The moderating role of allergy immunotherapy in asthma progression: Results of a population-based cohort study
Abstract
Background
Allergic asthma causes substantial morbidity and constitutes a public health burden, which increases with asthma severity. There is evidence that allergy immunotherapy (AIT) prevents the progression of allergic rhinitis (AR) to asthma. However, evidence is missing on the potential of AIT to prevent progression from milder to more severe asthma.
Methods
This population-based cohort study utilized healthcare data (2005 to 2014) from a statutory health insurance in Germany. The severity of asthma was classified according to the treatment steps recommended by the global initiative for asthma (GINA). The effect of AIT on the transition between the GINA steps was analyzed using multivariable Cox regression models adjusted for age and sex.
Results
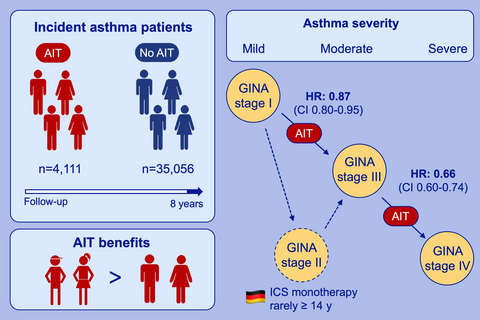
From the total cohort of 1,739,440 patients, 39,167 individuals aged 14 years or older were classified as having incident asthma during the observation period and were included in the study. From these, 4111 patients (10.5%) received AIT. AIT exposure was associated with a significantly decreased likelihood of asthma progression from GINA step 1 to GINA step 3 (HR 0.87; 95% CI 0.80-0.95) and GINA step 3 to GINA step 4 (HR 0.66; 95% CI 0.60-0.74). GINA medication for step 2 and step 5 was rarely prescribed.
Conclusions
This observational study in a real-world setting indicates that patients with allergic asthma who receive AIT are less likely to experience progression of asthma severity than asthma patients not receiving AIT.
Graphical Abstract
In a large cohort study of 39,167 asthma patients, those with AIT had a decreased likelihood for disease progression, measured in symptomatic medication according to GINA. Younger patients benefited most from AIT, which is reflected in prevention or delay of asthma progression. Inhaled corticosteroids (ICS) monotherapy (GINA stage 2) is rarely prescribed in Germany for asthma patients over 14 years.
1 INTRODUCTION
Allergic rhinitis (AR) has been recognized as the most important risk factor for the development of asthma.1 AR and asthma cause substantial public health burden due to high prevalence, adverse impact on quality of life, and high direct and indirect costs for the healthcare system. Costs attributable to asthma substantially increase with disease severity.2-5 Therefore, medications that prevent the progression from AR to asthma, and/or disease progression of existing asthma are of particular relevance to tackle the current public health burden of allergic diseases.
Allergen-specific immunotherapy (AIT) is currently the only causal treatment modality that not only reduces the symptoms of prevalent AR and asthma. AIT modifies the course of these allergic diseases in restoring allergen tolerance and reducing the tendency to produce immunoglobulin E (IgE).6 A large cohort study demonstrated that AIT with native unmodified allergens led to a significant risk reduction in asthma development.7 Similar data were obtained for grass tablet immunotherapy.8 A large, double-blind, placebo-controlled 5-year trial with a SQ standardized grass immunotherapy tablet showed a significant preventive effect on the development of asthma symptoms and/or use of asthma medications.9 Despite the beneficial potential of AIT, only 7% of patients with AR and 5% of patients with asthma receive AIT in Germany, which has been interpreted as an underutilization of this treatment method.10
While there is evidence that AIT prevents the development/ manifestation of asthma in patients with prevalent AR,7 studies on the effect of AIT on the course of disease in patients with prevalent asthma are missing. The value of AIT would be even higher if it not only prevented the progression from AR to asthma (AA), but also modified the course of asthma, that is, prevented asthma progression. To generate new evidence for this highly relevant research question, we undertook a large cohort study. We hypothesized that patients with asthma, who are exposed to AIT, are less likely to experience progression of asthma severity than patients not receiving AIT.
2 METHODS
2.1 Study design and participants
We undertook a longitudinal cohort study based on comprehensive routine healthcare data from Germany. In Germany, approximately 90% of the population is covered by statutory health insurances. We used routine healthcare claims data from AOK PLUS, a large statutory health insurance in Saxony (area ~ 18 000 km2, population ~ 4 Mio.), which covers approximately half of the local population. The database was used previously for several analyses in the field of allergy and other disease areas.11-13 The database includes information on outpatient care using diagnosis based on the International Statistical Classification of Diseases, 10th edition (ICD-10 Code), prescriptions according to the Anatomical Therapeutic Chemical classification (ATC code) as well as information on age and sex of the insured population.
The study population consisted of patients aged 12 years or older who were continuously insured from January 1, 2005, until December 31, 2014, or until death, and who were classified as having incident asthma during the observation period. Case validation methods were applied as suggested by the Guidelines “Good Practice Secondary Data Analysis” of the German Society of Epidemiology.14 Accordingly, an individual was classified as having asthma if asthma had been diagnosed at least twice (ICD-10 Code J45) in outpatient care by a physician (both primary and/or specialized physician) and if the subject had received at least two prescriptions of short-acting beta agonists (SABA), inhaled corticosteroids (ICS) or combinations of inhaled corticosteroids and long-acting beta agonists (LABA) within four consecutive quarters (For further details please refer to Table S1). Individuals who did not meet this definition in the years 2005-06, but did in the following years, were classified as incident cases of asthma. These patients with incident asthma constituted the study population. The study population was further stratified into three sub-cohorts according to their age in 2005: adolescents 12-17 years, younger adults 18-50 years, and older adults and elderly ≥50 years. Children younger than 12 years were not included as asthma severity could not be adequately defined based on the available data.
2.2 Primary outcome
Progression of disease severity in asthma over time was the primary outcome. The prescribed asthma medication for each patient according the Global Initiative for Asthma (GINA) classification was used as a marker for asthma severity.15 The GINA guidelines are accepted worldwide and provide recommendations on how to diagnose and treat asthma with a strong focus on disease control. GINA defines 5 treatment steps ranging from step 1 to step 5. If a patient is not well controlled, treatment escalation is recommended. If a patient is well controlled, a step down in asthma medication is recommended.15 All asthma medications prescribed for individuals of the study population were classified according to these 5 GINA steps. Allocation to low and moderate/high ICS medication was done by a professional pharmacist. An increase of asthma severity was defined as a step up in asthma medication according to the GINA recommendations.
2.3 Exposure to AIT and confounding variables
AIT exposure was defined as receiving at least one AIT prescription. A step up in symptomatic asthma treatment according to the GINA steps was only attributed to the AIT group, if the corresponding change in asthma treatment occurred after AIT initiation. Likewise, transitions between GINA steps were allocated to the non-AIT group if AIT was initiated after or at the same time as the corresponding change in symptomatic asthma treatment. There was no adjustment for the duration of AIT therapy prior to a change in asthma severity.
Age (continuously) and sex were considered as confounders. One potential limitation to internal validity in nonrandomized trials is confounding by indication. Patients with nonallergic asthma should not receive or benefit from AIT. As part of our sensitivity analyses, the population was limited to individuals who had at least two outpatient diagnoses for allergic rhinitis (ICD-10 J30) within 12 months in 2005-06.
2.4 Statistical analysis
In addition to standard descriptive statistics, we applied time-to-event analyses with nonparametric cumulative event curves and semiparametric Cox proportional hazard models.16 Time in days since January 1st, 2007, was used as the process variable. A step up in asthma medication was handled as the failure variable. The end of the observation period on December 31, 2014, or death was modeled as censored events. Follow-up could therefore be up to 8 years. Results were displayed as Hazard Ratios (HR) with corresponding 95% confidence intervals and adjusted for age and sex. A corresponding number needed to treat (NNT) after 5 years was calculated. Analysis was conducted using the program R, version 3.3.2 with the packages survival and survminer.17, 18
3 RESULTS
The study database included a total of 1 739 440 patients, with 54% being female and an average age of 49 years. A total of 39 167 patients (63% female; mean age: 49 years) with incident asthma met all eligibility criteria and were included in the study (Figure 1). A total of 4111 patients (10.5%) were exposed to AIT during the observation period. Patients receiving AIT were younger and more frequently suffering from comorbid allergic rhinitis than patients not exposed to AIT (Table 1).

| Variable | All subjects (percent) | AIT group (percent) | non-AIT group (percent) |
|---|---|---|---|
| Total | 39 167 (100) | 4111 (100) | 35 056 (100) |
| Male | 24 623 (37) | 1548 (38) | 12 996 (37) |
| Female | 14 544 (63) | 2563 (62) | 22 060 (63) |
| Adolescents | 2586 (7) | 653 (16) | 1933 (6) |
| Young adults | 16 950 (43) | 2916 (71) | 14 034 (40) |
| Older adults/elderly | 19 631 (50) | 542 (13) | 19 089 (54) |
| Allergic rhinitis | 1733 (44) | 3871 (94) | 13 461 (38) |
| No allergic rhinitis | 21 835 (56) | 240 (6) | 21 595 (62) |
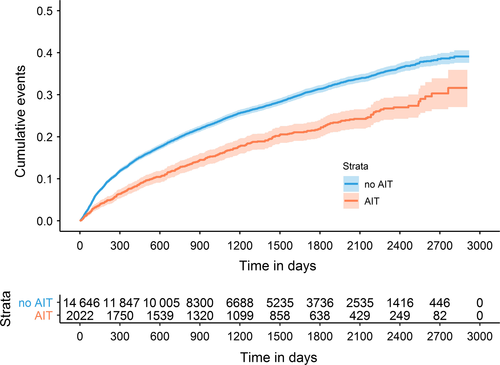
Medications of GINA step 2 (3.5%) and GINA step 5 (0.03%) were rarely prescribed so that the transition between GINA steps 1 and 2, step 2 and 3, and step 4 and 5 could not be analyzed. A total of 8726 patients had at least one transition between GINA steps 1, 3, or 4, and 1085 had two. Not all 39 167 patients were under risk of progression into all GINA steps. Some patients did not receive a GINA step 3 medication within the observation period and were therefore not at risk for step 4 medication. Others initially received treatments allocated to GINA step 3 or step 4 so that progression to these steps was impossible. Table 2 describes the populations at risk for the transition between the different GINA steps and proportions of patients transitioning between GINA steps stratified by AIT exposure. Generally, the proportions of patients experiencing a step up in asthma therapy as an indicator of asthma progression were lower in the subgroup of patients exposed to AIT than in patients not exposed to AIT (Table 2, Figure 2).
| AIT exposure | Transition 1-3 | Transition 3-4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| At risk for transition (n) | With transition (n/ %) | Without transition (n/ %) | At risk for transition (n) | With transition (n/ %) | Without transition (n/ %) | ||||||
| All | Yes | 2526 | 630 | 25 | 1896 | 75 | 2022 | 395 | 20 | 1627 | 80 |
| No | 20 891 | 4865 | 23 | 16 026 | 77 | 14 646 | 3921 | 27 | 10 725 | 73 | |
| Adolescents | Yes | 427 | 110 | 26 | 317 | 74 | 337 | 68 | 20 | 269 | 80 |
| No | 1344 | 450 | 33 | 894 | 67 | 965 | 243 | 25 | 722 | 75 | |
| Young adults | Yes | 1792 | 444 | 25 | 1348 | 75 | 1413 | 266 | 19 | 1147 | 81 |
| No | 8752 | 2290 | 26 | 6462 | 74 | 6335 | 1719 | 27 | 4616 | 73 | |
| Older adults/elderly | Yes | 307 | 76 | 25 | 231 | 75 | 272 | 61 | 22 | 211 | 78 |
| No | 10 795 | 2125 | 20 | 8670 | 80 | 7346 | 1959 | 27 | 5387 | 73 | |
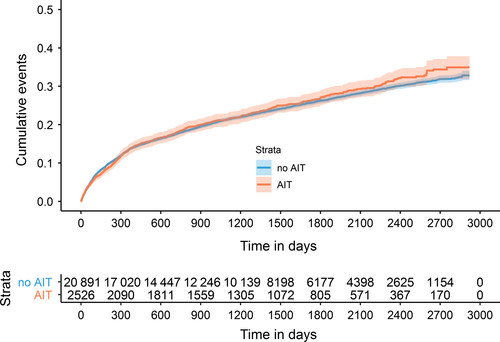
Multivariable Cox regression analyses indicated that AIT exposure was associated with a significantly decreased risk of asthma progression from GINA step 1 to GINA step 3 with a HR of 0.87 (95% CI 0.80-0.95). Male sex and higher age were also related to a lower likelihood of transition to step 3 GINA medications. When stratifying the cumulative event curves (S3-S5) and models by age group the effect of AIT was strongest in adolescents (HR 0.72: 0.58-0.88), followed by young adults (HR 0.89: 0.80-0.98). For the patients 50 years and older, no risk reduction was observed (HR 1.09: 0.87-1.38). In this age group, a high proportion (37.8%) of patients had comorbid chronic obstructive pulmonary disease (COPD).
For the transition from GINA step 3 to step 4, the preventive effect of AIT was more pronounced and resulted in a HR of 0.66 (95% CI 0.60-0.74) for the total cohort. Significant preventive effects of AIT on asthma progression from GINA step 3 to GINA step 4 were observed in all age groups. Effect estimates were similar in the three age groups (Table 3 Figure 3). The number needed to treat (NNT) to prevent 1 patient from moving from GINA step 3 to GINA step 4 after 5 years was 10.9 (95% CI 8.2-16.2).
| Hazard ratio (95% CI) | ||||
|---|---|---|---|---|
| ALL | Adolescents | Young adults | Older adults/elderly | |
| Disease progression (GINA step 1 to step 3) stratified for different age groups | ||||
| AIT | 0.87 (0.80-0.95) | 0.72 (0.58-0.88) | 0.89 (0.80-0.98) | 1.09 (0.87-1.38) |
| Male (Ref. Female) | 0.86 (0.82-0.91) | 0.84 (0.71-1.00) | 0.86 (0.79-0.93) | 0.88 (0.81-0.96) |
| Age (y) | 0.99 (0.99-0.99) | 0.96 (0.91-1.01) | 0.99 (0.99-1.00) | 0.98 (0.98-0.99) |
| Disease progression (GINA step 3 to step 4) stratified for different age groups | ||||
| AIT | 0.66 (0.60-0.74) | 0.76 (0.58-0.99) | 0.63 (0.56-0.72) | 0.72 (0.56-0.94) |
| Male (Ref. Female) | 1.01 (0.95-1.08) | 1.07 (0.85-1.35) | 0.96 (0.88-1.06) | 1.05 (0.96-1.16) |
| Age (y) | 1.00 (1.00-1.00) | 0.99 (0.93-1.06) | 1.01 (1.00-1.01) | 1.00 (0.99-1.00) |
As part of the predefined sensitivity analysis, the cohort was restricted to those with incident asthma and allergic rhinitis (n = 17 332). The results were similar to the ones from the overall cohort indicating robustness of our findings. The HR for the transition from GINA step 1 to GINA step 3 was 0.81 (CI 0.74-0.89) with an HR of 0.68, 0.81, and 1.01 for adolescents, younger adults, and elderly, respectively. For the transition from GINA step 3 to GINA step 4 medication, the HR was 0.70 (CI 0.62-0.78) in the total cohort of patients with comorbid allergic rhinitis and 0.76, 0.65, and 0.82 for the three age groups (Table S2).
4 DISCUSSION
This study is the first to investigate the effect of AIT on asthma progression in a large population-based cohort. Although this study was observational and conclusions have to be drawn with caution, our findings consistently indicate that AIT exposure is associated with a decreased risk of asthma progression, particularly in younger patients. Our study therefore suggests that AIT prevents the progression of incident asthma under routine care conditions.
In the younger age group, the effect for the prevention of progression from GINA step 1 to GINA step 3 was more pronounced. This result corresponds well with the results of a large double-blind placebo-controlled asthma prevention trial on 812 children. Patients participating in this trial had a history of grass pollen allergic rhinoconjunctivitis (no asthma diagnosis at start of the study). Furthermore, this study demonstrated that children treated with a SQ standardized grass pollen tablet had a significantly reduced risk of asthma symptoms and/or the need for asthma medication at the end of the five years trial.8 The NNT to prevent asthma symptoms and asthma medication in the two treatment-free follow-up years was decreased with the age of the children at randomization. The results of this study and the results presented in this paper therefore consistently confirm the current recommendations from AIT guidelines to start AIT as early as possible after disease onset.19
The limited effect in elderly on progression from GINA step 1 to GINA step 3 may be explained by a significant proportion of comorbid COPD. The diagnosis of asthma in primary care is prone to overdiagnosis,20 and asthma medication is also used to treat chronic obstructive pulmonary disease (COPD). About 46% of patients with asthma medication in a Swedish study neither had a diagnosis of asthma nor of COPD. Major indications for off-label prescriptions of asthma medication were cough, airway obstruction, dyspnea, and acute bronchitis.21 In addition to that, disease prevalence might be overestimated and diagnostic and pharmacological treatments for asthma and COPD might be underestimated when relying only on diagnosis codes.22 In our cohort, the proportion of comorbid asthma and COPD was 37% among patients age 50 years or older, and thus larger than the proportion of 20% reported elsewhere.23 AIT intervention is not effective if the progression of symptoms is driven by COPD. Particularly, in elderly patients, it is possible that asthma occurrence was not incident in some patients despite our efforts for a valid definition of incident cases. We do not have any information on morbidity and clinical care before 2005, and it may be possible that some patients without asthma treatment and diagnosis in 2006 and 2007 had asthma manifestation before the observation period of this study. If this was the case, it can be assumed that the remodeling of the pulmonary tissue is more advanced due to more chronic inflammation.24
Only few patients were prescribed GINA step 2 medications, so that it was not possible to investigate the effect of AIT on progression from GINA step 1 to GINA step 2. The low rate of GINA step 2 prescriptions indicates that, when a patient first develops asthma symptoms, SABA is prescribed (GINA 1). If asthma is not fully controlled an immediate step up to a GINA step 3 medication seems to be common, which seems to be only rarely reduced to GINA 2 even if patients have full asthma control. The German guideline “Diagnostics and therapy of patients with asthma” recommends low dose ICS alone and low dose ICS in combination with LABA as interchangeable alternatives for adult asthma patients.25
AIT effectively prevented a step up from GINA step 3 to GINA step 4 therapy with a number needed to treat of 10.9. This indicates that, despite restrictive guidelines for the use of AIT in severe and uncontrolled asthma,19 a preventive effect of AIT even in these patients seems to be possible. There is only one large, double-blind, placebo-controlled trial investigating the treatment effect of a SQ standardized sublingual house dust mite (HDM) SLIT tablet on moderate-to-severe asthma exacerbations in patients having uncontrolled asthma despite the use of asthma medication corresponding to GINA step 2-4. This trial demonstrated a significant reduction in the risk of having a moderate-to-severe asthma exacerbations combined with a good safety profile even in patients with uncontrolled asthma.26 Based on this study, GINA recommended the use of HDM SLIT for asthmatic patients.27
Meta-analyses investigating the clinical efficacy of AIT products revealed a substantial heterogeneity between different studies. Therefore, the World Allergy Organization recommends a product-based evaluation.28 This was not possible in the current study as the sample size for individual products would have become too small. Therefore, the results of this study should be considered supportive of product-specific efficacy documented in randomized clinical trials.
4.1 Study strengths and limitations
An important strength of our study is that it is based on a large and relatively unselected proportion of individuals from the general population in Saxony, Germany. Patients switching their health insurance within the observation period were excluded which may have resulted in a disproportional underrepresentation of young people who moved into or out of the federal state of Saxony within the observation period. Another major strength of our study is its robustness against selection bias due to missing data. Neither a nonresponse nor recall bias is possible due to the study design and the use of routine healthcare data. Confounding by age or sex was prevented by multivariable adjustments in the Cox models. Co-medication, noncompliance or comorbidities, which could enhance or reduce the effectiveness of AIT, were taken into account, ensuring that the results are not attributable to an artificial setting but reflect routine care. Established methods of internal case validation of administrative healthcare data as recommended by the guidelines for good practice secondary data analysis have been utilized.14 However, not all cases are incident cases according to epidemiologic standards. Access to care by an allergy/respiratory care specialist is most likely associated with a higher probability that existing asthma is diagnosed. With routine healthcare data, it is not possible to rule out misclassification of individual patients (patients suffering from the investigated disease, who do not consult a physician or who are falsely diagnosed with a disease). No data on the results of neither spirometry, peak expiratory flow (PEF), nor bronchial challenge testing were available in the healthcare database. The accuracy of the severity classification of a patient depends on the prescription behavior of his treating physician. If present, the resulting misclassification is most likely nondifferential and may therefore not explain the overall findings of our study. Furthermore, the type of allergy and the history of asthma disease treatment before the year 2005 are unknown.
4.2 Implications for clinicians and health policymakers
In accordance with findings from clinical trials which demonstrated effectiveness of AIT to treat AR and asthma symptoms, this study indicates that AIT may modify the course of asthma. Our study supports the assumption that treatment with AIT may prevent the progression from mild to more severe asthma. In addition to that, the results are also suitable to support product-specific efficacy documented in randomized clinical trials. In concordance with existing literature, our data also show stronger benefits in the younger age groups which supports current recommendations to start causal treatment with AIT in the early stage of the disease.
Unfortunately, the present study design lacks an alternative measure of asthma severity as lung function or the number of patient-reported exacerbations. We highly recommend the collection of relevant data on the potential preventive effect on AIT on asthma progression during future clinical trials. Due to the positive effects of AIT on the reduction in disease symptoms and its potential to prevent disease progression, AIT should be used to a greater extent. Furthermore, interventions for effective treatment compliance need to be evaluated and eventually implemented.
FUNDING
This study was independently planned and undertaken by the center of Evidence-based Healthcare, TU Dresden, Germany. The study was financially supported by ALK-Abelló. The views expressed are those of the authors, and not necessarily those of ALK-Abelló.
ACKNOWLEDGMENTS
The authors thank the Compulsory Health Insurance AOK PLUS for their cooperation in data use and technical support. We also thank Luise Mocke for conducting data queries.
CONFLICT OF INTEREST
E. G. Wüstenberg, V. Mücke and N.-S.-Hansen were all employees of ALK-Abelló while conducting this work. E. G. Wüstenberg has stock/stock options in ALK-Abelló. J. Schmitt has received institutional funding for investigator initiated research from ALK-Abelló, during the conduct of the study, and MSD, Novartis, Pfizer, Sanofi outside the submitted work. D. B. Küster and F. Tesch report grants from ALK-Abelló, during the conduct of the study.