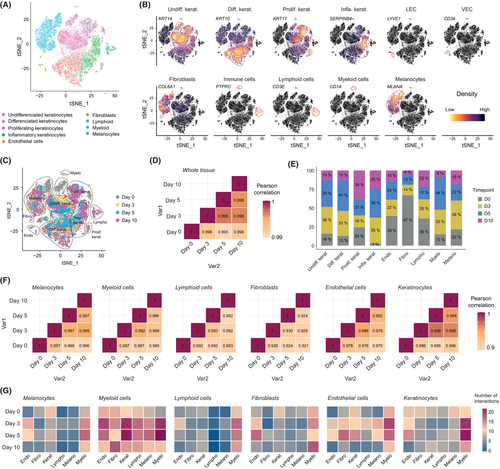
We previously observed a thickening of the cornified layer over the culture period in the absence of mechanical exfoliation (Figure 1B), which suggests a constant turnover of keratinocytes in the human skin module environment. We further tested this hypothesis by using Monocle3 pseudotime analysis18 on keratinocyte single-cell datasets. We found that the generated pseudotime trajectory recapitulated the normal differentiation of keratinocytes through the following consecutive states: undifferentiated, proliferating, inflammatory, and differentiated (Figure S4). In line with these findings, we could identify the progressive loss or acquisition of key genes characteristic of keratinocyte maturity states and epithelial barrier along the pseudotime axis, such as KRT14, ITGA6, and ITGB1 (i.e., markers of immaturity), and DSG1, KRT1, and KRT10 (i.e., markers of maturity and barrier) (Figure S4C), in accordance with previous findings.19
Subsequently, we once again utilized a pseudo-bulk transformation method to assess the correlation between the transcriptomic profiles of Days 0, 3, 5, and 10, but at the level of individual populations to potentially detect subtle changes over time. We observed a high correlation of more than 0.985 across all time points for most populations. However, slightly lower correlations of 0.970 and 0.921 were found in endothelial cells and fibroblasts, respectively (Figure 2F). Finally, we applied the algorithm CellPhoneDB, a statistical framework coupled with a ligand–receptor interaction repository which allowed us the exploration of cell–cell communication within complex cellular ecosystems.20 We found that myeloid cells exhibited a significant capacity of interaction with a wide range of structural and immune cell types within the tissue, suggesting their potential pivotal role within the skin ecosystem (Figure 2G). Consistently with our earlier correlation findings (Figure 2F), we observed that endothelial cells displayed a propensity for intercellular communication with various cell types within the tissue, although this communication significantly decreased by Day 10.
Taken together, these data demonstrate that the intercellular ecosystem naturally present in the ex vivo human skin modules remained relatively stable over 10 days of culture, at least at the microscopic and transcriptomic levels, although some variability seemed to emerge in the fibroblast and endothelial cell compartments.
2.3 Quantitative and qualitative validation of reactogenicity in response to mRNA vaccination in human skin modules
We decided to benchmark the effective performance of the ex vivo human skin modules as a proxy to understand subtle cellular and molecular changes in response to subcutaneous infusion of the well characterized commercially available Moderna mRNA-1273 COVID-19 vaccine,21 as an example of immunogenic drug. To achieve this, we implemented a flexible analytical pipeline composed of integrated multimodal analyses specifically adapted to extract qualitative and quantitative information from the biostabilized skin explants (Figure S1). We performed bulk RNA-Seq analyses to characterize sequential changes in gene expression profiles at the tissue level, at 8 h and 24 h after s.c. administration of the vaccine or control clinical-grade water. As potential interindividual variations and gender-based differences can introduce experimental biases, we applied a well-established Combat-Seq batch effect adjustment tool from R library sva version 3.48.22 We next analyzed our datasets and detected 65 and 332 differentially expressed genes (DEGs) at 8 h and 24 h, respectively, after injection of the vaccine (Figure 3A). We first interrogated publicly available databases to delineate putative biological pathways associated with the detected DEGs at 8 h and 24 h. These DEGs were related to nine main pathways categories which were find both at 8 h and 24 h in reaction to vaccine injection (Figure 3B). We found conserved signatures of pathways associated with stress response (e.g., ATF6 activation of chaperone genes), signal transduction and transcription/translation regulation (e.g., TCF/LEF:CTNNB1 mRNA binding proteins or p38MAPK or RUNX3 activation), modulation of blood vessels (e.g., modulation of platelet activation or fibrin clot formation), neuroinflammatory processes (e.g., modulation of Na/Cl-dependent neurotransmitters and Sema3A PAK-dependent axons), immune response and antigen processing (e.g., endocytosis, phagosome acidification, monocytes chemotaxis, or NF-kB activation), adjustment of cell metabolism (e.g., global modulation of lipids metabolism or ChREBP activation of metabolic genes expression), pro-inflammatory cytokine production/regulation (e.g., negative regulation of IL-17 production and positive regulation of Type 2 cytokines or JAK–STAT signaling pathway), skin homeostasis and cell adhesion (e.g., regulation of fibroblasts proliferation and homotypic cell–cell adhesion). Interestingly, at 8 h after vaccine injection, we observed the early expression of genes involved in the detection and response to xenobiotic agents, that is, molecules that are not normally present in animal's life (Figure 3C; Table S1).
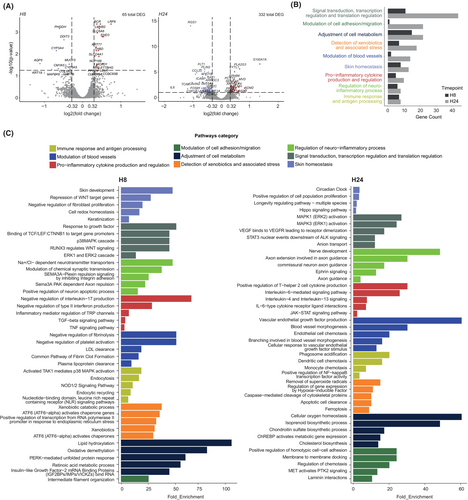
We next quantitatively analyzed the expression of genes associated with each biological pathway (Figure S5A). At both 8 h and 24 h, we found that most DEGs were associated with the biology of keratinocytes or other structural cells. The most upregulated DEGs (25 at 8 h and 52 at 24 h) were reported to be involved in the global regulation of skin homeostasis such as late cornified envelope 2C23 and A (LCE2C and LCE2A),24 pepiplakin-1 (EPPK1), the protease inhibitor serpin family B member 13 (SERPINB13),25 aspartate beta-hydroxylase domain-containing 2 (ASPHD2), lanosterol synthase (LSS), and arachidonic acid 12-lipoxygenase (ALOX12). A large proportion of the other DEGs was associated with the activation of the immune response in keratinocytes, RNA processing, vascular response, and stress in keratinocytes such as thermo-sensitive transient receptor potential M4 (TRPM4),26 cleavage and polyadenylation specificity factor subunit 1 (CPSF1),27 metallothionein-1G (MT1G), and calmodulin-like protein 5 (CALML5),28 respectively. Interestingly, upon injection of the COVID-19 vaccine, we found a global downregulation of IL-6 signaling (i.e., Il6 and IL6R) and a conserved strong expression of genes associated with the transport of lipoprotein, such as low-density lipoprotein receptor (LDLR) and low-density lipoprotein receptor-related protein 8 (LRP8), two pathways previously suggested to be involved in LNP particle incorporation.29 The genes adrenomedulin-2 (ADM2), that encodes a member of the calcitonin gene-related peptide (CGRP)/calcitonin family of hormones, and protein kinase C Zeta (PRKCZ) were also strongly upregulated and previously reported to be associated with skin inflammatory processes. Finally, we also found a global signature of MYC, a key gene that participates in many cellular functions, including cell cycle, survival, protein synthesis, cell adhesion, and microRNA expression30-32 (Figure S5A).
Taken together, these data demonstrate that after s.c. injection of the COVID-19 vaccine in natural human skin modules, a global modulation of the human skin ecosystem can be detected at the transcriptomic level. By assessing subtle alterations in gene expression, we can discern the precise sequential events occurring between the exogenous substance and the skin's biological processes. These events encompass the recognition and response to a foreign agent, the adjustment of skin homeostasis, the activation of stress responses, and the initiation of immune reactions.
We next injected the vaccine in skin modules generated from six different donors and measured 36 cytokines and chemokines released from the injected tissue in the model matrix after 8 h and 24 h using an electrochemiluminescence assay.33 When compared to control modules from the same donors injected with clinical-grade water, following vaccine administration, we observed a general increase in the levels of interleukin (IL)-4, IL-13, IL-17A, IL-16, IL-1α, IL-8(HA), IL-5, vascular endothelial growth factor (VEGF), macrophage inflammatory protein (MIP)-1α, MIP-1β, granulocyte-monocyte–colony-stimulating factor (GM–CSF), interferon gamma-induced protein (IP)-10 (also known as CXCL10), human macrophage/monocyte chemotactic protein (MCP)-4, and eotaxin. The following cytokines were not detected under our experimental conditions: TNF-β, IL-31, IL-27, IL-23, IL-22, IL-21, and eotaxin 3. Conversely, five cytokines and chemokines known to be secreted by activated T cells, DCs, LCs, and macrophages were found to be significantly increased 8 h after vaccination. This conserved secretomic signature triggered by the injection of the vaccine across the six donors tested includes IFN-γ, the thymus and activation-regulated chemokine (TARC, also known as CCL17), MCP-1 (also known as CCL2),34 IL-6, and the macrophage-derived chemokine (MDC, also known as CCL22)35 (Figure 4A,B; Figure S5B), the last four being previously reported by the KEGG database to possibly interact with viral proteins.

In addition, we performed an experiment of global shotgun proteomics with the same samples to further extend our analyses. We found a precise signature of 40 differentially expressed protein (with an adjusted p value of 0.05 and a fold change set to 1.2) at 8 and 24 h after vaccinations. We then integrated together both secretomic (Figure 4A,B) and proteomic (Figure 4C,D) signatures to infer which biological pathways could be regulated at the tissue level using a combination of protein-based publicly available databases, such as KEGG, GO-bp, and Reactome. We found a common signature of pathways (Figure 4E) which recapitulated, at least in part, that observed with the transcriptomic analysis of the same samples (Figure 3B): stress response and detection of xenobiotics (e.g., cellular response to organic cyclic compounds, maturation of spike protein, or regulation of apoptosis), signal transduction and transcription/translation regulation (e.g., activation of STAT proteins, regulation of cytoplasmic translation, or regulation of RUNX3 expression), immune response and antigen processing (e.g., macrophage chemotaxis, positive regulation of leukocytes adhesion, Th17 cells differentiation, or cross-presentation of soluble exogenous antigens), adjustment of cell metabolism (e.g., nitric oxide synthase, protein-N-linked glycosylation, or auto degradation of the E3 ubiquitin ligase COP1), and pro-inflammatory cytokine production/regulation (e.g., IL-10, IL-6, IL-17, and IL-1 signaling) (Figure 4F; Table S2).
Taken together, these findings strongly suggest that immune and/or structural cells, which naturally reside in natural human skin, maintain the ability to release immunomodulatory substances in response to vaccine administration in the modules.
2.4 Single-cell profiling of skin-resident immune cells after subcutaneous vaccination enables the tracking of vaccine sequences and associated modulation of transcriptomic profiles
To gain insight into precise innate mechanisms occurring at the site of vaccine injection, we performed a scRNA-Seq analysis on dissociated human skin modules enriched in skin-resident CD45+ immune cells at 8 h and 24 h post s.c. vaccine injection (Figure S1). Using the Uniform Manifold Approximation and Projection (UMAP) approach, we analyzed the expression patterns of 26,248 genes in 15,564 single cells from the aggregated dataset of the two time points and could unambiguously identify the presence of 14 global immune cell clusters (Figure 5A,B). All populations were annotated based on their combined expression of cardinal marker genes as previously described.36-38 We next developed an analytical pipeline adapted to the amount of biological material contained in each natural human skin module, to sequentially track and quantify the presence of SARS-CoV-2 spike mRNA copies into cells and investigate associated modulations of transcriptomic states at the single cell level (Figure S6). As the SARS-CoV-2 spike sequence is not naturally present in the 24 chromosomes of the human genome reference database (GRCh38),39 we built an “in-silico 25th chromosome” at the end of the GRCh38 that corresponds to the sequence of the vaccine (Figure S6). To this end, we created an entry into the GRCh38-associated GTF annotation files and added the entire spike sequence as CDS (CoDing Sequence) and an exonic entry from positions 58 to 3879 to suppress the putative UTR (UnTranslated Region) defined by NAanalytic (the detailed protocol is described in the Methods section). We then aligned the scRNA-Seq raw data to the custom reference transcriptome, and proceeded to perform conventional analyses. We found that 8 h after s.c. injection of the vaccine, the DC/macro cluster, and more surprisingly the mast cell cluster, expressed significant levels of spike mRNA (Figure 5C–E). These data strongly suggest that, in our experimental conditions, the LNPs that vehicle the spike mRNA sequences will be preferentially incorporated into DC/macrophage and mast cell compartments after s.c. injection.
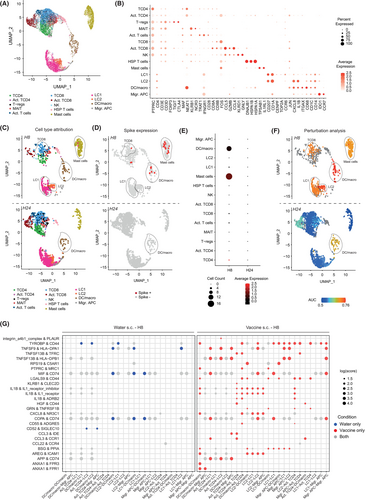
To evaluate the potential impact of s.c. vaccination in different immune populations, we used the machine learning-based algorithm Augur.40 After 8 h, the Augur analysis revealed significant transcriptomic changes in immune compartments, including DC/macrophages, mast cells (both having detectable levels of spike mRNA), LC1s, LC2s, and CD8 T cells (these last populations having very low/undetectable levels of spike mRNA), while at 24 h, only the DC/macrophage compartment exhibited continued statistical differences (Figure 5F). We next extracted the list of DEGs associated with our analysis and inferred putative biological pathways regulated in each immune compartment over time. We found conserved signatures of pathways associated with mRNA processing and translation machinery, Type I immune response, Roundabout (Robo) receptors signaling (i.e., involved in actin cytoskeleton remodeling), SARS-CoV-1/2 antiviral response, and cellular stress in most cell types (Figure S7A). Interestingly, DC/macrophage and LC2 subsets shared some common additional signatures of genes associated with clathrin-mediated endocytosis (i.e., reported to be involved in LNP incorporation),41 RHO GTPase signaling, MAPK signaling, transcriptional regulation, cell migration, and regulation of neuronal and vascular compartments. However, some pathways were found specifically upregulated in the DC/macro subset, such as FLT3L/FLT3 axis, NF-κB signaling, MHC-I-mediated cross-presentation,42-44 epidermal growth factor (EGF)/EGF receptor signaling, response to interferon, and reactive oxygen species (ROS) production (Figure S7A). Finally, we found that only the DC/macro subset exhibited enhanced expression of genes encoding TLR2, TLR4, and TLR8 (Figure S7B), previously reported to be involved in the priming of potent antiviral response by DCs.45
Taken together these data demonstrate that upon s.c. injection of the vaccine, spike mRNA sequences were detected at 8 h, but not 24 h following injection, and mainly in two innate immune clusters harboring DC/macro and mast cell signatures. Nonetheless, we could detect cell-specific transcriptomic modulations in multiple skin-resident immune cells after vaccination.
2.5 Subcutaneous and intradermal deliveries of the vaccine foster the development of mature antigen-presenting cells and cell–cell interactions
Prior researches have indicated that diverse routes of vaccine administration may selectively target specific immune subsets, leading to distinct cellular and humoral immune responses.46, 47 We generated several human skin modules from the same donor studied in Figure 5 to investigate potential changes in immune cells transcriptomic profiles and incorporation of spike sequences when the mRNA-1273 vaccine was administered i.d. versus s.c. Again, we detected the presence of significant levels of the spike sequence mostly at 8 h, but less at 24 h, after i.d. vaccine injection in DC/macro and mast cell clusters (Figure S8A–C), confirming a natural capacity for these cells to uptake/be targeted by the LNP-loaded mRNA particles. We next used Augur to investigate potential transcriptomic changes in the immune compartment. We found that 8 h after i.d. vaccination, DC/macrophages, LC1s, and CD8 T cells, but not mast cells nor LC2s, exhibited significant transcriptomic changes (Figure S8D). However, compared to results observed upon s.c. administration, all cells of the myeloid compartment, including LC2, migratory APC and mast cell clusters, and a large proportion of CD4 and CD8 T cells underwent significant transcriptomic changes 24 h after i.d. administration of the vaccine.
We next applied the algorithm CellPhoneDB to explore differences in cell–cell communication and ligand–receptor interactions between different immune cells when the vaccine was injected s.c. or i.d.20 8 h after s.c. injection of the vaccine, we found that myeloid cells, including DC/macrophages, LC1s, LC2s, and migratory APCs exhibited an increased capacity of interaction with other immune cell types within the tissue, including CD4 T-cell subset, as compared to the clinical-grade water control (Figure 5G; Figure S7C). Such a pattern of interaction was then significantly decreased at 24 h (Figure S7C). Conversely, 8 h after i.d. injection of the vaccine, the pattern of interaction of myeloid cells with other immune cell subsets was less pronounced than that observed after s.c. injection. However, 24 h after i.d. injection most myeloid cells exhibited a significant increase in their capacity to form interactions with lymphoid cells such as CD4 T-cell subset (Figure S9A). We next inferred the predicted ligand–receptor pairs that could be engaged between the different interacting immune cells. We found that most of the predicted interactions were either between myeloid cells or between myeloid cells and CD4 T cells, independent of the route of administration (Figures S7C and S9A). Interestingly, we found significantly more predicted pairs of ligand–receptor after s.c. injection than after i.d. injection of the vaccine. Among the predicted interactions, we observed that the s.c. vaccination could favor interactions between CD4 T cells and antigen-presenting cells (i.e., clusters DC/macro, LC1, LC2, and migratory APCs). Notably, the interactions between CD4 T cells and DC/macrophages specific to vaccine condition were CD44 (i.e., involved in cell–cell adhesion) and TYROBP (i.e., protein tyrosine kinase involved in cell activation), TNFSF13B (i.e., BAFF involved in lymphocytes activation) and TFRC (i.e., transferrin receptor involved in T cell activation), PTPRC (i.e., CD45) and MRC1 (i.e., mannose receptor C type 1 or CD206), LGALS9 (i.e., Galectin 9) and CD44, IL1B, and IL1 receptor (and IL1 receptor inhibitor), IL1B and ADRB2 (i.e., adrenergic receptor beta 2 involved in immune modulation), HGF (i.e., hepatocyte growth factor) and CD44, CXCL8 (i.e., interleukin 8), and NR3C1 (i.e., glucocorticoid receptor), CD55 (i.e., a potent activator of naïve T cells), and ADGRE5 (i.e., CD97 involved in T-cell activation), CCL3, and CCR1 (Figure 5G; Figure S9B).
We finally investigated changes in the activation status of skin-resident APCs at the protein level using Multiplex ANnotated Tissue Imaging System (MANTIS®),48 an interactive analytical system based on multiplexed confocal imaging, that automatically generates a digitized version of the skin immune landscape and enables single-cell quantitative data visualization. Using MANTIS®, we generated an attribution matrix to automatically annotate dermal DCs (dDCs, CD45+ CD1c+ CD207−), langerin+ dDCs (CD45+ CD1c+ CD207+) and Langerhans cells (LCs, CD45+ CD1c− CD207+)49 (Figure 6A,B) and analyzed the expression of well-established activation/maturation markers such as HLA-DR, CD80, CD86, CD83, CD40, and CCR7 in modules generated from four donors and injected s.c. with the vaccine or water for injection control. A total of 8166 single CD45+ immune cells were identified with the following distribution 7.4% dDCs, 26.7% langerin+ dDCs, 33.9% LCs, and 32% other immune cells across the donors (Figure 6C). We found that LCs and langerin+ dDCs significantly upregulated most of the maturation markers mentioned above, especially at 24 h, and to a lesser extent 8 h following vaccination (Figure 6D; Figure S10A). However, dDCs seemed to be less responsive to the vaccine and upregulated CD83 and pan-HLA after 24 h.
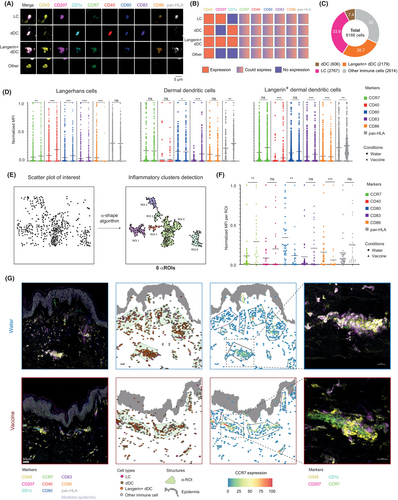
Finally, we recently developed an algorithm based on α-shape that enables to automatically detect and quantify the main cellular clusters in the human dermis often located in perivascular areas (i.e., named hereafter “α-ROIs”) to provide a high-level view of the in situ immune architecture of the skin after the injection of water or vaccine.48 Using this method, we investigated the anatomical location of APCs after vaccination and the expression of key biomarkers within cell clusters (Figure 6E). While the numbers of α-ROIs or relocation of dDCs, langerin+ dDCs, or LCs after vaccination did not seem to be changed, we found that such cells within α-ROIs upregulated significant amounts of CCR7 at both 8 and 24 h (Figure 6F,G; Figure S10B,C), a critical chemokine receptor involved in DCs homing to draining lymph nodes after vaccination.50 Taken together, these results provide robust evidence that skin-resident myeloid cells acquire a mature phenotype of APCs after vaccination (in particular LCs and langerin+ dDCs), while increasing their predicted interactions with surrounding CD4+ T cells with specific pairs of ligands and receptors. Here we show that such pipeline is uniquely positioned to assess numerous scientific questions in the field of human innate immune response to vaccines, not only in DCs, but also in a wider range of native tissue-resident myeloid and lymphoid cell populations depending on the route of administration.
3 DISCUSSION
Here we introduce a versatile framework primarily designed for longitudinal profiling of the human skin ecosystem in response to vaccines at the injection site. Each individual human skin module consists of the matrix–skin biopsy–silicone ring association, allowing s.c. or i.d. delivery using standard clinical syringes while preserving the integrity of native adipose tissue. Compared to classical skin-related data obtained during clinical trials in humans, this method offers a remarkable scalability, enabling multiple parallel experiments in one donor and extension to cohorts based on selectable human biological diversity. The platform incorporates an analytical pipeline tailored to the amount of biological material found in a single module.
Using this platform, we characterized the biological response and immunocompetency of the skin, at both organ and single-cell levels, in response to injection of the mRNA-1273 COVID-19 vaccine, as a model of immunogenic drug. Such vaccine is recommended for intramuscular injection, it is important to notice that in the absence of blood flow, the subsequent analysis of immune response is restricted to skin-resident cells. One should thus be careful in extrapolating data to the in vivo situation. At the organ level, we could decipher precise sequential events that unfold between the exogenous substance and the skin biology. These events encompass the recognition and response to the foreign agent, adjustments in skin homeostasis, activation of stress responses, and initiation of immune reactions. It is very interesting to speculate that the injection of a vaccine into the skin not only affects APCs, but also generates a complex biological response at the organ level that is likely to influence the efficacy of the immune response to carry out protection (Figure S11, left). Exploring and manipulating the mechanisms that govern the global regulation of the skin ecosystem following vaccination, extending beyond the targeted immune cells, holds great promise for future investigations.
When analyzed through the lenses of immunology at the single-cell level, we found that, under our experimental conditions, the vaccine consistently targets dermal DC/macrophages and mast cells, irrespective of the administration route in natural human skin modules (Figure S11, right). Mast cells are highly granular/vesicular cells,51-53 it would be interesting to gain a better understanding of how they could incorporate such quantities of LNPs. These findings are in line with previous reports that LNPs could efficiently transfect in vitro-cultured mast cells.54, 55 Previous work suggests that mast cells could activate antigen-experienced CD4 T cells in mice and humans,56-58 to which extent such findings are applicable in the context of vaccination is still unknown. The primary function of mast cells is to release granule-associated mediators, a process named “degranulation”59 and involved in drug-associated injection site reactions.60, 61 COVID-19 vaccines, vaccines excipients, anti-PEG antibodies, and skin mast cell-associated products (such as histamine) have been reported to be associated with redness, swelling, pain, anaphylactoid reactions, and sweet syndrome in the skin,62-70 the extent to which these clinical manifestations are directly associated with the capacity of mast cells to incorporate LNPs remains an open question.
Beyond the above-described spike-positive immune cells, the infusion of the vaccine also triggered substantial transcriptomic changes in the entire myeloid compartment, including LC2, migratory APC and mast cell clusters, and in a significant proportion of CD4 and CD8 T cells. It is possible that our in-silico detection of the spike is not sensitive enough to catch smaller incorporation of the vaccine in those cell types. Indeed, we found the presence of the spike mRNA at 8 h, but not anymore at 24 h, indicating that the spike mRNA is either completely degraded or partially degraded in less than 24 h after injection in the skin. However, in line with our organ level analysis, it is likely that the vaccine triggers a qualitative modification of the skin ecosystem and fine tuning of “bystander non-targeted cell types” at the site of injection.
Finally, previous reports in the field have shown that vaccine efficacy could be affected by the route of administration.46, 71, 72 We found that the i.d. injection route shows a prolonged modulation of both myeloid and lymphoid cells compared to the s.c. route, persisting from 8 h to 24 h after vaccine administration. These findings further demonstrate that the route of administration could lead to diverse effects on local immune cell populations within the skin, involving not only dermal DC/macrophages, but also a broader array of myeloid and lymphoid cells. In conclusion, we demonstrate that the use of the natural human skin module technology, coupled to a scalable analytical pipeline, has great potential to advance the study of skin physiology. In the context of drug and vaccine discovery and development, it will be especially valuable for the study of molecular mechanisms of action, prioritization of lead candidates, toxicity and safety testing, and biomarkers identification.
4 MATERIALS AND METHODS
4.1 Natural human skin modules: sourcing, production, treatment, and sampling
Natural human skin modules were obtained from Genoskin SAS (https://www.genoskin.com/). Genoskin has obtained all legal authorizations necessary from the French Ministry of Higher Education, Research and Innovation (AC-2017-2897) and the Personal Protection Committee (2017-A01041-52). Skin biopsies of 23 mm with 10 mm total thickness and 18/23 mm diameter silicon rings were embedded in a proprietary solid matrix, with the epidermal surface in direct contact with ambient air. The natural human skin biopsies were mounted on cell culture inserts, loaded in multi-well companion plates containing 3 mL of chemically-defined xeno-free Genoskin medium based on William's E (Pan Biotech) supplemented with 1 mM CaCl2, 1% of penicillin/streptomycin, 0.2% of amphotericin B, and 10 mg/mL of vitamin C. The modules were maintained at 37°C in a standard incubator (5% CO2) up to 10 days, and culture medium was renewed each day.
Modules viability (MV) study. At Days 0, 3, 5, and 10, the modules were sampled and preserved either in OCT for immunostaining, or fixed in formalin and paraffin-embedded (FFPE) for hematoxylin and eosin (H&E) staining, or processed for scRNA-Seq (Figure S1).
Early events of vaccination (EEV) study. At 0 h, natural human skin modules were injected subcutaneously either with clinical-grade water or mRNA-1273 COVID-19 vaccine (Moderna). Two sets of experiments were performed and both gathered water-injected and vaccine-injected conditions. In the first set of experiment, modules were sampled at 0, 8 and 24 h, and split into four pieces: 1/4 of the module (including the hypodermis) was saved for paraffin embedding, 1/4 of the module without hypodermis was frozen in RNA safeguard reagent (Bioer) and stored at −80°C, 1/4 of the module without hypodermis was embedded in OCT embedding matrix (Cellpath) and stored at −80°C, and 1/4 of the module without hypodermis was frozen and stored at −80°C. The matrix and the culture medium of the module were collected and stored at −80°C. In the second set of experiment, modules were collected at 0, 8, and 24 h and processed for scRNA-Seq. Two modules were used for each condition (Figure S1).
4.2 Histology
Five micrometer thick FFPE-tissue sections were rehydrated, stained with H&E, and dehydrated. The slides were covered with mounting medium (Eukitt®, Orsatec), and sealed with a coverslip. For each sample, representative images were acquired at 40x magnification with a DMi1 (Leica) equipped with a MC170 HD camera (Leica).
4.3 Injection and bolus observation
The natural human skin modules from at least four donors were injected subcutaneously or intradermally with 100 μL of patent blue V (E131), 0.5% (w/v) in PBS, or potassium iodide solution just before the picture acquisition or tomography acquisition respectively. For the tomography acquisition, an EasyTom X-ray microtomography machine (RX Solutions) was used. This machine was equipped with a 150-kV microfocus X-ray source (Hamamatsu).73 X-ray tomographies were obtained by acquiring 1440 images at different angles evenly distributed over 360°. The 3D volume was reconstructed using RX Solution Software (X-Act). Post-processing of the 3D images was performed using Imaris software (Oxford Instruments).
4.4 Thick section staining and two-photon imaging
Hundred micrometer thick cryosections from one donor cultured at different time points were fixed with paraformaldehyde. Slides were rinsed, blocked, permeabilized, and then incubated with fluorophore-coupled antibodies or unconjugated antibodies for 24 h at 4°C in the dark. The slides were rinsed and incubated with the secondary antibodies for 2 h at room temperature in the dark. Finally, slides were mounted in Mowiol medium (Sigma-Aldrich) and sealed with a coverslip. All conjugated and unconjugated antibodies used in this study were validated in single immunostainings (unpublished), and are listed in Table S3. Mosaic images were acquired using an Ultima 2P plus biphotonic microscope (Bruker Corporation) equipped with a laser Chameleon Discovery NX (Coherent Inc) operating at a 770 nm excitation wavelength, and a 20x objective, Numerical Aperture of 1A.U., 2 mm WD (Olympus). The acquired images were stitched and processed using the Imaris Software.
4.5 Human 36-plex cytokines assay
The human multiplex cytokine release analysis was performed on six donors, two conditions (water for injection or vaccine), and two time points (8 and 24 h after injection). Levels of cytokines secreted in the matrix were quantified using the 36-plex kit (K15089G) from Meso Scale Discovery (MSD),74 following manufacturer's instructions. Cytokine concentrations expressed in pg/mL were calculated by comparison to the standard curve which was generated in the same biological matrix as the samples. Only values that were in the detection range were considered. Significantly statistical cytokine concentration changes over the six analyzed donors were assessed.
4.6 Proteomic analysis
The proteomic analysis was performed on six donors, two conditions (water for injection or vaccine), and two time points (8 and 24 h after injection), on both culture medium and frozen skin explants. Briefly, skin explants were denatured by heating and 100 μg of proteins were digested with iST kits (PreOmics). Resulting peptides were cleaned using Phoenix kit (PreOmics). The eluted peptides concentration in each sample was determined using BCA method. For the culture medium samples, the same protocol was followed without the initial step of denaturation and extraction. 250 ng of peptides were injected in LC–MS/MS once for each sample. Chromatography was performed using a Vanquish Neo system using PepMap100 C18 column. Data were acquired using an Exploris 480 (Thermo Scientific) mass spectrometer using the experimental settings described in Table S4. Data were processed with Proteome Discoverer 3.0. Proteins were identified by SEQUEST-HT search algorithms. The database was made of the human reference proteome from Uniprot (UP000005640), the SARS-CoV-2 proteome (UP000464024_2697049), and a database of frequently observed experimental contaminants in mass spectrometry without human contaminants (cRAP database, https://www.thegpm.org/crap/). INFERYSTM, a deep learning prediction-based rescoring system (Thermo Scientific), was used to increase the number of MS/MS identifications. False discovery rate (FDR) determination was made using Percolator algorithm. The abundance was measured for each peptide, and summed for each protein, using the Minora Feature Detector and the Feature Mapper (Proteome Discoverer 3.0).
By using the version 1.24.0 of the DEP package75, data were transformed into a SummarizedExperiment R object. Two comparisons were conducted: water versus vaccine at H8, and water versus vaccine at H24 in both culture medium and explant data. Donors were treated as biological replicates. First, the potential presence of outliers was investigated with different methods (Hubert PCA,76 interquartile range, and Rosner test) and led to the removal from the analysis of the sample Water_Donor14 in the explant_H8. Due to poor data quality missing values were filtered: if for protein X, a quantification value is found in at least three samples of either the water or the vaccine condition, then the protein is retained for further analysis; otherwise, it is removed from the dataset. Then, data were normalized using the variance-stabilizing transformation method implemented in the R package limma77 (version 3.58.1). Imputation of the remaining missing values was performed by random draw from a Gaussian distribution centered on the minimal value. The contrast was defined by setting the control as the water samples. Proteins with significantly different abundance between the two conditions and an adjusted p-value <0.05 were defined as differentially expressed proteins (DEPs).
4.7 Integration of proteomic and cytokine release data
Proteins and cytokines which were identified as statistically differentially concentrated between the water and vaccine conditions were combined in an enrichment pathway analysis conducted with PathfindR R package78, using the protein interaction network STRING, as well as databases such as gene ontology (biological process only), KEGG, and Reactome.
4.8 Bulk RNA sequencing
The bulk RNA sequencing was performed on four donors, two conditions (water for injection or vaccine), and two time points (8 and 24 h after injection). Briefly, a 4-mm punch of skin (without hypodermis) was digested in Trizol using a GentleMACS™ Octo Dissociator (Miltenyi Biotec) to extract RNA from the tissue. The RNA was then purified using a RNAeasy Plus Mini Kit (Qiagen) following the manufacturer's instructions. The concentration and quality of RNA were determined in each sample using a NanoDrop (ThermoFisher Scientific). mRNA library preparation was realized following manufacturer's recommendations (Illumina Stranded mRNA Prep from Illumina). Final samples pooled library prep was sequenced on NovaSeq 6000 Illumina with SP-200 cycles cartridge (2 × 800 millions of 100 bases reads), corresponding to 2 × 30 millions of reads per sample after demultiplexing. These sequence data have been submitted to the GenBank databases under accession number PRJNA1135157. Quality control was performed with MultiQC79 and reads were aligned with STAR80 on the human GRCh38 reference genome. Aligned reads were ordered and indexed using SAMtools.81 Genes counts were computed with HTSeq82 counts. DESeq283 was used to process raw count. DESeq object was created from a sample table. Donor and gender biases were corrected using Combat-seq from R library sva.22, 84 Biological process of gene ontology, KEGG, and Reactome databases were used to compute the pathway enrichment analysis.
4.9 Single-cell RNA sequencing
For the MV study, a total of four scRNA-Seq (0, 3, 5, and 10 days of culture) were performed using one donor. For the EEV study, a total of eight scRNA-Seq were performed in one donor: two conditions (water for injection or vaccine) at two time points (8 and 24 h after injection) and injected subcutaneously or intradermally. Briefly, skin was harvested in a predigestion medium, fragmented into pieces, and incubated at 37°C. The samples were digested with Liberase (Sigma Aldrich) and DNAse I (Sigma Aldrich). Samples were further dissociated with the GentleMACS™ Octo Dissociator. Cells were then enriched with a Percoll (CYTIVA) gradient and washed. For the MV study, the cell suspension resulting from the dissociation was sorted using a FACSymphony (BD Biosciences) cytometer to isolate Sytox negative alive cells. For the EEV study, the cell suspension resulting from the dissociation was enriched in CD45+ cells with an EasySep Release Human CD45 Positive Selection Kit (Stemcell Technologies) following the manufacturer's instructions. For scRNA-Seq, cells were encapsulated into droplets using Chromium Next GEM Single Cell 3′ Reagent Kits v3.1 with single indexing, according to manufacturer's protocol (10× Genomics). All the libraries were finally controlled on a Fragment Analyzer HS-NGS run (Agilent). 10× libraries were pooled and charged on one SP lane of the NovaSeq 6000 instrument (Illumina) using the NovaSeq 6000 SP Reagent Kit v1.5. These sequence data from the EEV study have been submitted to the GenBank databases under accession number PRJNA1137828.
For the MV study, raw sequencing data were processed with Cellranger.85 Transcripts were mapped on the reference human genome GRCh38 and all samples were aggregated. For the EEV study, water samples were processed similarly to the MV study data. Regarding the vaccine-injected samples, a custom genome of reference was created with Cellranger mkref function in order to detect the exogenic spike COVID-19 mRNA. The sequence retro-engineered by NAalytics86 was added at the end of the GRCh38 genome as an artificial vaccine “chromosome” (Figure S6). Removal of ambient RNA was performed on EEV data with Cellbender87 version 0.2.2. The datasets were filtered using the Seurat R toolkit88 (Table S5). Normalization and variance stabilization were performed with SCtransform seurat function89 and data were integrated using Harmony.90 Pseudotimes of keratinocytes differentiation were calculated with the Monocle3 R package.91 Perturbation analysis was performed using the cell type prioritization R library Augur.40 Interactome study was performed with CellphoneDB.20 For each cluster, only genes expressed in at least 25% of the cells of the cluster were conserved. For each condition, a table gathering all interaction pairs between all cell types was created. Only cell types with more than 10 interactions (in absolute value) were kept.
4.10 MANTIS analysis
The MANTIS analysis was performed as previously described48 on five donors, two conditions (water for injection or vaccine), and two time points (8 and 24 h after injection). A specific “activation of antigen-presenting cells” panel (Table S6) was designed based on cell type attribution markers (CD45, CD207, and CD1c) and activation markers (CCR7, CD40, CD80, CD83, CD86, and pan-HLA). Cell types were attributed based on the MFI of CD45, CD207, and CD1c. Distinct thresholds were determined for each attribution marker and depending on the skin compartment (epidermis and dermis) to allow the removal of autofluorescence signal and background that can be different depending on the compartment (Table S7).
For each time point, vaccine and water MFI of activation markers were statistically compared following MFI min–max normalization. The transformation was made using the formula: (MFI_Marker − min(MFI_Marker))/(max(MFI_Marker) − min(MFI_Marker)) to represent all the data (all donors and conditions) between 0 and 1.
An analysis based on 3D location of APCs was also performed. The epidermis was identified using its natural autofluorescence as previously described.48 α-shape algorithm was used to model the epidermis (alpha = 0.05) and immune cell clusters (0.07 for Donors 12, 13, and 14, and to 0.06 for Donors 8 and 9) as previously described.48 For each alpha-region of interest (α-ROI), different parameters were determined: area, total number of cells, total number of cells of each cell type, and the mean MFI of each marker. The shortest distance between α-ROI and the neighboring cells was computed using a k-dimensional tree method. For non-normalized data, paired by donor comparisons were performed (volumes of tissue by acquisition were compared, no statistical difference was measured).
4.11 Statistics
For each couple vaccine–water samples, preliminary parametric tests of the adequacy to a normal distribution (Shapiro test) and variance homogeneity (Fisher F-test) were performed to determine the most relevant statistical test to compare means (Student t-test, Welch's t-test, or Wilcoxon signed-rank test). Statistical tests were performed using Prism 8 (GraphPad Software) and the Rstats R package.
4.12 Data visualization
Visualization charts were obtained using the ggplot2,92 Pigengene,93 pheatmap, DEP, and ComplexHeatmap94 R packages, and matplotlib95 and seaborn96 Python packages.
AUTHOR CONTRIBUTIONS
N.G. conceived the project. M.S., M.P., E.B., J.M., E.P., and N.G. were involved in experimental design. M.S., M.P., E.B., J.M., E.P., and N.G. performed most experiments and compiled the data. N.S. A.L., A.B., and L.B. provided important help with experiments. E.M. and P.D. provided expertise. All authors participated in analyzing the data and writing or editing the paper.
ACKNOWLEDGMENTS
We thank Anthony David, Julie Charpentier, Margot Romero, Anne Dalapa-Amana, Lévi Da Silva, Alexandra Ochando, and Hervé Huchon at Genoskin SAS, all the members of the Gaudenzio Lab at Infinity, and Paul Duru from the IMFT and Laurent Malaquin from the LAAS CNRS for discussions and technical assistance. We thank Sophie Allart and Simon Lachambre for technical assistance at the cellular imaging facility of Inserm UMR 1291, Toulouse. We thank Anne-Laure Iscache, Valerie Duplan-Eche and Fatima-Ezzahra L'Faqihi for technical assistance at the flow cytometry core facility of INSERM UMR1291, Toulouse. We thank the GeT-Santé facility (I2MC, Inserm, Génome et Transcriptome, GenoToul, Toulouse, France) for the advice and technical contribution to the single-cell and bulk RNA sequencing experiments. This work also benefited from equipment and services from the iGenSeq core facility (genotyping and sequencing), at ICM.
FUNDING INFORMATION
This work was supported by the Agence Nationale pour la Recherche (ANR) (to N.G and E.P), by Genoskin SAS (to E.P) and co-funded by the European Union (ERC-2018-STG, IMMCEPTION, #802041 and ERC-2023-COG, PRENATALL, #101124255 to N.G). Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the European Research Council. Neither the European Union nor the granting authority can be held responsible for them.
CONFLICT OF INTEREST STATEMENT
Co-patent applications between Inserm and Genoskin have been filed related to the subject matter of this publication. N.G. acts as chief scientific officer, E.M is chief innovation officer and P.D is founder and chief executive officer at Genoskin. N.G., E.M., and P.D. are shareholders at Genoskin. M.S, E.M, E.B, M.P, and E.P are employees at Genoskin.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.