The global incidence and prevalence of anaphylaxis in children in the general population: A systematic review
Funding information: YW was supported by the Melbourne International Research Scholarship (MIRS) and Melbourne International Fee Remission Scholarship (MIFRS) from The University of Melbourne and MCRI Top-up Scholarship from Murdoch Children's Research Institute. Noor H.A. Suaini's PhD scholarship was funded by National Health and Medical Research Council of Australia (NHMRC) funded Centre for Food and Allergy Research (CFAR).
Abstract
Background
Despite an increasing number of publications from individual countries and regions, there is still no systematic review of the global epidemiology of anaphylaxis in the general paediatric population.
Methods
We conducted a systematic review, using a protocol registered and published with the international prospective register of systematic reviews (PROSPERO). Results were reported following PRISMA guidelines. The search strategy was designed in Medline (ovid) and modified for Embase (ovid) and PubMed. Papers were screened by two independent reviewers following selection and exclusion criteria. Data extraction and risk of bias assessment were completed by the same two reviewers. Studies in adults only or those that did not report data in children separately were excluded.
Results
A final total of 59 articles were included. Of these, 5 reported cumulative incidence, 39 reported incidence rate and 17 reported prevalence data. The incidence of anaphylaxis in children worldwide varied widely, ranging from 1 to 761 per 100 000 person-years for total anaphylaxis and 1 to 77 per 100 000 person-years for food-induced anaphylaxis. The definition of anaphylaxis from NIAID/FAAN was the most commonly used. Gender and ethnicity were demographic risk factors associated with anaphylaxis in children. Increasing total or food-induced anaphylaxis incidence over time was reported by 19 studies.
Conclusion
The reported incidence of anaphylaxis in children varied widely. Studies in developing countries are underrepresented. To accurately compare anaphylaxis incidence between countries and investigate the time trends, further studies using a standardized definition across different countries are required.
Abbreviations
-
- ASCIA
-
- Australasian Society of Clinical Immunology and Allergy
-
- ED
-
- emergency department
-
- FIA
-
- food-induced anaphylaxis
-
- NIAID/FAAN
-
- National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network
1 INTRODUCTION
Anaphylaxis is a serious and rapid-onset allergic reaction with the involvement of multiple body systems that can lead to death.1 In 2015, Tejedor-Alonso reported an increase in both the number and quality of studies on the epidemiology of anaphylaxis over the past 10 years.2 An increase in hospital presentation rates for anaphylaxis has been reported in Western countries such as Canada,3 Finland, Sweden,4 Australia,5 the United Kingdom6, 7 and the United States8 using data from hospital administrative and national healthcare databases. Emerging studies from Asian regions also show a rising anaphylaxis incidence in South Korea9 and Hong Kong10 using national insurance claims data and hospital admission databases, respectively.
A previous systematic review in 2013 by Panesar et al11 summarized the epidemiology of anaphylaxis in Europe, but there has been no systematic review of anaphylaxis outside of Europe, and several additional studies published since late 2012 were not included in this review. Another systematic review in 2015 by Umasunthar et al12 reported the risk of food-induced anaphylaxis in patients with food allergy, but not in a general population and not including other non-food triggers of anaphylaxis. By only reporting anaphylaxis among patients with a previous diagnosis of food allergy, patients who presented with anaphylaxis as their first food reaction might have been missed. A detailed description of the epidemiology of anaphylaxis worldwide using the latest data could help us better understand and compare the overall disease burden caused by anaphylaxis in different regions. Additionally, some countries are challenged by the fact that it is expensive and time-consuming to estimate food allergy prevalence using the gold standard method of oral food challenge. As previous studies have shown that food was the most frequent trigger of anaphylaxis in children,6, 13 food-related anaphylaxis in children could be a good proxy for food allergy in those countries without convincing food allergy information although it may underestimate food allergy prevalence.10 Finally, by comparing the risk of anaphylaxis in children by subgroups, we could obtain better insights into the aetiology and risk factors of anaphylaxis.
Despite an increasing number of publications from individual countries and regions, there is still no systematic review on the global epidemiology of anaphylaxis at any age. The absence of a systematic review in the paediatric population is problematic because this is where there is a dramatic rise in hospital admission rates for food-induced anaphylaxis.14 Although incidence is the most adequate and frequently used measurement of anaphylaxis in the general population, prevalence is also reported in studies and can be used as a complementary method.2 Hence, we aimed to describe the current epidemiology of anaphylaxis including incidence, prevalence and eliciting triggers among children in the general population worldwide, and to investigate whether there was evidence of changing time trends of anaphylaxis and whether this differed by region.
2 METHODS
The protocol of this systematic review has been registered and published with the international prospective register of systematic reviews (http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016042080). We followed the PRISMA guidelines to report our results.15
2.1 Search strategy
The search strategy was formed following terms and methods from previous studies,12, 16 designed in Ovid MEDLINE and modified for Embase and PubMed. Although Medline and PubMed are essentially the same databases, we included both in our search strategy as PubMed includes e-publications and additional journals that were not included in Medline. Our search strategy was developed in conjunction with a librarian from the Royal Children's Hospital, Melbourne. The exact search strategies for Ovid MEDLINE, Embase and PubMed are outlined in Table S1. The search was conducted in the above databases on 19 September 2018. The reference lists of identified papers were reviewed for additional studies. We included only published literature that has undergone peer review in our systematic review; however, we also reviewed conference abstracts during the screening process to retrieve in-progress publications. Other sources of literature were not considered in our study.
2.2 Study selection
Identified articles were independently screened via titles and abstracts according to the selection and exclusion criteria by two reviewers (YW and NS). Screening results from the two reviewers were compared. Discrepancies were resolved by reading of the full-text and discussions between the two reviewers. Reference lists of identified studies were reviewed for additional studies. Finally, full-text review was undertaken by the same reviewers for all identified articles. Discrepancies were resolved by discussions between the two reviewers, if necessary, a third reviewer (JK) arbitrated.
The screening process was developed and piloted by YW. To pilot the title and abstract screen, the first 50 manuscripts were screened by title and abstract using EndNote X7; then, the full text was retrieved to ensure that the initial screen had not missed any potentially relevant articles. The full-text screening process was tested on 10 manuscripts. These manuscripts were labelled by YW after reading the full text and were checked again to make sure the articles had been assigned to the correct label. Then, the results were discussed with NS to ensure all relevant articles had been included. Both YW and NS followed the same screening process.
Inclusion criteria were as follows:
- Original observational studies, including cross-sectional studies, cohort studies, registries (prospective/retrospective/historical cohort design) and hospital databases;
- Studies reporting the incidence and/or prevalence of anaphylaxis in a general population or studies reporting hospital and/or emergency department (ED) admission rate by using the total population in the catchment area as the denominator;
- Studies were conducted in children. Studies conducted in the whole population (adults and children) were included if they provided a breakdown by age groups, with results for children reported separately.
Exclusion criteria were as follows:
- Systematic and non-systematic reviews, non-research letters, case reports, randomized controlled trials, comments and editorials;
- Studies reporting anaphylaxis rates in patients with specific diseases (including allergic disorders) or under specific condition (eg, anaesthesia, immunization);
- Studies that did not state age group of participants or did not provide incidence or prevalence data in children separately or studies reporting hospital and/or ED admission rate by using the number of patients admitted as the denominator;
- Studies reporting food-dependent exercise-induced anaphylaxis only.
2.3 Risk of bias assessment
Risk of bias of included studies was assessed independently by two reviewers (YW and VM) using 10 questions assessing both external validity and internal validity modified from the risk of bias tool established by Hoy et al.17
2.4 Data extraction
Data from included articles were extracted by two reviewers (YW and VM) using the same extraction form. Any discrepancy was resolved by checking original articles and discussion. We also contacted authors of original studies to request original incidence data in children if these were not provided. Of 7 authors contacted, 2 authors18, 19 replied with the requested information and these data were included in our review. We summarized the reference details, such as study design, study population, data sources, extracted data type, denominator, data collection years, country, age of target population, definition of anaphylaxis and outcome confirmation, reported type of anaphylaxis, risk of bias, numerator of incidence and response rate of included studies. Incidence (including cumulative incidence, incidence rate and admission rate) and prevalence estimates with 95% confidence intervals (CI) of anaphylaxis for each year and/or time period were extracted. In our review, studies that reported hospital and/or ED admission rate of anaphylaxis per 100 000 person-years were considered as a measure of incidence if they used the total population in the catchment area as the denominator. The number of cases and person-time at risk or size of sample population were also extracted if the incidence or prevalence was not provided. Incidence rate ratios were calculated based on extracted data to assess the association between demographic factors (eg, sex and ethnicity) and anaphylaxis where possible. Other risk ratio results (eg, odds ratio) reported by the studies were extracted directly if there was not enough original data to calculate incidence rate ratios.
2.5 Outcomes
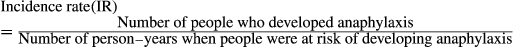
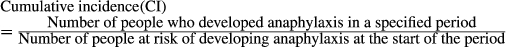
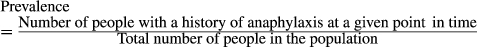
2.6 Data synthesis and analysis
Incidence rate, cumulative incidence or prevalence estimates were extracted where available, or calculated from available data if these estimates were not provided in the paper. A random-effects model using the method of Der Simonian and Laird was applied for the meta-analysis. The heterogeneity was estimated by the Mantel-Haenszel model. We use I2 statistic to examine and quantify between-study heterogeneity. Very high heterogeneity was found in all analyses. The I2 statistic was above 95% for all primary and subgroup analyses. According to the Cochrane handbook, if substantial heterogeneity (I2 > 50%) is found, pooling data using meta-analysis is not recommended.21 Hence, we have not pooled the results. All anaphylaxis definitions were eligible for including studies. We performed sensitivity analyses to examine the effect of using different definitions of total anaphylaxis and food-induced anaphylaxis on our results.
To assess gender differences, we calculated incidence rate ratios (IRR) and 95% confidence intervals (CI) using Poisson regression models. Statistical analyses were undertaken using STATA 15 (StataCorp, College Station, TX).
3 RESULTS
3.1 Study selection
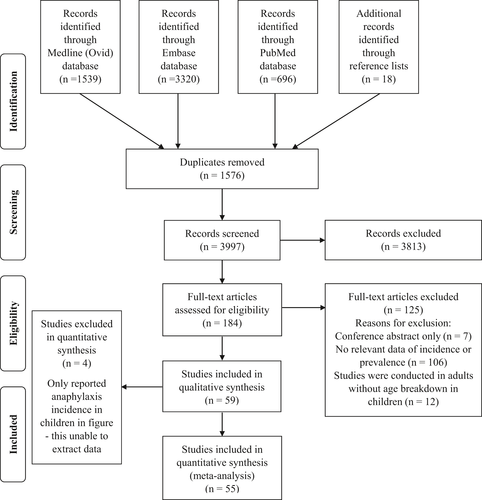
Figure 1 outlines the search results and results of the article screening. We identified 3997 original references from 3 databases after removing duplicates, and additional articles from the reference lists of identified papers. Title and abstract review excluded 3813 articles as they did not meet the inclusion criteria. The remaining 184 articles underwent full-text screening. A final total of 59 articles were included in the qualitative synthesis, and 4 of them were excluded from quantitative analysis as they only reported anaphylaxis incidence in children in their figures. Among the included studies, 42 reported anaphylaxis incidence, 15 reported anaphylaxis prevalence, and 2 studies reported both anaphylaxis incidence and prevalence.
3.2 Study characteristics and risk of bias
The main characteristics of the studies are listed in Table 1. Of the included studies, seventeen only measured and reported total anaphylaxis, nineteen studies only reported food-induced anaphylaxis, and nineteen studies measured total anaphylaxis and also reported anaphylaxis separately by specific triggers. The remaining four studies measured only anaphylaxis induced by hen's egg, peanut, hazelnut and drugs specifically.
| Studies reported incidence | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study ID | Study Design | Study Population | Data source characteristic | Extracted data type (original reported type in the study) | Denominator (population) | Data collection years | Country | Age of study population | Definition of anaphylaxis and outcome confirmation | Reported type of anaphylaxis | Risk of bias | Numerator of incidence |
| Andrew, E. 2018 | Registry | Paediatric patient from emergency medical services (EMS) | EMS is a statewide provider in Victoria | Incidence rate (incidence) | Victorian population in relevant year and the population in 2001 as the standard population | 01/07/2008-30/06/2016 | Australia | 0-16 y | NIAID/FAAN equivalent (patients with a sudden onset of two or more of: respiratory distress, abdominal symptoms, skin/mucosal symptoms or hypotension); patients received emergency treatment with epinephrine | Total anaphylaxis and specific agents triggered anaphylaxis | Low | NA |
| Michelson, K. A. 2018 | Registry | Children from ED visits | Nationwide Emergency Department Sample (NEDS) | Incidence rate (incidence) | National population estimates | 01/01/2008-31/12/2014 | United States | 0-18 y | NA; serious diagnosis | Total anaphylaxis (only) | Low | NA |
| Okubo, Y. 2018 | Registry | Hospitalization patients (inpatient) for anaphylaxis, not include ED patients | National representative Kids’ Inpatient Database (KID), National estimates of hospitalizations was calculated using discharge-level weight variables (DISCWT) | Incidence rate (hospitalization rate) | Not mentioned | 2006, 2009, 2012 | United States | 0-20 y | ICD-9 (995.6x); primary diagnoses for hospitalization discharge records | Only food-induced anaphylaxis | Low | NA |
| Osterlund, J. 2018 | Registry | Children presented to Umea University hospital | Paediatric emergency visits to Umea University hospital in Vasterbotten County, Sweden | Cumulative incidence (hospitalization rate) | Population data from Statistics Sweden | 01/01/2006-31/12/2015 | Sweden | 0-18 y | NIAID/FAAN; ICD-10 diagnostic code | Only food-induced anaphylaxis | Low | NA |
| Speakman, S. 2018 | Registry | Paediatric ED presentations from public hospital for food-induced anaphylaxis | Routine coded discharge data from the Ministry of Health's National Minimum Dataset (NMDS) | Incidence rate (admission rate) | Population data from NZ census | 01/01/2006-31/12/2015 | New Zealand | 0-14 y | ICD-10 (T78.0, T78.2); First two diagnostic fields | Only food-induced anaphylaxis | Low | NA |
| Wang, Y. 2018 | Registry | Hospital and ED admission for anaphylaxis in paediatric population | Clinical Data Analysis and Reporting System coving records for all public hospitals in Hong Kong, coving 78% of total inpatients | Incidence rate (incidence) | Population estimated from Centre for Health Protection, Department of Health, Hong Kong SAR. | 01/07/2001-30/06/2015 | Hong Kong, China. | 0-18 y | ICD-9 (995.0, 995.60-995.69); Diagnosis codes | Total anaphylaxis and specific agents triggered anaphylaxis | Low | Yes, first onset |
| Ruiz Oropeza, A. 2017 | Registry | All patients seen at the ED and the Acute Paediatric Ward (APW), Odense University Hospital (OUH) | OUH served for a mixed rural–urban population of 288 587 persons | Incidence rate (incidence) | Population living in the hospitals catchment area from the StatBank Denmark website | 01/05/2013-30/04/2014 | Denmark | 0-18+ y, breakdown with 0-17 age group | NIAID/FAAN equivalent (WAO/EAACI diagnostic criteria); Review records in the ED | Total anaphylaxis and specific agents triggered anaphylaxis | Low | Yes, first onset |
| Yang, M.S. 2017 | Registry | All patients from Korean National Health Insurance (NHI) claims database | Korean NHI is a mandatory health insurance programme and covers 97.9% of the population in Korea | Incidence rate (incidence) | Number of beneficiaries from National Health Insurance Statistical Yearbook | 01/01/2008-31/12/2014 | South Korea | 0-70+ y breakdown with 0-19 age group | ICD-10 (T78.0, T78.2, T80.5, T88.6); ICD-10 Principal diagnoses | Total anaphylaxis (only) | Low | NA |
| Lee, S. 2017 | Registry | All Olmsted County residents from Rochester Epidemiology Project (REP) | REP captures 98.7% of the population in Olmsted County | Incidence rate (incidence) | Population of Olmsted County, adjusted to US 2010 white population | 01/01/2001-31/12/2010 | United States | 0-60+ y breakdown with 0-9, 10-19 age groups | NIAID/FAAN; Review records | Total anaphylaxis and specific agents triggered anaphylaxis | Low | NA |
| Liu, F.C. 2017 | Registry | Citizens from the National Health Insurance (NHI) research database | NHI is a single-payer programme and covers 99% of the population | Incidence rate (incidence) | Population of Taiwan in 2012 | 01/01/2005-31/12/2012 | Taiwan | 0-80+ y breakdown with 0-9, 10-19 age groups in figures | ICD-9 (995.0, 995.4, 995.6); ICD-9 diagnostic code | Total anaphylaxis and specific agents triggered anaphylaxis | Low | Yes, first onset |
| Xepapadaki, P. 2016 | Cohort Study | Children from EuroPrevall birth cohort | Children recruited from 9 countries across Europe | Cumulative incidence (NA was calculated based on cases and person-time at risk) | Number of children recruited in the birth cohort | 01/10/2005-31/03/2007 | UK, the Netherlands, Germany, Poland, Lithuania, Spain, Italy, Greece | 2 y | Not mentioned | Hen's egg–induced anaphylaxis only | Moderate | Yes, first onset |
| Jeppesen, A. N. 2016 | Registry | Hospitalization for anaphylaxis, not include ED patients | Danish National Patient Registry and Danish Civil Registration System | Incidence rate (hospitalization rate) | Population living in Denmark between 1995 and 2012 (person-time at risk) | 01/01/1995-31/12/2012 | Denmark | 0-75+ y breakdown with 0-14 y | ICD-10 (T78.0, T78.2, T80.5, T88.6, T634F); ICD-10 primary and secondary diagnoses | Total anaphylaxis (only) | Low | Yes, first onset |
| Vetander, M. 2016 | Cohort Study | People from the birth cohort | BAMSE, a population-based, unselected birth cohort of children in 1994-1996 | Incidence rate (incidence) | The number of adolescent in the study population (person-time at risk) | 01/01/2009-31/12/2011 | Sweden | 16 y old | NIAID/FAAN; Questionnaire of survey | Only food-induced anaphylaxis | Moderate | NA |
| Kim, S. H. 2016 | Registry | Inpatient and outpatient for anaphylaxis | Health Insurance Review and Assessment Service (HIRA) | Incidence rate (incidence) | Korean population in 2012 | 01/01/2011-31/12/2013 | South Korea | 0-60+ y, breakdown with 10-19 y | ICD-10 (T78.0, T78.1, T78.2); ICD-10 diagnostic code | Only food-induced anaphylaxis | Low | NA |
| Kivisto, J. E. 2016 | Registry | Hospitalization for anaphylaxis, not include ED patients | National Hospital Discharge Register (NHDR) in Finland and the National Patient Register (NPR) in Sweden | Incidence rate (incidence) | Mid-populations from the Official Statistics of Finland and Statistics Sweden | 01/01/1999-31/12/2011 | Finland, Sweden | 0-19 y | ICD-10 (T78.0, T78.2); ICD-10 diagnostic code | Total anaphylaxis (only) | Low | NA |
| Wright, B. L. 2015 | Registry | North Carolina public school students reported of anaphylaxis | North Carolina Annual School Health Services Report | Incidence rate (incidence) | Total students in all North Carolina public schools | 01/01/2004-31/12/2014 | United States | Students from elementary school to high school | Not mentioned | Total anaphylaxis (only) | High | NA |
| Turner, P. J. 2015 | Registry | Hospital admissions for anaphylaxis, not include ED visits only | Hospital Episodes Statistics database and the Patient Episode Database for Wales | Incidence rate (hospital admission rate) | Population in mid-2001 and mid-2006 from the Office for National Statistics | 01/01/1992-31/12/2012 | United Kingdom | 0-85+ y, breakdown with 0-4, 5-9, 10-14, 15-19 age groups in figures | ICD-9 (995.0, 995.6), ICD-10 (T78.0, T78.2, T88.6); ICD-9 and ICD-10 diagnostic code | Total anaphylaxis and specific agents triggered anaphylaxis | Low | NA |
| Tejedor-Alonso, M. A. 2015 | Registry | Hospital admissions for anaphylaxis | Spanish Minimum Basic Data Set (MBDS) | Incidence rate (incidence) | Population in Spain | 01/01/1998-31/12/2011 | Spain | 0-75+ y, data received for age 0-14 y from author | NIAID/FAAN; ICD-9 diagnostic code | Total anaphylaxis and specific agents triggered anaphylaxis | Low | NA |
| Parekh, D. 2015 | Registry | Hospital admissions for food-induced anaphylaxis, did not state whether include ED patients | Database of the Italian Ministry of Health, Hospital admissions | Incidence rate (incidence) | Italian paediatric population | 01/01/2006-31/12/2011 | Italy | 0-14 y | ICD-9 (did not provide detailed codes); ICD-9 diagnostic code | Only food-induced anaphylaxis | Low | NA |
| Mullins, R. J. 2015 | Registry | Hospital admissions for anaphylaxis (include ED and inpatient) | Australian Institute of Health and Welfare (AIHW) | Incidence rate (hospital admission rate) | National population estimates from Australian Bureau of Statistics | 01/07/1998-30/06/2012 | Australia | 0-30+ y, breakdown with 0-4, 5-14 y | ICD-10 (T78.0, T80.5, T78.2, T88.6, L50, T78.3, J45, J46); ICD-10 diagnostic code | Total anaphylaxis and specific agents triggered anaphylaxis | Low | NA |
| Dyer, A. A. 2015 | Registry | Hospital admissions for food-induced anaphylaxis (include ED and inpatient) | Illinois hospital discharge data (COMPdata) | Incidence rate (admission rate) | Illinois population estimated from the US Census Bureau | 01/01/2008-31/12/2012 | United States | 0-19 y | ICD-9 (995.60-995.69); ICD-9 diagnostic code | Only food-induced anaphylaxis | Low | NA |
| Buka, R. J. 2015 | Registry | ED attendances for anaphylaxis | National Health Service (NHS) organizations in the UK | Incidence rate (incidence) | Population in catchment area | 01/01/2012-31/12/2012 | United Kingdom | 0-90 y, breakdown with 0-15 y | NIAID/FAAN equivalent (WAO diagnostic criteria); Electronic database search for key words | Total anaphylaxis (only) | Moderate | NA |
| Hoyos-Bachiloglu, R. 2014 | Registry | Hospital admissions for anaphylaxis, did not state whether include ED patients | National hospital discharge database | Incidence rate (admission rate) | Chilean population | 01/01/2001-31/12/2010 | Chile | 0-97 y, breakdown with 0-9, 10-19 y | ICD-10 (T78.0, T78.2, T88.6, T78.3 to avoid miscoding as angioedema); ICD-10 diagnostic code | Total anaphylaxis (only) | Low | NA |
| Liew, W. K. 2013 | Registry | Hospital admissions, Department of Children's Emergency, Allergy service outpatient clinics in KK Women's and Children's Hospital | The largest paediatric tertiary referral centre in Singapore | Incidence rate (incidence) | Singapore residents ≤ 18 y | 01/01/2005-131/12/2009 | Singapore | 0-18 y | NIAID/FAAN; Review records | Total anaphylaxis and specific agents triggered anaphylaxis | Low | Yes, first onset |
| Rolla, G. 2013 | Registry | Patients reporting severe allergic reactions in Reference Centre | Reference Center for Severe Allergic Reactions in Piemonte Region | Incidence rate (incidence) | Population in Piemonte during 2010 | 01/01/2010-31/12/2010 | Italy | 0-87 y, breakdown with 0-17 y | NIAID/FAAN (Brighton Collaboration); Patients reported severe allergic reactions | Only food-induced anaphylaxis | Low | NA |
| Vetander, M. 2012 | Registry | ED attendances for anaphylaxis | Three paediatric hospitals in Stockholm County | Incidence rate (incidence) | The population of all children in Stockholm | 01/01/2007-31/12/2007 | Sweden | 0-17 y | NIAID/FAAN equivalent (Modified EAACI task force anaphylaxis paper); ICD-10 diagnostic code | Total anaphylaxis and food-induced anaphylaxis | Low | Yes, first onset |
| Tejedor Alonso, M. A. 2012 | Registry | Anaphylaxis patients from primary care, ED, Inpatient and outpatient clinic | Cases from public health settings in Alcorcon | Incidence rate (incidence) | The population in Alcorcon | 01/01/2004-31/12/2005 | Spain | 0-85+ y, breakdown with 0-4, 5-9, 10-14, 15-19 y | NIAID/FAAN; Retrieve from database by alphanumeric strings searching | Total anaphylaxis and specific agents triggered anaphylaxis | Low | No |
| Canani, R. B. 2012 | Registry | Hospital admissions for anaphylaxis, did not state whether include ED patients | Hospital episode statistics system database | Cumulative incidence (hospital admission rate) | Italian population under 14 y of age | 01/01/2001-31/12/2005 | Italy | 0-14 y | ICD-9 (995.60-995.68); ICD-9 diagnostic code | Only food-induced anaphylaxis | Low | NA |
| Mulla, Z. D. 2011 | Registry | Hospitalization statistics (inpatient) for anaphylaxis, not include ED patients | Texas Department of State Health Services | Incidence rate (hospitalization rate) | Texas resident population estimate | 01/01/2004-31/12/2007 | United States | 0-24 y, breakdown with 0-4, 5-9, 10-14, 15-19 y | ICD-9 (995.61); ICD-9 principal discharge diagnosis or one of the secondary diagnosis | Peanut-induced anaphylaxis only | Low | NA |
| Moro Moro, M. 2011 | Registry | Emergency department attendances for anaphylaxis | Hospital Universitario Fundacion Alcorcon (HUFA) | Cumulative incidence (incidence) | Catch population of HUFA in 2005 | 01/01/2004-31/12/2005 | Spain | 0-69+ y, breakdown with 0-4, 5-9, 10-14, 15-19 y | NIAID/FAAN; Electronic clinical records search using alphanumeric strings | Total anaphylaxis and specific agents triggered anaphylaxis | Moderate | NA |
| Harduar-Morano, L. 2011 | Registry | Emergency department attendances for anaphylaxis | Florida Agency for Health Care Administration | Incidence rate (incidence) | Florida population estimated for 2005 and 2006 | 01/01/2005-31/12/2006 | United States | 0-85+ y, breakdown with 0-4, 5-14 y | NIAID/FAAN; ICD-9 (995.60-995.69, 995.0) diagnostic code | Total anaphylaxis and specific agents triggered anaphylaxis | Low | NA |
| Iribarren, C. 2010 | Registry | Hospital admissions for anaphylaxis (include ED and inpatient) | Kaiser Permanente of Northern California (KPNC) | Cumulative incidence (incidence) | The population in the cohort | 01/01/1996-31/12/2006 | United States | 0-65+ y, breakdown with 0-11, 12-18 age groups in figures | NIAID/FAAN; ICD-9 (995.6, 999.40, 995.0, 708.0, 989.5, 995.1) diagnostic code | Total anaphylaxis and specific agents triggered anaphylaxis | Low | Yes, first onset |
| Ho, M. 2010 | Registry | Hospital admissions for anaphylaxis (include ED and inpatient) | Hospital Authority central computer system CDARS | Incidence rate (admission rate) | Hong Kong population | 01/01/1997-31/12/2007 | Hong Kong, China | 0-17 y | NIAID/FAAN equivalent (Defined by author); ICD-9 diagnostic code | Total anaphylaxis and specific agents triggered anaphylaxis | Low | NA |
| Gonzalez-Perez, A. 2010 | Registry | Individuals enrolled for at least 1 y with a general practitioner | The Health Improvement Network (THIN) database | Incidence rate (incidence) | Each member of the cohort as the time contributed to the study period (person-time at risk) | 01/01/1996-31/12/2005 | United Kingdom | 10-79 y, breakdown with 10-19 y by gender | ICD-9 (995.0, 995.4, 995.6, 693.1, 695.1, 708.0, 708.9, 989.5, 995.1, 995.3); Contacting general practitioner with a completed questionnaire | Total anaphylaxis (only) | Low | Yes, first onset |
| Sheikh, A. 2008 | Registry | Patients had a computer-recorded diagnostic read code for anaphylaxis | QRESEARCH database | Incidence rate (incidence) | Number of patient years of observation standardized by mid-year population estimates for UK | 01/01/2001-31/12/2005 | United Kingdom | 0-90+ y, breakdown with <5, 5-9, 10-14 age groups in figures | NA; Computer-recorded diagnostic read code for anaphylaxis in the electronic health record | Total anaphylaxis (only) | Low | Yes, first onset |
| Lin, R. Y. 2008 | Registry | Hospital admissions for anaphylaxis, did not state whether include ED patients | Statewide Planning and Research Cooperative System (SPARCS) database | Incidence rate (hospitalization rate) | The resident population estimates in New York State from the US Census Bureau | 01/01/1990-31/12/2006 | United States | 0-19 y | ICD-9 (995.6, 999.4, 995.0, 708.0, 995.1, 995.3) and Common Procedural Terminology; ICD-9 diagnostic code | Total anaphylaxis (only) | Low | NA |
| Decker, W. W. 2008 | Registry | Patients from all medical care providers (inpatient and outpatient) | The Rochester Epidemiology Project | Incidence rate (incidence) | Population in Olmsted County, Minnesota, adjusted for US population in 2000 | 01/01/1990-31/12/2000 | United States | 0.8-78.2 y, breakdown with 0-9, 10-19, 0-19 y | NIAID/FAAN equivalent (Defined by author); ICD-9 diagnostic code and hospital adaptation of the ICD-2 codes. | Total anaphylaxis (only) | Low | Yes, first onset |
| Calvani, M. 2008 | Registry | Hospital admissions for anaphylaxis (ED+inpatient) | Sistema Informativo Ospedaliero (SIO) and the Sistema informativo Emergenza Sanitaria (SIES) system | Incidence rate (incidence) | The average children resident for 2 subsequent years (person-time at risk) | 01/01/2000-31/12/2003 | Italy | 0-17 y | ICD-9 (995.0, 995.4, 995.60-995.69, 999.4); ICD-9 diagnostic code | Total anaphylaxis and specific agents triggered anaphylaxis | Low | Yes, first onset |
| Poulos, L. M. 2007 | Registry | Hospitalization for anaphylaxis (inpatient), did not include ED patients | National Hospital Morbidity Database | Incidence rate (hospitalization rate) | Australian population in mid-2001 | 01/07/1993-30/06/2005 | Australia | 0-65+ y, breakdown with 0-4, 5-14 y | ICD-9 (995.0, 995.6, 999.4),ICD-10 (T78.2, 88.6, 78.0, 80.5); ICD-9 and ICD-10 principal diagnosis | Total anaphylaxis (only) | Low | NA |
| Braganza, S. C. 2006 | Registry | Emergency department attendances for anaphylaxis | One Australian paediatric emergency department | Incidence rate (incidence) | Local catchment population in Brisbane, Australia | 01/07/1998-30/06/2001 | Australia | 0.2-14.1 y | ASCIA definitions; ICD-9 diagnostic code | Total anaphylaxis and specific agents triggered anaphylaxis | Moderate | NA |
| Bohlke, K. 2004 | Registry | Patients for anaphylaxis from automated hospital, emergency department and outpatient clinic | Group Health Cooperative | Incidence rate (incidence) | Each member of the cohort as the time enrolled in the HMO during study period (person-time at risk) | 01/03/1991-31/12/1997 | United States | 0-17 y | Defined by author own algorithm; ICD-9 (995.0, 995.6, 999.4, 995.4) diagnostic code | Total anaphylaxis and specific agents triggered anaphylaxis | Low | NA |
| Ruffoni, S. 2015 | Registry | Phone calls and medical visits for anaphylaxis | Liguria Medical emergency service | Incidence rate (NA, was calculated based on cases and person-time at risk) | Population in Liguria in 2013 | 01/01/2013-31/12/2013 | Italy | 0-17 y | NIAID/FAAN equivalent (defined by author); Calls due to suspected anaphylaxis recorded by Liguria Medical Emergency Service | Total anaphylaxis (only) | Low | NA |
| West, S. L. 2007 | Registry | ED visits and hospital admissions for drug-related anaphylaxis | South Carolina Emergency Room Hospital Discharge Database (SCERHDD) | Incidence rate (admission rate) | SC paediatric population from 2000 US census | 01/01/2000-31/12/2002 | United States | 0-18 y | The algorithm defined by author based on ICD-9 and E-codes; ICD-9 (995.0, 995.3, 785.50, 708.0, 708.1, 708.9, 995.1, 478.75, 478.8, 786.05, 786.07, 786.09, 786.1, 458.9, 785.0, 693.0, 995.2) diagnostic code | Drug-related anaphylaxis | Low | NA |
| Gupta, R. 2004 | Registry | Hospital admissions for anaphylaxis, did not state whether include ED patients | Health Survey for England, Scottish Health Survey, International Study of Allergies and Asthma in Childhood (ISAAC) and the European Community Respiratory Health Survey (ECRHS) | Incidence rate (hospital admission rate) | Mid-year population estimated from National Statistics | 01/07/2000-30/06/2001 | United Kingdom | 0-45+ y, breakdown with 0-14 y | ICD-9 (995.0, 999.4), ICD-10 (T78.0, T78.2, T80.5, T88.6); ICD-9 and ICD-10 diagnostic code | Total anaphylaxis (only) | Low | NA |
| Studies reported prevalence | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study ID | Study Design | Study Population | Data source characteristic | Extracted data type (original reported type in the study) | Denominator (population) | Data collection years | Country | Age of study population | Definition of anaphylaxis and outcome confirmation | Reported type of anaphylaxis | Risk of bias | Response Rate (%) |
| McWilliam, V. L. 2018 | Cross-sectional study | Students reported to have experienced food-induced anaphylaxis in the past 12 mo | SchoolNuts study, students from 229 schools from greater metropolitan Melbourne area | Prevalence | 9663 consented students with completed questionnaires | 01/07/2011-31/12/2014 | Australia | 10-14 y | ASCIA definition; Questionnaire | Only food-induced anaphylaxis | Low | 51.1 |
| Jeong, K. 2018 | Registry | Patients designated as “absolutely confirmed” and “confirmed” of anaphylaxis | Korean National Health Insurance (NHI) database covers about 98% of the overall Korean population | Prevalence | Korean population from beneficiaries of health insurance and medical aid in 2010-2014 NHI statistical yearbooks | 01/01/2010-31/12/2014 | South Korea | 0-80+ y, breakdown with 0-2, 3-6, 7-12, 13-17, 18-19 age groups in figures | Defined by authors, using ICD-10 codes and combine with EAI management information; ICD-10 diagnostic codes | Total anaphylaxis (only) | Low | 100.00 |
| Ruiz Oropeza, A. 2017 | Registry | All patients seen at the ED and the Acute Paediatric Ward (APW), Odense University Hospital (OUH) | OUH served for a mixed rural–urban population of 288 587 persons | Prevalence | Population living in the hospitals catchment area from the StatBank Denmark website | 01/05/2013-30/04/2014 | Denmark | 0-18+ y, breakdown with 0-17 age group | NIAID/FAAN equivalent (WAO/EAACI diagnostic criteria); Review records in the ED | Total anaphylaxis and specific agents triggered anaphylaxis | Low | 100.00 |
| Kim, M. 2017 | Cross-sectional study | Children reported to have food-induced anaphylaxis in questionnaire | Survey in 50 000 schoolchildren from 17 cities and provinces. | Prevalence | 29 842 children returned questionnaire with valid responses | 01/09/2015-30/09/2015 | South Korea | 6-16 y | NIAID/FAAN; Questionnaire | Only food-induced anaphylaxis | Low | 59.70 |
| Chan, J. C. K. 2017 | Cohort study | Children were confirmed to have anaphylaxis during oral food challenge | HealthNuts study recruited 5276 12-mo-old children across Melbourne between September 2007 and August 2011 | Prevalence | 5276 children agreed to participate in this study | 01/09/2007-31/08/2015 | Australia | 1 and 4 y | Defined by author, Anaphylaxis was defined as evidence of circulatory or respiratory compromise (eg, hypotension, persistent cough or wheeze); oral food challenge–induced anaphylaxis | Only food-induced anaphylaxis (peanut, raw egg, sesame-induced anaphylaxis) | Low | 74.00 |
| Ontiveros, N. 2016 | Cross-sectional study | Children reported to have food-induced anaphylaxis in questionnaire | Survey in 10 elementary schools (private and public schools) in Culiacan | Prevalence | 1049 children returned questionnaire with valid responses | 01/09/2014-31/08/2015 | Mexico | 5-13 y | NIAID/FAAN (WAO); Questionnaire and parent reported | Only food-induced anaphylaxis | Low | 84.00 |
| Protudjer, J. L. 2016 | Cohort study | Children reported to have anaphylaxis at 16 y | BAMSE project, a birth cohort of 4089 children between 1994 and 1996 | Prevalence | 2572 children with available information from follow-up questionnaires | 01/01/2010-31/12/2012 | Sweden | 16 y | NIAID/FAAN; Questionnaire | Only food-induced anaphylaxis | Low | 62.90 |
| Dereci, S. 2016 | Cross-sectional study | Schoolchildren in the city of Rize | 20 800 school children | Prevalence | 15 783 people returned questionnaire | 01/01/2013-29/02/2013 | Turkey | 6-18 y | NA; Questionnaire | Hazelnut-induced anaphylaxis | Moderate | 75.90 |
| Kilger, M. 2015 | Cross-sectional study | Children reported to have primary anaphylactic reactions | 16 644 children from 86 primary school and kindergartens | Prevalence | 5981 children returned questionnaire and were included in the study | 01/03/2011-30/06/2011 | Germany | Children from primary school and kindergarten, average age was 7 y | NIAID equivalent (EAACI position paper); Questionnaire | Total anaphylaxis (only) | Low | 35.93 |
| Gaspar, A. 2015 | Cross-sectional study | Children diagnosed as anaphylaxis in an allergy outpatient clinic | Allergy outpatient clinic | Prevalence | 3646 children from allergy outpatient clinic | 01/01/2011-31/12/2011 | Portugal | 0-17 y | NIAID/FAAN; Questionnaire | Total anaphylaxis (only) | Moderate | NA |
| Park, M. 2014 | Cross-sectional study | Children reported to have current food allergy with anaphylaxis as the symptoms | 16 982 children were recruited from 301 public childcare centres | Prevalence | 16 749 children returned valid questionnaires | 01/09/2011-31/10/2011 | South Korea | 0-6 y | NIAID/FAAN; Questionnaire | Only food-induced anaphylaxis | Low | 98.63 |
| Gaspar-Marques, J. 2014 | Cross-sectional study | Children reported to have anaphylaxis in questionnaire | Children health questionnaire in the frame of the ENVIRH Project (Environment and Health in Children Day Care Centres) | Prevalence | 1217 children returned questionnaire | 01/01/2014-31/12/2014 | Portugal | 0-6 y | NIAID equivalent (EAACI position paper); Questionnaire | Only food-induced anaphylaxis | Low | 54.60 |
| Lao-araya, M. 2012 | Cross-sectional study | Children from selected kindergartens reported having anaphylaxis in questionnaire | 9 kindergartens selected by multistage random sampling | Prevalence | 452 children returned questionnaire | 01/01/2010-31/12/2010 | Thailand | 3-7 y | NIAID equivalent (anaphylaxis defined as ≥2 organ systems involved); Questionnaire | Only food-induced anaphylaxis | Low | 82.80 |
| Ho, M. H. 2012 | Cross-sectional study | Children reported having anaphylaxis to foods | Child Health Survey (CHS) | Prevalence | 7393 land-based non-institutionalized children | 01/09/2005-31/08/2006 | Hong Kong, China | 0-14 y | NA; Reported anaphylaxis as symptom | Only food-induced anaphylaxis | Moderate | 73.30 |
| Sheikh, A. 2008 | Registry | Patients had a computer-recorded diagnostic read code for anaphylaxis | QRESEARCH database | Prevalence | Number of patients observed standardized by mid-year population estimates for UK | 01/01/2001-31/12/2005 | United Kingdom | 0-90+ y, breakdown with <5, 5-9, 10-14 age groups in figures | NA; Computer-recorded diagnostic read code for anaphylaxis in the electronic health record | Total anaphylaxis (only) | Low | 100.00 |
| Touraine, F. 2002 | Cross-sectional study | Children were reported as anaphylactic shock | 1086 questionnaires were distributed to 4 primary schools, 2 colleges and public high school, 1 private college and high school in Limoges | Prevalence | 748 returned questionnaire | 2000-2001 school year | France | 5-17 y | NA; Questionnaire | Only food-induced anaphylaxis | High | 69.00 |
| Boros, C. A. 2000 | Cross-sectional study | Children from selected schools for the project | Children from preschools, schools and childcare centres | Prevalence | 4173 South Australian children | 01/01/2000-31/12/2000 | Australia | 3-17 y | Defined by author: Anaphylaxis was defined as rapid onset with symptoms of airway tract obstruction, skin rash, gastrointestinal involvement and cardiovascular involvement; Questionnaire and parent reported | Total anaphylaxis and specific agents triggered anaphylaxis | Moderate | 60.00 |
- NA, refers to relevant information was not available in including studies.
The risk of bias assessment results are listed in Table S2. High risk of bias was found in 2 (3.4%) studies, moderate risk in 8 (13.6%) and low risk of bias in 49 (83.1%) studies.
The majority of studies (42/44) reporting anaphylaxis incidence were conducted based on registry databases, and most (34/42) were from hospital and/or ED admission databases. Only two studies were conducted in population-based birth cohorts. Only a minority of studies (12/44) stated that only first-onset anaphylaxis episodes were used to calculate incidence. Most of the studies (12/17) that reported anaphylaxis prevalence were cross-sectional studies, and more than half (8/12) were conducted in a school environment. Among these studies, nine had a participation rate above 70%, six between 50% and 70%, and two below 50% or unknown.
The age of the study population varied in included studies, from 0 to 90 years of age, although only data from children were included in this study. The data collection period of studies was also different, from 1995 to 2016.
3.3 Anaphylaxis in children by trigger
The incidence of anaphylaxis in children by trigger is shown in Figure 2A. Of 44 studies, 29 reported total anaphylaxis with a wider incidence range of 1-761 per 100 000 person-years, 17 reported incidence of food-induced anaphylaxis, ranging from 1 to 77 per 100 000 person-years, and 6 reported incidence of drug-induced anaphylaxis, with range between 0.3 and 10.6 per 100 000 person-years. The incidence of anaphylaxis triggered by other agents such as insect, anaesthesia, venom, serum and individual foods (peanut, nuts, fruit, milk, seafood and egg) are also depicted in the figure. Anaphylaxis induced by individual food triggers had higher incidence (0.1-9.7 per 100 000 person-years) compared with insect, anaesthesia and serum triggers.

The prevalence of anaphylaxis by trigger is listed in Figure 2B. Prevalence estimates for total anaphylaxis ranged from 0.04% to 1.8% (four studies). The prevalence of food-induced anaphylaxis ranged from 0.3% to 1.2% (ten studies).
3.4 Total and food-induced anaphylaxis in children by definition of anaphylaxis
The incidence of total and food-induced anaphylaxis in children stratified by definition of anaphylaxis is shown in Figure 2C,D. The definition from NIAID/FAAN was the most widely applied definition in included studies (n = 17) although 17 studies relied on ICD-9/ICD-10 codes alone to define anaphylaxis.
3.5 Total and food-induced anaphylaxis in children by region
Figure 2E,F summarize the incidence of total anaphylaxis and food-induced anaphylaxis by region. Most studies were from Europe (n = 16), followed by America (n = 10) and Oceania (n = 5). Additionally, 5 studies from Asia reported a lower incidence of total and food-induced anaphylaxis in children. The range for total anaphylaxis incidence in studies from Europe was between 2.3 and 761 per 100 000 person-years, and in America was between 0.8 and 70 per 100 000 person-years. Incidence of food-induced anaphylaxis was higher in studies from Europe (from 1.4 to 76.7 per 100 000 person-years) compared with studies from other regions.
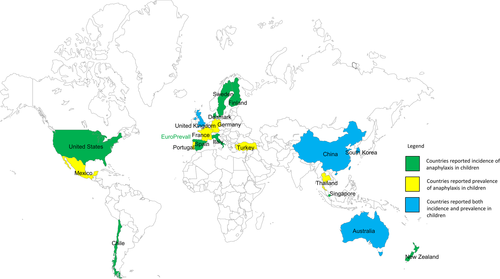
Figure 3 shows countries with available data on anaphylaxis incidence or prevalence. The United States, Chile, Spain, Finland, Italy, Singapore, New Zealand and countries from EuroPrevall study (UK, the Netherlands, Germany, Poland, Lithuania, Spain, Italy and Greece) reported anaphylaxis incidence in children, while Mexico, Portugal, Turkey, Germany and Thailand reported anaphylaxis in children using prevalence. The United Kingdom, China (Hong Kong SR), Denmark, Sweden, South Korea and Australia have provided both incidence and prevalence information of anaphylaxis in children.
3.6 Demographic factors associated with anaphylaxis (male gender and ethnicity)
Eleven studies reported incidence rate of anaphylaxis by gender as shown in Table S4. Boys had a higher incidence rate of total anaphylaxis than girls based on results from studies by Wang et al and Bohlke et al (P < 0.001, IRR = 1.31, 95% CI: 1.10-1.55). Boys under 10 years of age had higher anaphylaxis incidence than girls. However, as the children grew older (≥10 years), girls tended to have a comparable or even higher rate of anaphylaxis compared with boys.
A study published recently explored the association between anaphylaxis and ethnicity and found South Asian children living in the United Kingdom were more likely to have anaphylaxis compared with white children living in the United Kingdom (OR: 2.37, 95% CI 1.83-2.90).22 Another study in the United States reported the rates of hospital presentation due to food-induced anaphylaxis were highest in Asian children, followed by black children, white children and then Hispanic children.23 The study in New Zealand also found paediatric food–induced anaphylaxis hospital presentations were highest in Asian children, followed by Pacific people.24
3.7 Time trends of anaphylaxis in included studies
Increasing anaphylaxis incidence over time was reported by studies from the United States,25, 26 Spain,18 Australia,5, 27, 28 Denmark,29 South Korea,9 Hong Kong,10 Finland and Sweden4 for total anaphylaxis, and from United Kingdom,6 Spain,18 Italy,19, 30, 31 Australia,5, 27 Hong Kong,10 New Zealand,24 Sweden32 and United States23, 33, 34 for food-induced anaphylaxis. The incidence of total anaphylaxis and the incidence of food-induced anaphylaxis for available years from 1990 to 2013 were extracted from these studies and are shown in Figures S1 and S2.
Studies by Bohlke et al, Lee et al (not shown in the figure as data was not available) and Hoyos-Bachiloglu et al (not shown in the figure as data were not available) reported no significant increase in total anaphylaxis incidence during 1991-1997 in western Washington State, USA,35 during 2001-2010 in Olmsted County, USA,8 and during 2001-2010 in Chile.36
4 DISCUSSION
This systematic review identified 59 studies measuring incidence and prevalence of anaphylaxis from more than 20 countries located in four continents. Seventeen studies reported incidence or prevalence of total anaphylaxis and did not distinguish by sub-type of anaphylaxis triggers. In childhood, male gender was associated with a higher incidence of anaphylaxis. Increasing trends of total anaphylaxis or food-induced anaphylaxis incidence between 1990 and 2013 were reported by 18 studies, while three studies did not find an increase in anaphylaxis incidence over a similar time period.
Our review is the first study to investigate worldwide anaphylaxis data in children in the general population up to 2018 using a robust systematic review methodology. We stratified the incidence and prevalence of anaphylaxis in children by triggers, definitions, regions and time trends. However, we are unable to provide a single overall estimate of anaphylaxis for children worldwide according to the Cochrane Handbook because the heterogeneity in our results was high (I2 > 95%).21 It is difficult to compare between studies due to differences in study design, anaphylaxis definitions, and the regions and years in which the study was conducted. To explore potential sources of the observed heterogeneity, we conducted subgroup analyses, for example, by anaphylaxis definition, region and study design; however, substantial heterogeneity remained (I2 > 90% in all subgroups). Even among studies that used the same definitions of anaphylaxis, misclassification is possible and could affect the estimates. Since most of the studies reporting anaphylaxis incidence were registry or hospital admission databases, the characteristics of the databases will affect the estimates. Given that food allergy prevalence and triggers vary by region, it is likely that anaphylaxis prevalence and triggers would vary by region too.37, 38 An additional factor that could affect incidence of anaphylaxis, which was not measured in most of the included studies, is the presence of coexisting conditions such as asthma. A limitation of the publications identified by our systematic review was the use of hospital databases, which rely on both optimal recognition by clinicians and optimal coding by database staff which may be challenging in a busy hospital setting. When hospital presentation of anaphylaxis is used as the proxy of anaphylaxis incidence, it would be more reliable if patient signs and symptoms were also captured so that cases could be confirmed and validated using standardized objective criteria. Another limitation was that some of the studies which asserted to report incidence of anaphylaxis did not state whether the cases in the numerator were new cases or repeat admissions. Overestimation of anaphylaxis incidence (new cases only) in these studies was possible because some children might be counted more than once. It is important to differentiate whether the reported cases were first onset or not. Including the first onset of anaphylaxis estimates the incidence of anaphylaxis, that is, new cases of anaphylaxis, whereas including all occurrences of anaphylaxis without differentiating whether the anaphylactic reaction was first or subsequent reactions would estimate the rate. Interpreting the rate as anaphylaxis incidence, and counting some anaphylaxis patients more than once, may overestimate the true incidence. We conducted a subgroup analysis including only studies that specified that they used first-onset anaphylaxis as the numerator; however, few studies made this distinction (n = 9) and there was still substantial heterogeneity between studies (I2 = 100%, P < 0.001). Additionally, among those studies reporting hospital presentations of anaphylaxis, different patient groups were included, such as outpatients, hospitalized patients and/or ED patients. Furthermore, time trends of anaphylaxis incidence in our review could only be shown in individual studies rather than by combining results because of the limited number of studies that provided the risk data for each year and also the high heterogeneity of these studies.
The range of total anaphylaxis incidence in our review (from 1 to 761 per 100 000 person-years) was wider than the range reported (range 1.5-32 per 100 000 person-years) by Panesar in their systematic review of European studies in 2013.11 The highest incidence of total anaphylaxis (761 per 100 000 person-years) was reported in Sweden by Vetander et al39 using parent-reported questionnaire in a population-based birth cohort. There could be overestimation in the study by Vetander et al due to the reliance on parent-report, although they defined anaphylaxis according to symptoms recorded in the questionnaire using NIAID/FAAN criteria. Their study was not included in any previous systematic reviews because it was only published in 2016. Their study was also the only one among included studies that reported anaphylaxis estimates based on parent-reported survey. We did a sensitivity analysis removing this study to investigate the heterogeneity in the remaining subset and still observed a high heterogeneity (I2 > 95%, P < 0.001) by using random-effects model.
Panesar et al11 performed a meta-analysis of anaphylaxis prevalence, reporting a pooled anaphylaxis prevalence of 0.3%. However, there was significant heterogeneity (I2 = 94.6%, P < 0.0001) in their meta-analysis, and it was based on only three studies. We chose not to present a pooled estimate due to the limited number of comparable studies, referring to studies undertaken applying the same definition within the same trigger from same region.
In our review, we found evidence of an association between gender and ethnicity and risk of anaphylaxis, consistent with previous individual studies.40 Relationships between ethnicity and risk of food allergy have also been reported by previous studies. Children with Asian ethnicity who were born in Australia were reported to have higher risk of eczema, egg allergy and peanut allergy compared with children of other ethnicities.41, 42 Similar to food allergy, our systematic review also identified a higher risk of anaphylaxis in children with Asian ethnicity compared with other ethnicity from 3 studies.22-24
The incidence and prevalence reported in this systematic review provide us with improved clarity about anaphylaxis, including its frequency in several specific subgroups by trigger, definition and region. However, high heterogeneity (I2 > 90%) limits our interpretation of an overall incidence and prevalence. Studies in developing countries are also underrepresented. Future studies across different countries using a consistent, accurate definition of anaphylaxis and using the correct epidemiological method to define incidence and prevalence would help to identify any true difference between countries, and help to provide an overall estimate of prevalence.
ACKNOWLEDGMENTS
We would like to acknowledge Miguel A. Tejedor-Alonso and Roberto Berni Canani for replying and sharing their original data with us. We acknowledge Poh Chua from the Royal Children's Hospital for her help with developing and revising the search strategy for this review. The authors also would like to acknowledge Jing Wang for her suggestions on this study.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
YW, JK and KA conceived this review. The review was undertaken by YW, NS and VM. JK, KA and RP helped YW with the data analysis and interpretation of data. YW led the drafting of the manuscript. All authors critically commented on the drafts of manuscript and finally approve this version to be published.




