A new framework for the interpretation of IgE sensitization tests
Abstract
IgE sensitization tests, such as skin prick testing and serum-specific IgE, have been used to diagnose IgE-mediated clinical allergy for many years. Their prime drawback is that they detect sensitization which is only loosely related to clinical allergy. Many patients therefore require provocation tests to make a definitive diagnosis; these are often expensive and potentially associated with severe reactions. The likelihood of clinical allergy can be semi-quantified from an IgE sensitization test results. This relationship varies though according to the patients’ age, ethnicity, nature of the putative allergic reaction and coexisting clinical diseases such as eczema. The likelihood of clinical allergy can be more precisely estimated from an IgE sensitization test result, by taking into account the patient's presenting features (pretest probability). The presence of each of these patient-specific factors may mean that a patient is more or less likely to have clinical allergy with a given test result (post-test probability). We present two approaches to include pretest probabilities in the interpretation of results. These approaches are currently limited by a lack of data to allow us to derive pretest probabilities for diverse setting, regions and allergens. Also, cofactors, such as exercise, may be necessary for exposure to an allergen to result in an allergic reaction in specific IgE-positive patients. The diagnosis of IgE-mediated allergy is now being aided by the introduction of allergen component testing which may identify clinically relevant sensitization. Other approaches are in development with basophil activation testing being closest to clinical application.
Allergies are the most frequent chronic diseases in Europe today, affecting the daily lives of more than 60 million people 1. Typical manifestations are asthma, rhinitis, eczema, food allergy and anaphylaxis. Many nonallergic disorders present with symptoms that are similar to allergic diseases making it important to have good tests for allergy. There are also many situations where a patient may be exposed to more than one putative allergen, so tests to identify the triggering allergen are required. Allergic diseases can be divided into IgE-mediated and non-IgE-mediated allergy 2. IgE-mediated allergy, with rapid onset of symptoms after exposure to the allergen, is associated with specific IgE to the relevant allergen. This report will focus on IgE-mediated allergy as we have a number of routine clinical tests that can detect the presence of specific IgE. Skin prick testing (SPT) can provide immediate information about the presence of IgE sensitization to specific allergens in clinic 3. Serum-specific IgE testing requires blood to be sent to a laboratory and can be tested to allergen extracts or individual allergen components 4. Atopy patch testing has been developed over the last decade to understand the relationship between IgE sensitization and the cell-mediated response that characterizes atopic eczema but is not widely used 5. Other approaches to testing for IgE-mediated allergy, such as basophil activation test (BAT), have been used in the research setting, and their application for clinical use is in development 6. The drawback of IgE sensitization tests is that they report the presence of specific IgE, not the presence of clinical allergy (Fig. 1). However, when used by clinicians trained in their interpretation, they are useful to support a diagnosis of clinical allergy and identify the likely triggering allergens within the context of a full clinical history.
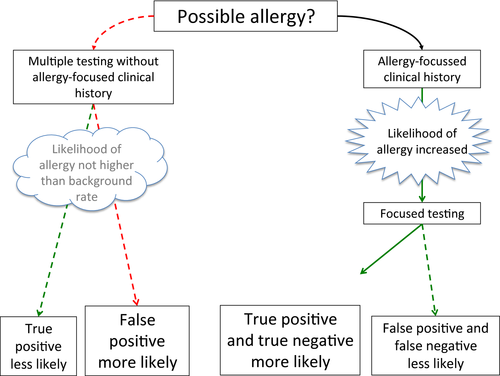
There have been considerable advances in allergy over the last decade. Firstly, we now have a better understanding of the pathophysiology of allergic disease and immunological impact of therapies. Secondly, technological advances have allowed us to detect individual allergen components. Finally, with better data sets, we are beginning to understand the relationship between specific presentations, IgE sensitization test results and clinical allergy. For these reasons, the European Academy of Allergy and Clinical Immunology (EAACI) formed a task force to reassess how we employ IgE sensitization tests. The task force involved a wide range of clinicians, scientists and patient groups. The aim of the task force was to refine our conceptual approach to interpreting IgE test results by integrating information from the patient history with the test results and to consider additional tests to improve our ability to accurately diagnose clinical allergy to allow us to improve patient care. Although many of the examples used are from food allergy, given that we have much better evidence in this area, the approach is a generic one that should be applicable to any allergic disease. This position paper builds on the activities of other EAACI diagnostic task forces including those focused on children 7, rhinitis 8 and food allergy 9, 10. While this position paper is primarily aimed at allergists, the task force is planning to disseminate the concepts more widely using focused materials aimed at different audiences including specialty physicians, primary care doctors and patients.
The relevance of IgE sensitization to clinical allergy
Pathophysiology of IgE-mediated allergy
An understanding of the pathophysiology of IgE-mediated allergy is useful for considering how to best interpret allergy test results. Mast cell degranulation commences with cross-linking of IgE receptors by allergen, functional autoantibodies against IgE or the high-affinity IgE receptor (FcεRI), or FcεRI-independent such as opiates or C5a complement. This results in calcium-dependent series of intracellular signalling events causing a piecemeal or anaphylactic degranulation of tissue mast cells and circulating basophils. Preformed mediators, such as histamine and the generation of vasoactive eicosanoids, induce an acute cellular inflammatory response. Long-term effects on inflammation are influenced by local tissue factors and structures.
The immediate effects of histamine release depend on the organ affected (Box 1). They include itching, redness, wheals and angioedema of the skin; wheezing of the lung; rhinorrhoea, nasal blockage and sneezing; lacrimation and itching of the eye; vomiting, diarrhoea and abdominal pain of the bowel and faintness; and tachycardia of the circulation, culminating in collapse if the reaction is severe. Understanding how organs are affected differently by the same downstream stimulus–secretion events in the mast cell and basophil resulting in allergic disease and how IgE-induced hypersensitivity may be abrogated by the development of immunological tolerance is central to understanding allergy.
Box 1. Examples of organ-specific effects of immediate hypersensitivity reactions
Skin
The relevance of specific IgE sensitization is often unclear in patients with atopic dermatitis without a history of associated urticaria closely related to allergen exposure as patients often have multiple sensitizations to inhalant and food allergens. Delayed eczematous reactions up to 24 h after exposure to relevant allergens, such as house dust mite, grass pollen or cows’ milk, are thought to be due to T-cell activation through allergen binding to specific IgE on FcεRI of inflammatory dendritic epidermal cells (IDEC) or Langerhans’ cells by antigen focusing. Atopy patch testing may be used, but this has low sensitivity although specificity is reasonable.
Although some acute urticaria is caused by immediate hypersensitivity reactions to foods or drugs, the majority of cases appear to relate to acute viral infection (usually of the upper respiratory tract) or remain idiopathic. Chronic urticaria, by contrast, is almost never the result of specific IgE sensitization even though activation of FcεRI appears to be a key pathogenic event in some patients with functional autoantibodies directed against FcεRIα or cytophilic IgE. Immunological contact urticaria due to environmental exposures or body fluids, for example, is probably common but underreported in the community and must be distinguished from nonimmunological urticaria that appears to be mast cell and IgE independent.
Airways
Acute symptoms of allergic asthma and rhinitis develop after exposure to a number of triggers. One trigger is inhalant allergens to which the patient has developed IgE sensitization. In the nose, this leads to immediate itching, sneezing, rhinorrhoea and congestion, whereas the lower airways respond with narrowing, oedema and mucus secretion. Chronic changes result from the influx of inflammatory cells, including eosinophils and damage to epithelial cells. Upper and lower airway symptoms and/or exacerbations can also occur with other triggers, for example viral respiratory tract infections, cold air or pollution.
Digestive tract
The oral allergy syndrome is characterized by immediate oral mucosal symptoms of itching, burning and swelling of the mouth after eating fresh fruits, nuts or vegetables containing pan-allergens (profilins, pathogenesis-related protein-10) that are also present in tree or grass pollens to which patients with allergic rhinitis/conjunctivitis have become sensitized. Skin prick testing and serum-specific IgE assays may be negative due to loss of allergenicity in standardized reagents. However, prick-to-prick testing with fresh fruits or vegetables will be positive.
Immediate hypersensitivity (food allergy) reactions in the digestive tract present acutely with vomiting, diarrhoea and/or abdominal pain. A chronic pattern of inflammation analogous to allergen-induced atopic eczema exacerbations with eosinophilic inflammation has been linked with IgE sensitization, especially in eosinophilic oesophagitis, but defining sensitizations by skin prick and specific IgE testing is inconsistently predictive of a response to specific dietary restrictions. Atopy patch testing is often negative.
Systemic reactions
Anaphylaxis is an acute severe systemic reaction involving the skin, airways, bowel and circulation that often presents with syncope or difficulty with breathing. It is usually caused by an allergy to a food, drug or venom. Similar presentations can result from nonallergic mechanisms, with iodinated contrast material for example.
Development of tolerance in IgE-mediated allergy
The development of natural tolerance is complex and poorly understood, but the events involved in specific allergen immunotherapy have been closely studied 11. They involve the induction of peripheral T-cell tolerance by the promotion of regulatory Treg and Tr1 cells. These directly or indirectly suppress pro-inflammatory cells including eosinophils, mast cells and basophils by the formation of specific IgG4, followed by reduction in specific IgE.
Diagnosis of IgE sensitization in clinical practice
The spectrum of clinical disease linked with IgE sensitization includes anaphylaxis, asthma, atopic dermatitis, conjunctivitis, drug reactions, eosinophilic enteritis, food allergies, oral allergy syndrome, rhinitis, urticaria and venom allergy (examples in Box 1). The primary tool for assessing immediate hypersensitivity reactions is the history. Investigations are, to some extent, disease specific (Table 1). They include SPT with whole allergen extracts, prick-to-prick testing with fresh fruits/vegetables, atopy patch testing and serum-specific IgE for whole allergen extracts and component-resolved diagnostic (CRD) testing. Challenges may be undertaken in an appropriate clinical context.
| Disease | Skin prick tests | Intradermal tests | Atopy patch test (ATP) | Serum-specific IgE | Challenge |
|---|---|---|---|---|---|
| Acute urticaria | (+) | − | − | (+) | (+) |
| Histaminergic angioedema (without wheals) | (+) | − | − | (+) | (+) |
| Atopic dermatitis | + | − | + | + | + |
| IgE-mediated food allergies | + | − | − | + | + |
| Oral allergy syndrome | +a | − | − | + | (+) |
| Eosinophilic oesophagitis | + | − | (+) | + | − |
| Asthma | + | − | − | + | (+) |
| Rhinitis | + | − | − | + | (+) |
| Conjunctivitis | + | − | − | + | (+) |
| Anaphylaxis | + | (+) | − | + | (+) |
| Venom allergy | + | + | − | + | (+) |
| Drug reactions | +/− | +/− | − | +/− | (+) |
- Symbols: +: indicated; (+) consider when symptoms are likely to have been precipitated by an allergen or other tests have not provided a clear allergic diagnosis; −: not indicated.
- a Skin prick tests using fresh fruits or vegetables require a prick-to-prick technique.
Careful history taking is essential for targeted specific IgE testing. It is recommended that SPT for anaphylaxis, especially to foods and drugs, should be performed with facilities available for resuscitation because severe systemic allergic reactions and rare deaths have occurred 12. Multifactorial anaphylaxis due to cofactors may involve challenge with the suspected triggers concurrently if allergen testing is negative. Testing for specific IgE to omega-5 gliadin in cereal grain may be relevant to patients with a history of exercise-induced anaphylaxis or tropomyosin in patients with house dust mite sensitization.
The relevance of patient and environmental factors in interpreting IgE sensitization test results
Personal, family and medical features of a patient's history are the paramount considerations in determining when to test for allergic sensitization because these factors contribute significantly to the ability of the clinician to correctly interpret these tests.
Personal factors
It is well known from both clinic- and population-based studies that the prevalence of the disease in a population strongly influences the usefulness of the disease-specific tests. Screening of nonsymptomatic individuals with specific IgE tests has a low positive predictive value, in the range of 50%. In clinic populations, where the likelihood of the disease being present is higher, the positive predictive value of SPT or specific IgE testing may be higher than 85% 13. In contrast, in both unselected and referred populations, the tests have high negative predictive value and specificity.
Individuals from different ethnic backgrounds may have different tendencies to develop or demonstrate positive skin test and specific IgE results. This has been shown recently in the screening data from the LEAP study where higher rates of specific IgE were found in black individuals than in other ethnic groups 14. Also specific IgE levels for peanut are higher in African American subjects than in non-African American subjects in US studies 15.
It has been extensively documented that a subject's age has a considerable impact on the interpretation of tests; for example, a difference of a year may double the likelihood of clinical allergy in preschool children 16.
Other patient factors include the presence or absence of associated diseases such as atopic dermatitis/eczema. There is a strong association between eczema severity and food allergy 14, 17; this may relate to underlying filaggrin mutations 18.
Environmental factors
Geographical location of patients may make considerable differences. Important factors are presumably temperature, humidity and pollen exposure. In Australia, rates of peanut allergy are higher in the south than in the north 19 and specific IgE testing for peanut shows different patterns from north and western to southern Europe 20, 21; Ara h 2 is the dominant allergen in the north and west, but being of less significance in southern Europe 22. Similarly, the EPAAC study showed wide variations in peanut-specific IgE levels across European countries in a preschool population with moderately severe eczema 23.
Other factors
Potential cofactors also need to be considered when interpreting the clinical history in relation to IgE test results. Exercise in the few hours after eating a specific food to which the individual is sensitized might be necessary in order to trigger a food-related reaction 24. Other cofactors include physical and emotional stress, infections and medications. Coexisting morbidities such as mastocytosis in beekeepers can definitely modify the pattern of reaction and may influence IgE testing interpretation 25.
Finally, IgE testing may also be used for the follow-up of an allergic disease. Changes in results with sequential testing in individual patients can indicate whether food allergies are likely to persist or resolve 26, 27. They can be used to decide whether repeat food challenge is needed for assessing whether a child has outgrown a specific food 28.
Maximizing the available diagnostic information by treating SPT and specific IgE results as continuous measures
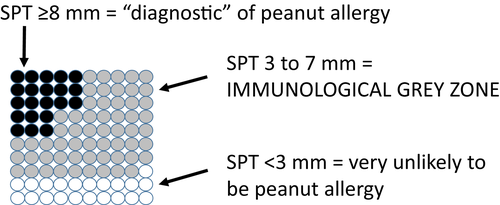
Traditionally, SPT and specific IgE results have been dichotomized into positive or negative according to an arbitrary cut-off value. Typically, this was a 3-mm wheal diameter for SPT and 0.35 kU/l for specific IgE. This has given rise to the use of sensitivity (proportion of true positives that are correctly identified) and specificity (proportion of true negatives that are correctly identified) as a way of describing the utility of the test 10. There is a trade-off in terms of how useful tests are and those with good sensitivity usually have poor specificity and vice versa. Sensitivity and specificity provide a population perspective, while this may be useful for public health screening tests, and it is less helpful for interpreting test results for individual patients. So a patient with a SPT ≥3 mm or specific IgE of ≥0.35 kU/l to a food only has an approximate 50% chance of being allergic; meanwhile, another patient with a 0 mm SPT or <0.35 specific IgE result still has approximately a 10% chance of being allergic to that food.
The introduction of 95% positive predictive values 29 has helped clinicians to provide more clinically relevant recommendations from the test results. An example would be 15 kU/l for peanut, children with a result at or above this value have a 95% chance of having peanut allergy 30, and there are many other examples in the literature 3. This approach provides a patient rather than a population perspective. However, most patients have results that fall into the grey area below the 95% positive predictive value 29 (Fig. 2) and the confidence intervals associated with these positive predictive values are wide, so they are not as precise as they may seem to be 31 and they do not take into account other factors such as presenting history.
In a further refinement of the interpretation of allergy test results, the relationship between the result and the probability of clinical allergy has been modelled using logistic regression. This approach gives curves where the probability of clinical allergy can be estimated for different test results 30, 32. While this appears to maximize the information available to guide clinical management, these probability curves only relate to the relationship between test result and clinical allergy in an ‘average’ patient. Patients seen in an allergy clinic are very heterogeneous; for example, some may have a nonallergic underlying disorder and others may be sensitized but not clinically allergic.
Many groups have looked to see whether IgE test results can provide information about the likely severity of future allergic reactions. In general, no relationship has been found 32 although one study found a weak relationship with more severe reactions being associated with slightly larger SPT results 33.
Utilizing allergen components to improve our diagnostic approach
Traditional diagnostic tests in allergy are directed towards crude extracts which are made up of a number of different allergenic molecules. These different molecules can be individually assayed using CRD 34. Component-resolved diagnostics can discriminate between binding to potent, more relevant allergens and cross-reactive, clinically less relevant allergens. In allergen extracts, some allergens are present in a suboptimal concentration. Allergen extracts contain, in addition to the allergens, many other nonallergenic components that might hamper the analysis of IgE binding to the relevant allergens; the CRD approach avoids this issue.
Component-resolved diagnostics has introduced improvements in allergy diagnostics, especially in the areas of respiratory 35 and food allergies. For instance, more than 10 different allergenic peanut proteins are described: Ara h 1 to Ara h 13. Within this group, it seems that IgE binding to Ara h 2 and Ara h 6 allergens, belonging to the conglutin protein family, is the most relevant to clinical peanut allergy 36. The Ara h 8 allergen in peanut belongs to the PR-10 family, like Bet v 1 the major allergen in birch pollen. This can cause false-positive test results when patients with birch pollen allergy are tested with whole peanut extract. Additionally, some of the allergens in food can predict severe type of reactions. This has been shown for apple where Mal d 1-specific IgE is associated with mild, local allergic reactions, while Mal d 3-specific IgE is associated with more severe, systemic reactions. Also in hazelnut allergy, Cor a 1 is associated with mild while Cor a 9 and Cor a 14 are associated with more severe allergic reactions 37, 38.
Component-resolved diagnostics can also be used to define the most relevant allergen for allergy immunotherapy. For instance, in the case of insect venom allergy with clinical nonrelevant cross-reactivity between bee and wasp allergens, the determination of IgE to the bee venom protein Api m 1 and wasp venom allergens Ves v 1 and Ves v 5 can be helpful in determining the clinically relevant sensitization 39.
In a diagnostic setting, there are a number of ways that CRD can be utilized. The most widely used approach is determination as single component-specific IgE (e.g. ImmunoCAP, ImmuLite, HyTech) 40. Single determination can also be performed by the BAT, although the latter is less available in routine settings. To fully utilize the potential of CRD, a number of IgE components need to be assayed per patient in a multiplex analysis. This can be carried out in a multiplex system, currently available as the semi-quantitative ImmunoCAP–ISAC system which enables the determination of IgE binding to 112 allergens in one test run using only 30 μl of serum. An important difference between single and multiplex systems is the amount of allergen, and the ISAC system uses approximately 100 000-fold less allergen than the ImmunoCAP 40, 41. This may underlie its lower sensitivity at low sIgE levels as there will be more competition between allergen-specific IgG and IgE to bind to the lower amount of immobilized allergen on the ISAC chip 40. Also the allergens coupled to the single allergen system are not always the same as on the multiplex system and allergens will differ between manufacturers.
The multiplex analysis approach generates multiple data points per patient, and this has important implications for how these data are interpreted. Results for 112 individual allergens might be too complicated to interpret with many clinically irrelevant sensitizations. An approach where the focus is on IgE binding to specific groups of allergens may be more helpful and diagnostic algorithms are currently being developed to do this.
Potential for IgG to confound the result of sIgE assay systems
The level of specific IgE may be underestimated by the presence of other allergen-specific antibodies, particularly by specific IgGs including IgG4. It is also likely that allergen-blocking antibodies mitigate the clinical effects of specific IgE 42. For this reason, it has been argued that susceptibility to interference makes the outcome of a specific IgE test clinically more relevant. However, such interference by allergen-blocking antibodies is undesirable from the analytical point of view, because the outcome of the assay is not accurately reflecting the level of specific IgE in the sample. With high levels of allergen in the testing system (e.g. UniCAP system), interference by allergen-blocking antibody is unlikely. Where allergen levels are lower (e.g. ISAC), the risk of interference increases 43. This may particularly important following specific immunotherapy where specific IgG levels are elevated.
Integrating patient, environmental and allergen factors with IgE test results to predict the likelihood of clinical allergy
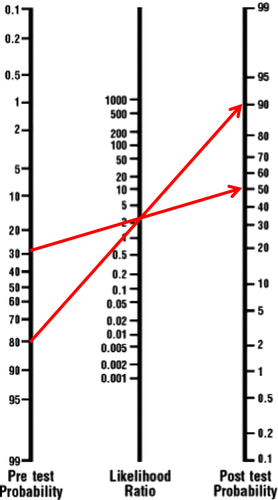
Probability curves provide an estimate of the risk of clinical allergy for specific IgE test results for the ‘average’ patient. The chance of clinical allergy can though be more precisely estimated from a test result by taking into account the presenting features of a patient; this is the so-called pretest probability 9, 44. A nomogram approach to interpreting test results in relationship to the pretest probability has been developed 45. This calculates the chance of clinical allergy using a likelihood ratio which is the likelihood of a given test result in clinically allergic subject over the likelihood of a similar result in normal subject (see example in Fig. 3). Although this approach maximized the available clinical information, it has not been widely adopted by clinicians, perhaps because it is too time-consuming, too complicated or clinicians do not have good estimates for pretest probabilities. There are likelihood ratio data for a limited number of foods 46 and almost no data for aeroallergens. A problem with many of the studies in which likelihood ratios (or other diagnostic parameters) are calculated is that subjects with very different pretest probabilities are mixed 47. Additionally, we do not know whether pretest probabilities are stable across different populations.
An alternative approach would be to use modern computer power to generate programmable calculator systems to synthesize presenting features together with the allergy test results to give a prognostic indication of the likelihood of clinical allergy. These are underpinned by logistic regression models built from clinical cases. An example is the Cork/Southampton prognostic model for food allergy 49. A proto algorithm was generated using data from Southampton food challenges (n = 429, peanut, egg, milk). It was then retrospectively applied, evaluated and modified using data from Cork food challenges (n = 289, peanut, egg, milk). Finally, it was prospectively validated in a blinded study in a further group of Cork children undergoing food challenges (n = 70, peanut, egg, milk). The clinical features that were found to predict clinical allergy were symptoms, sex, age, skin prick test, specific IgE and total IgE. The model had 97% accuracy for allergy and 94% for tolerance with an area under the curve of 0.97 for peanut, 0.95 for egg and 0.94 for milk in the prospective validation study. The model has since been validated in other settings 50.
The major drawback of the both the nomogram and calculator approach is the need to be able to derive a pretest probability for individual patients. While these have been developed for specific settings 49, we do not know how they differ between different setting and regions 50. Without a good estimate of the pretest probability, the predictive power of the system will be poor. Further data from a diverse range of setting, regions and allergens are required before this approach can be used routinely. It does though show promise.
IgE sensitization test results from the patient perspective
Patients generally regard IgE sensitization tests as allergy tests and they directly self-translate results into clinical diagnoses. To help patients understand the meaning of their IgE sensitization test results, clinicians need to explain the nature of these tests before ordering them and not refer to them as allergy tests. Such a clear understanding is essential if patients are going to have the confidence to implement changes to their self-management plans. On the face of it, a negative IgE sensitization test result might be expected to exclude clinical allergy. Patients need to understand that these tests cannot completely exclude allergy, especially when their clinical history is very suggestive of a clinical allergy. This situation needs to be managed with the patient, for example would it be safe to reintroduce the food at home in a graded manner or should a food challenge be ordered? Patients need to understand that sometimes tests give inconclusive results that do not allow clinicians to rule out or rule in clinical allergy. This situation would warrant a discussion about the merits of a provocation challenge to establish a firm conclusion. Finally, even where the IgE sensitization test is positive, a dialogue with the patient is needed as not all will have results that are overwhelming predictive of clinical allergy. Is the level of certainty sufficient for an individual patient to be happy to avoid the allergen or is a provocation challenge required to firmly establish the diagnosis? Individual patients may have different preferences about which IgE sensitization test is used and how they are subsequently managed in the context of different test results.
Potential new advances in allergy diagnostics
Novel approaches to the diagnosis of IgE-mediated allergic disease have been researched in the recent years, including the BAT, IgE and IgG4 binding to linear allergen peptides, mediator release and genetic profiling.
Basophil activation test is a flow cytometry-based assay that assesses changes in the expression of activation markers on the surface of basophils following allergen stimulation 51. Being a functional assay, it has the potential to more closely resemble the clinical phenotype of patients than methods that merely detect the presence of IgE. Basophil activation test has been applied to the diagnosis of allergy to food as well as drug, venom and environmental allergens 52. Its utility seems to be best in cases where conventional allergy tests have failed to diagnose allergy and provocation tests are to be considered 53. Basophil activation test could allow a reduction in the need for provocation tests 53. It seems to be valuable also in monitoring patients undergoing immunomodulatory treatments such as allergen-specific immunotherapy 54, 55 and omalizumab 56, and in monitoring natural resolution of food allergy 57, 58.
IgE and IgG4 binding to linear peptides has been studied in food and respiratory allergies. IgG4 do not seem to have a role in diagnosing allergies 59. Allergic patients tend to have greater IgE binding and broader epitope diversity than tolerant patients 60. Higher epitope diversity has also been associated with more severe reactions to food 61. For different allergens, immunodominant epitopes have been identified. Technical challenges as well as cost-effectiveness, role of tertiary structures and need for validation have hampered its wider use in clinical practice 47.
Other methods such as mediator levels, such as PAF 62, 63, and genetic profiling 64 may be useful in predicting patients at risk of developing more severe allergic reactions in the future.
Summary and future perspectives
Over the last decade, there have been considerable advances in our understanding of the immunological changes underlying clinical allergy and the relationship between skin prick and specific IgE test results and clinical manifestations of allergy. We have therefore reconsidered our approach to interpreting specific IgE test results and how we utilize them in clinical practice (Box 2). The meaning of a specific IgE sensitization test results varies according to the prevalence of allergy in the test population, a patients’ age, their ethnicity and any coexisting clinical diseases such as eczema (Fig. 4). The history may also indicate that a cofactor must be present for a clinical reaction to occur when a patient with specific IgE to the allergen to which they are exposed. We now have data to map specific IgE sensitization test results to the probability of clinical allergy for several foods. Unfortunately, these do not take into account patient-specific factors, neither do the 95% positive predictive values that are routinely used in clinical practice.
Box 2. Summary
Relevance of IgE sensitization to clinical allergy
- Immediate hypersensitivity (type I) reactions commence with cross-linking of IgE receptors by allergen.
- Specific symptoms of clinical allergy depend on the organ affected.
- Diseases other than allergy may give rise to allergy type symptoms.
Relevance of patient and environmental factors in interpreting IgE sensitization test results
- IgE sensitization tests perform better in clinic populations than the community as the prevalence of symptomatic allergy in a clinic population is higher.
- The meaning of specific IgE sensitization test results varies by patients’ age, ethnicity, presenting features of the putative allergic reaction and coexisting clinical diseases such as eczema.
- The presence of cofactors, such as exercise, may be necessary for contact with an allergen to results in an allergic reaction in specific IgE-positive patients.
Maximizing the available diagnostic information by treating SPT and specific IgE results as continuous measures
- 95% positive predictive values provide a patient perspective, but most patients’ result are below these levels and they do not take into account other factors such as the presenting history.
- Probability curves allow the chance of clinical allergy to be estimated from different test results, but they do not take into account other factors such as the presenting history.
Utilizing allergen components to improve our diagnostic approach
- Component-resolved diagnosis (CRD) can discriminate between binding to potent, more relevant allergens and cross-reactive, clinical less relevant allergens.
- Multiplex CRD systems can generate results for over a hundred component allergens; specific diagnostic algorithms are required to focus attention on the clinically relevant sensitization.
- As current allergen panels are still inadequate, both in number and in biosimilarity, we still depend on allergen extracts for IgE sensitization testing.
Potential for IgG to confound the result of sIgE assay systems
- The level of specific IgE may be underestimated in the presence of high levels of specific IgG, particularly in assay systems using low amounts of allergen.
Integrating patient, environmental and allergen factors with IgE test results to predict the likelihood of clinical allergy
- The chance of clinical allergy can be more precisely estimated from a test result, by taking into account the presenting features of a patient (pretest probability).
- A nomogram can be used which employs likelihood ratios for specific test results to translate pretest probabilities to the chance of clinical allergy (post-test probability).
- Computer-based calculator systems have been developed to calculate the chance of clinical allergy based on the presenting features of a patient and their IgE sensitization test result.
- This approach is currently limited by a lack of data to allow us to derive pretest probabilities for diverse setting, regions and allergens.
IgE sensitization test results from the patient perspective
- Patients generally see IgE sensitization tests as being synonymous with diagnostic allergy tests.
- The interpretation of the IgE sensitization test result should be explained to the patient, and then, there needs to be a two-way discussion about subsequent clinical management.
Potential new advances in allergy diagnostics
- Basophil activation test may potentially better reflect the presence of clinical allergy than other IgE sensitization tests, but further validation is required.
- Other approaches, such as binding to linear peptides and platelet-activating factor, still require further developmental work before they can be assessed for their role in the clinic.
The chance of clinical allergy can be more precisely estimated from an IgE sensitization test result, by taking into account the presenting features of a patient (pretest probability). The presence of each of these patient-specific factors may mean that a patient is more or less likely to have clinically allergy with a given test result (Fig. 4). Together, they may considerably modify the interpretation of IgE sensitization test results. We have presented two approaches to including pretest probabilities in the interpretation of results. The nomogram approach employs likelihood ratios for specific test results to translate pretest probabilities into the chance of clinical allergy (post-test probability). This approach has not been widely implemented, perhaps because of the lack of likelihood ratio data and its apparent complexity. Computer-based calculator systems can overcome these issues, but they rely on internal models to generate pretest probabilities from similar patient information; these are limited by a lack of data from a wide range of diverse setting, regions and allergens. We need more studies to determine to what extent the meaning of test results differs in different populations. Finally, a simpler approach has been proposed where each of the presenting history and IgE sensitization test results are divided into three levels of likelihood for clinical allergy and these are synthesized in a 3-by-3 table 9. This approach has not though been formally validated.
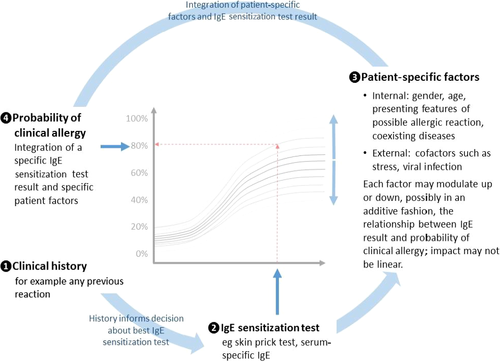
 The nature of the clinical history should determine the best IgE sensitization test.
The nature of the clinical history should determine the best IgE sensitization test.  Each IgE sensitization test result is associated with a specific probability of clinical allergy as shown by the interrupted red arrows.
Each IgE sensitization test result is associated with a specific probability of clinical allergy as shown by the interrupted red arrows.  The exact relationship between test result and probability of allergy varies according to internal and external factors which may make clinical allergy more or less likely in a potentially additive fashion.
The exact relationship between test result and probability of allergy varies according to internal and external factors which may make clinical allergy more or less likely in a potentially additive fashion.  So the likelihood of clinical allergy can determine from the IgE sensitization test result and a knowledge of other important patient factors; these precise relationships still need to be established.
So the likelihood of clinical allergy can determine from the IgE sensitization test result and a knowledge of other important patient factors; these precise relationships still need to be established.It is very apparent that there are gaps in our knowledge about the role of IgE in allergic disease and our interpretation of IgE sensitization tests. In IgE-mediated allergy, IgE is necessary but not sufficient to elicit an allergic reaction. A better understanding of the mechanisms of IgE-mediated allergic disease would help us to interpret false-positive IgE levels. This includes tissue factors that may play a role at the effector cell level but also patient factors (e.g. ethnicity, coexisting inflammatory diseases) and environmental factors (e.g. migration, climate change, cultural characteristics) that can function as a promoter or an enhancer of the allergic response.
Component-resolved diagnosis represents an important advance in specific IgE testing. Clinically, the ability to be able to discriminate between binding to potent, more relevant allergens and cross-reactive, clinical less relevant allergens is exciting. The implementation of the technology is currently limited by the lack of studies that focus on whether the CRD approach can improve the diagnostic experience of patients. We also await the availability of commercial CRD SPT reagents. For multiplex CRD systems, we also need to develop better approaches to interpreting IgE sensitization data on over 100 allergens to avoid over diagnosing clinical allergy.
Lastly, we need better systems and approaches to identify clinical important IgE sensitization. The BAT and IgE binding to linear allergen peptides are new diagnostic tests currently in development. Many patients have symptoms of IgE-mediated allergy without evidence of specific IgE sensitization. This may be due to the absence of the allergens that are driving the symptoms in the diagnostic extracts or to local production of allergen-specific IgE in the target organ. The utility of tests other than IgE sensitization tests, such as the atopy patch test, in the diagnosis of conditions with mixed IgE and non-IgE-mediated pathogenesis, such as atopic dermatitis and eosinophilic esophagitis, should also be explored further.
Acknowledgments
We would like to acknowledge the support of EAACI in developing this position paper.
Conflicts of interest
Moira Austin has received support from ALK-Abello to attend BSACI meeting. Philippe Eigenmann has received lecture honorarium and research grants from ThermoFisher Scientific and is on the scientific advisory board of MicrotestDX. Edward F. Knol has received speakers’ fee and travel reimbursement from Thermo Fisher Scientific. Graham Roberts has received funding for his research programme and acted as a scientific consultant for ALK-Abello and Thermo Fisher. Alexandra F. Santos has received research funding from the Medical Research Council, UK, the NIAID and the Immune Tolerance Network, USA, and the National Peanut Board, USA. She has received research support for a multicentre project using basophil activation test from Bühlmann Laboratories AG, Switzerland, through King's College London. Rob Aalberse has no conflict of interest to declare.
Author contributions
This paper was generated by a pan-EAACI task force supported by the Asthma Section, Dermatology Section, ENT Section, Immunology Section, Pediatric Section, Allergy Diagnosis Interest Group, Allied Health Interest Group, Drug Allergy Interest Group, Food Allergy Interest Group, JMA Working Group and Patient Group. The task force chair was Graham Roberts and the secretary was Markus Ollert. Each author drafted a section which Graham Roberts edited into the final document. All authors reviewed and discussed the final document and approved the final version. The final version was evaluated and endorsed by the EAACI Executive Committee.




