Circulating delta-like Notch ligand 1 is correlated with cardiac allograft vasculopathy and suppressed in heart transplant recipients on everolimus-based immunosuppression
Abstract
Cardiac allograft vasculopathy (CAV) causes heart failure after heart transplantation (HTx), but its pathogenesis is incompletely understood. Notch signaling, possibly modulated by everolimus (EVR), is essential for processes involved in CAV. We hypothesized that circulating Notch ligands would be dysregulated after HTx. We studied circulating delta-like Notch ligand 1 (DLL1) and periostin (POSTN) and CAV in de novo HTx recipients (n = 70) randomized to standard or EVR-based, calcineurin inhibitor-free immunosuppression and in maintenance HTx recipients (n = 41). Compared to healthy controls, plasma DLL1 and POSTN were elevated in de novo (P < .01; P < .001) and maintenance HTx recipients (P < .001; P < .01). Use of EVR was associated with a treatment effect for DLL1. For de novo HTx recipients, a change in DLL1 correlated with a change in CAV at 1 (P = .021) and 3 years (P = .005). In vitro, activation of T cells increased DLL1 secretion, attenuated by EVR. In vitro data suggest that also endothelial cells and vascular smooth muscle cells (VSMCs) could contribute to circulating DLL1. Immunostaining of myocardial specimens showed colocalization of DLL1 with T cells, endothelial cells, and VSMCs. Our findings suggest a role of DLL1 in CAV progression, and that the beneficial effect of EVR on CAV could reflect a suppressive effect on DLL1.
Trial registration numbers—SCHEDULE trial: ClinicalTrials.gov NCT01266148; NOCTET trial: ClinicalTrials.gov NCT00377962.
Abbreviations
-
- ANOVA
-
- analysis of variance
-
- BPAR
-
- biopsy-proven acute rejection
-
- CAV
-
- cardiac allograft vasculopathy
-
- CNI
-
- calcineurin inhibitor
-
- CsA
-
- cyclosporine A
-
- DLK1
-
- delta-like 1 homologue
-
- DLL1
-
- delta-like Notch ligand 1
-
- EC
-
- endothelial cell
-
- ECM
-
- extracellular matrix
-
- EVR
-
- everolimus
-
- HF
-
- heart failure
-
- hsCRP
-
- high-sensitivity C-reactive protein
-
- HTx
-
- heart transplantation
-
- IVUS
-
- intravascular ultrasound
-
- LVEDD
-
- left ventricular end-diastolic diameter
-
- MCP-1
-
- monocyte chemoattractant protein 1
-
- MIT
-
- maximal intimal thickness
-
- MMF
-
- mycophenolate mofetil
-
- mTORC
-
- mTOR complex
-
- mTOR
-
- mammalian target of rapamycin
-
- NF
-
- nuclear factor
-
- NT-proBNP
-
- N, terminal pro-B-type natriuretic peptide
-
- PAV
-
- percent atheroma volume
-
- PBMC
-
- peripheral blood mononuclear cell
-
- POSTN
-
- periostin
-
- sCD25
-
- soluble CD25
-
- SIR
-
- sirolimus
-
- TNF
-
- tumor necrosis factor
-
- VSMC
-
- vascular smooth muscle cell
1 INTRODUCTION
Heart transplantation (HTx) remains the definitive treatment for advanced heart failure (HF), offering a median survival after HTx of more than >13 years.1 Within 5 years, however, approximately 30% of the patients develop cardiac allograft vasculopathy (CAV), an important contributor to adverse outcome after HTx.2 Clinically, CAV resembles coronary heart disease, but it is pathologically distinct from the usual coronary atherosclerosis and may affect any graft vessel, with concentric, progressive thickening along the length of the vessels that ultimately leads to ischemia, HF, and death.3 Multiple factors contribute to CAV development, including immunologic (eg, chronic rejection process), and non–immune-related responses (eg, hyperlipidemia and viral infection), but the pathogenesis is incompletely understood.
Everolimus (EVR) is an alternative or supplement to calcineurin inhibitor (CNI)–based immunosuppressive regimen after HTx and reduces the incidence and progression of CAV.4-6 EVR, a mammalian target of rapamycin (mTOR) inhibitor, has antiproliferative effects on lymphocytes, vascular smooth muscle cells (VSMCs), endothelial cells (ECs), and fibroblasts7 and attenuates the activation and inflammatory response of neutrophils in vitro.8 However, the clinical benefit of EVR in HTx correlates poorly with changes in circulating inflammatory and vascular markers, and the mechanism by which EVR slows CAV progression remains unrevealed.5
The Notch signaling pathway is essential for communication between neighboring cells9 and processes known to contribute to CAV,10 like EC activation,11 accumulation and activation of immune cells,12 proliferation of VSMCs, extracellular matrix (ECM) remodeling13, 14 and T cell activation.15 Activation or inhibition of Notch signaling depends on the configuration and interactions of canonical Notch ligands.16 Proteolysis induces secretion of extracellular domains, which together with a large group of noncanonical ligands may modulate Notch signaling.17 The canonical delta-like Notch ligand 1 (DLL1) regulates the cell fate of monocytes,18 macrophages,19 T cells,20 and ECs,21 and in HF, circulating DLL1 is elevated and associated with impaired cardiac function and adverse outcome.22, 23 To our knowledge, DLL1 is the only detectable canonical Notch ligand in human plasma/serum. For noncanonical Notch ligands, delta-like 1 homologue (DLK1) is the most studied and has inhibitory effects on angiogenesis.24 Periostin (POSTN), a matricellular protein functioning as a noncanonical Notch ligand,25 is regulated in clinical myocardial injury,26 and is associated with cardiac dysfunction in end-stage HF.23 POSTN may modify Notch signaling through inhibition of DLK127 or maintenance of Notch1 receptor expression.28
Based on the involvement of Notch signaling in processes central to the development and progression of HF and CAV, and potential modulation of Notch signaling by mTOR inhibition,29 we hypothesized that (1) circulating Notch ligands would be dysregulated in HTx recipients, (2) EVR would affect levels of circulating Notch ligands in HTx recipients, and (3) circulating levels of Notch ligands would be associated with CAV progression. We tested our hypothesis by measuring the Notch ligands DLL1, POSTN, and DLK1 in plasma from 2 cohorts of HTx recipients with EVR-based and standard immunosuppression, at different timepoints and accompanied by assessment of CAV.
2 MATERIALS AND METHODS
2.1 Patient population
Two adult HTx cohorts were included: (1) de novo HTx recipients from the Scandinavian heart transplant everolimus de novo study with early CNI avoidance (SCHEDULE)4 and (2) maintenance HTx recipients from the NOrdic Certican Trial in HEart and Lung Transplantation (NOCTET).30
The SCHEDULE trial (ClinicalTrials.gov NCT01266148) was a 1-year, controlled and randomized multicenter trial and has been reported in detail.4 In brief the trial was conducted in 5Scandinavian HTx centers to evaluate if early initiation of EVR and early cessation of the CNI cyclosporine A (CsA) could improve renal function and hamper the progression of CAV. After antithymocyte globulin induction therapy, de novo HTx recipients were randomized to an immunosuppressive regimen consisting of either (1) low-dose EVR, CsA, mycophenolate mofetil (MMF), and corticosteroids with CsA withdrawal, and stepwise increments in EVR dose up to full dose after 7-11 weeks (hereafter: Everolimus group, EVR); or (2) standard regimen with CsA, MMF, and corticosteroids (hereafter: CNI group, CNI). The 2regimens were initiated no later than the fifth postoperative day. After the 1-year trial, immunosuppression was according to the investigator's preference, and the patients were reassessed at 3 years post-HTx.31 A total of 70 patients (CNI, n = 34; EVR, n = 36) with plasma collected at ≥3 timepoints: 7-11 weeks (i.e., baseline), 6 months, 1 year, and 3 years post-HTx, were included in the present follow-up substudy.
The NOCTET trial (ClinicalTrials.gov: NCT00377962) was a 1-year, controlled and randomized multicenter study conducted in 5Scandinavian transplant centers and reported in detail earlier.30 In brief; maintenance HTx recipients with renal impairment were randomized to either (1) EVR + reduced CNI (hereafter: Everolimus group, EVR) or (2) standard CNI-based regimen for maintenance immunosuppression ≥1 year post-HTx (hereafter: CNI group, CNI). The aim of the study was to evaluate whether the introduction of EVR with a simultaneous protocol-specified reduction in CNI exposure would improve renal function. After the 1-year core trial, the study was extended for 1 year and patients were reassessed at their annual visit 2 years after enrollment.32 A total of 41 patients (CNI, n = 22; EVR, n = 19) (mean 4.7 years, range 0.9-16.9 years post-HTx) with plasma collected at ≥3 timepoints: on inclusion (i.e., baseline), 6 weeks and 1 and 2 years after inclusion, were included in the present follow-up substudy.
For comparison, 20 age- and sex-matched healthy subjects were included.
For both studies, written informed consent was obtained after institutional review board approval. The studies were carried out in accordance with the ICH Harmonized Tripartite Guidelines for Good Clinical Practice, applicable local regulations, and the Declaration of Helsinki.
2.2 T cell isolation and activation
Peripheral blood mononuclear cells (PBMCs) were obtained from heparin-anticoagulated blood from healthy donors by Isopaque-Ficoll (Lymphoprep; Axis-Shield, Oslo, Norway) gradient centrifugation.33 Isolation of T cells from PBMCs was performed by negative selection using Pan T cell isolation kit and MACS Separator (Miltenyi Biotec, Bergisch Gladbach, Germany). Isolated T cells were resuspended in AimV medium (Gibco, ThermoFisherScientific, Waltham, MA), seeded into a 48-well plate (Costra, Cambridge, MA; 250.000 cells/well), and incubated for 40 minutes in a humidified CO2 incubator at 37°C before adding EVR (Sigma-Aldrich, St. Louis, MO), sirolimus (SIR) (Rapamycin, InvivoGen, San Diego, CA), or CsA (Sigma-Aldrich) in different concentrations. After 90 minutes of incubation, the cells were activated by beads coated with antibodies to CD2, CD3, and CD28 (T cell activation/expansion kit, Miltenyi Biotec). Cell-free supernatants and cell pellets were harvested at 24, 48, and 72 hours and stored at −80°C.
3 RESULTS
3.1 Patient population
Baseline demographics and characteristics were comparable between the 2treatment groups of de novo HTx recipients (SCHEDULE population), except for measured glomerular filtration rate, high-sensitivity C-reactive protein (hsCRP), and total cholesterol that were higher, and uric acid and terminal pro-B-type natriuretic peptide (NT-proBNP) that were lower in the EVR group (Table 1). For maintenance HTx recipients (NOCTET population), no differences in baseline demographics and characteristics were found (Table 1). For comparison, 20 healthy control subjects were recruited. Controls (mean age ± standard deviation [SD] 59.4 ± 8.8 years) were of similar age as the NOCTET population (58.9 ± 9.3), but not significantly older than the SCHEDULE population (50.5 ± 13.7, P = .11). Furthermore, creatinine in the controls was lower (76 ± 11 μmol/L) compared to both SCHEDULE (111 ± 41 μmol/L, P < .001) and NOCTET (121 ± 26 μmol/L, P < .001). Women represented 35% of controls, which was not statistically different from SCHEDULE (24%) or NOCTET (12%) (chi-square P = .11).
| SCHEDULE | NOCTET | |||||
|---|---|---|---|---|---|---|
| CNI (n = 34) | Everolimus (n = 36) | P | CNI (n = 22) | Everolimus (n = 19) | P | |
| Recipient demographics | ||||||
| Age(y) | 49.7 ± 13.7 | 51.4 ± 14.0 | .612 | 60.9 ± 7.8 | 56.6 ± 10.5 | .142 |
| Female gender (%) | 8 (24%) | 9 (25%) | .886 | 3 (14%) | 2 (11%) | .762 |
| BMI (kg/m2) | 25.3 ± 3.4 | 23.7 ± 3.5 | .080 | 25.5 ± 4.6 | 27.1 ± 6.0 | .339 |
| sBP (mm Hg) | 134 ± 15 | 134 ± 20 | .844 | 144 ± 15 | 145 ± 17 | .940 |
| dBP (mm Hg) | 82 ± 9 | 80 ± 12 | .394 | 90 ± 10 | 90 ± 14 | .928 |
| HR (beats/min) | 84 ± 12 | 92 ± 11 | .008 | 82 ± 11 | 88 ± 10 | .099 |
| Medical history | ||||||
| Diabetes (%) | 6 (18%) | 3 (8%) | .245 | 4 (18%) | 1 (5%) | .207 |
| Hypertension (%) | 5 (15%) | 6 (17%) | .822 | 15 (68%) | 16 (84%) | .233 |
| Current smoker (%) | 18 (53%) | 17 (47%) | .632 | 12 (55%) | 13 (68%) | .364 |
| Time since HTx (y) | 4.4 ± 3.4 | 5.1 ± 4.0 | .517 | |||
| Biochemistry | ||||||
| Hgb (g/dl) | 11.4 ± 1.1 | 11.1 ± 1.4 | .409 | 13.6 ± 1.2 | 14.2 ± 1.3 | .109 |
| mGFR (mL/min) | 55 ± 15 | 67 ± 18 | .003 | 58 ± 12 | 62 ± 15 | .389 |
| Creat (μmol/L) | 118 ± 32 | 105 ± 48 | .190 | 121 ± 26 | 119 ± 20 | .787 |
| hsCRP (mg/L) | 4 (2,8) | 6 (3,22) | .035 | 2.4 (0.9,3.4) | 2.7 (1.0,3.9) | .708 |
| Uric acid (μmol/L) | 421 (334,454) | 299 (253,382) | <.001 | 452 (387,520) | 450 (397,508) | .972 |
| Cholesterol (mmol/L) | 4.9 ± 0.9 | 5.5 ± 1.2 | .042 | 5.2 ± 0.7 | 5.3 ± 0.7 | .654 |
| NT-proBNP (pg/mL) | 155 (102,247) | 94 (54,204) | .041 | 50 (27, 118) | 32 (22, 58) | .202 |
| Donor age (y) | 44 ± 13 | 40 ± 15 | .182 | 35 ± 12 | 36 ± 13 | .927 |
| Female donor | 9 (30%) | 14 (42%) | .283 | 8 (36%) | 8 (42%) | .707 |
- Categoric data are reported as n (%) and continuous data as mean ± SD and median and interquartile range depending on distribution.
- sBP, systolic blood pressure, dBP, diastolic blood pressure; HR, heart rate; Hgb, hemoglobin; mGFR, measured glomerular filtration rate; Creat, creatinine; hsCRP, high-sensitivity C-reactive protein; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
3.2 Notch ligands in plasma at baseline
Compared to healthy controls and adjusting for differences in age and creatinine, baseline plasma DLL1 levels were higher in de novo (SCHEDULE) (P < .01) and in maintenance (NOCTET) (P < .001) HTx recipients. In SCHEDULE, DLL1 levels correlated with creatinine (r = −0.45, P < .001), hsCRP (r = 0.36, P = .004), NT-proBNP (r = 0.38, P = .004), and uric acid (r = 0.53, P < .001). Similar, but more modest associations were observed for baseline DLL1 in NOCTET (creatinine r = −0.27, P = .088; hsCRP r = 0.28, P = .078; uric acid r = 0.33, P = .042, and hemoglobin r = 0.32, P = .044). In addition, DLL1 levels at baseline in NOCTET were correlated with time since HTx (r = 0.35, P = .026) (Figure 1).
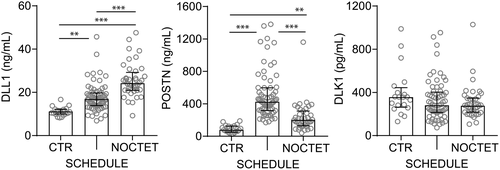
Baseline POSTN levels were also higher in both groups of HTx recipients compared to controls, but in contrast to DLL1, the highest levels of POSTN were seen in de novo HTx recipients (SCHEDULE). No significant correlations between POSTN and baseline biochemical variables were observed in SCHEDULE. For maintenance HTx recipients (NOCTET), baseline POSTN levels correlated with uric acid (r = 0.39, P = .014) but not with time since HTx (r = 0.28, P = .085) (Figure 1).
For DLK1, no significant differences were found (Figure 1).
3.3 Effect of EVR on Notch ligands in plasma
A significant overall treatment effect was observed in the temporal course of circulating DLL1 for each study population (Figure 2). In de novo HTx recipients (SCHEDULE) plasma levels of DLL1 decreased after 6 months of EVR treatment and remained low compared to baseline levels, but above those of healthy controls, throughout the study. Comparing the change in DLL1 after adjusting for baseline DLL1 levels and change in kidney function (ie, creatinine) in the same period revealed a significantly larger decrease in the EVR group at all timepoints. Among maintenance HTx recipients (NOCTET), a similar, but less distinct, pattern was observed with a significant treatment effect at 1-year follow-up.
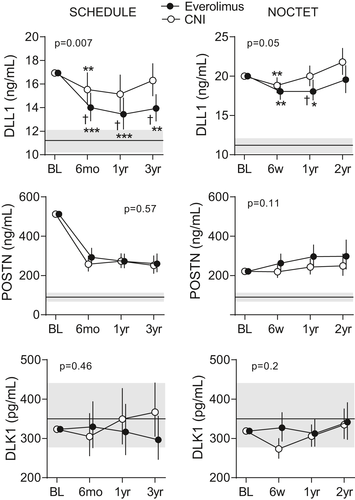
No treatment effects were observed for POSTN or DLK1 (Figure 2). POSTN levels declined after commencing on either of the immunosuppressive regimens in de novo HTx (SCHEDULE) recipients and remained low, but above those of healthy controls, for both treatment groups compared to baseline (Figure 2). In contrast, POSTN increased compared to baseline at all subsequent timepoints in both treatment groups in maintenance HTx recipients (NOCTET) (Figure 2). Plasma DLK1 remained stable and comparable to levels in healthy controls in both treatment arms and study populations (Figure 2).
Whereas DLL1 displayed similar profiles in SCHEDULE and NOCTET, POSTN exhibited a marked early decline in SCHEDULE, independent of study arm. The level of POSTN at 1 and 3 years in SCHEDULE was similar to that in NOCTET levels (ie, median 4.7 years post-HTx), and it seems that at 6 months, “steady state” levels of POSTN are achieved. The reason for this pattern is unclear, but because POSTN is induced during tissue injury and during ECM remodeling,34 we suggest that the initial high POSTN levels after HTx reflect ongoing ECM remodeling.
3.4 Notch ligands and acute rejections
Similar to the original report,31 there was a trend association between biopsy-proven acute rejection (BPAR) and the use of EVR (32% vs 13%, P = .056), and the use of CNI, respectively. As depicted in Table S1, we observed, however, no difference in change in DLL1 between patients with and without BPAR, although there was a trend toward lower DLL1 levels in patients who experienced BPAR within the first year (P = .090). No differences in change in or 1-year levels of DLL1 in relation to BPAR were observed within the treatment arms. For the other ligands, the decline in POSTN during 1-year follow-up was larger in those who experienced BPAR.
3.5 DLL1 and CAV development
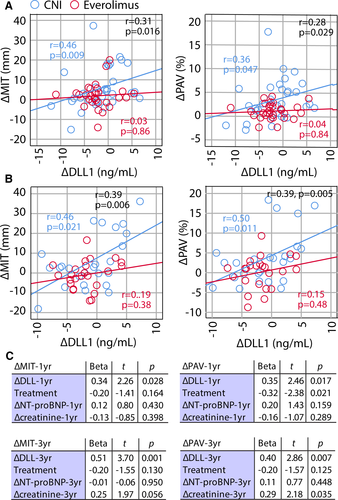
Recently, we reported attenuated CAV development in the EVR group of de novo HTx recipients (SCHEDULE).5 Herein, we observed a treatment effect for DLL1. We therefore evaluated the relationship between change in DLL1 (∆DLL1) and CAV development in the SCHEDULE population. A positive correlation between ∆DLL1 and change in maximal intimal thickness (∆MIT) from baseline to 1 (r = 0.31, P = .016) and 3 years (r = 0.39, P = .006) was noted, primarily reflecting changes in the CNI group (Figures 3A,B). However, we found no correlation between MIT and DLL1 levels at the individual time points. ∆DLL1 could potentially reflect improved allograft function, but correlations with changes in allograft function (ie, left ventricular ejection fraction [LVEF], left ventricular end diastolic diameter [LVEDD], peak early diastolic mitral inflow velocity/peak late diastolic mitral inflow velocity [E/A ratio], and NT-proBNP) were poor for both ∆MIT and ∆DLL1 (Table S2). However, ∆DLL1 correlated with change in CRP (r = 0.30, P = .018), mainly driven by a correlation in the CNI group (r = 0.58, P = .001), and creatinine (r = 0.49, P < .001) where the correlation was present in both treatment groups (Table S2). For the whole study population, the association between ∆DLL1 and ∆MIT was sustained when treatment, ∆NT-proBNP, and ∆creatinine were included in a linear regression model (Figure 3C). These data support that the association between changes in circulating DLL1 and MIT are independent of changes in allograft or kidney function. Moreover, changes in percent atheroma volume (∆PAV), an additional marker of CAV development also downregulated by EVR in the SCHEDULE population,5 were also significantly correlated with ∆DLL1 at 1 and 3 years, primarily reflecting changes in the CNI group, and again, independently of allograft or kidney function (Figure 3C).
3.6 Effect of EVR on Notch ligand DLL1 in myocardial tissue
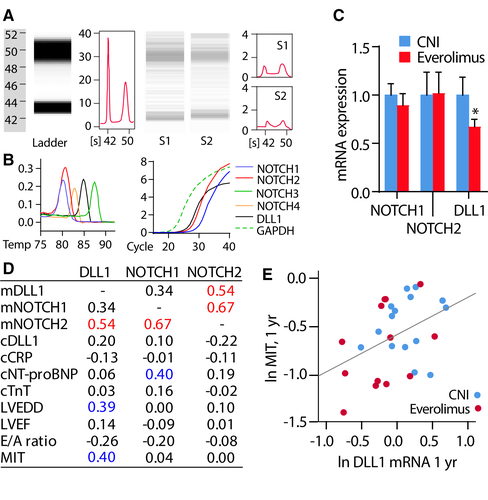
To assess whether the observed effects of EVR on DLL1 could be observed in the myocardium, we determined expression of mRNA for DLL1 and Notch receptors 1 and 2 in myocardial tissue from both study arms (EVR, n = 14; CNI, n = 18) in the SCHEDULE population. Whereas DLL1 was downregulated in the EVR group, NOTCH1/2 were similar between the groups (Figure 4C). It is notable that DLL1 correlated with MIT 1 year post-HTx for both study groups (n = 28, r = 0.40, P = .003) (Figures 4D,E), and a similar finding was also found between DLL1 and PAV (r = 0.36, P = .057). DLL1 also correlated with LVEDD, and NOTCH1 was correlated with NT-proBNP, suggesting some association with allograft function (Figure 4D).
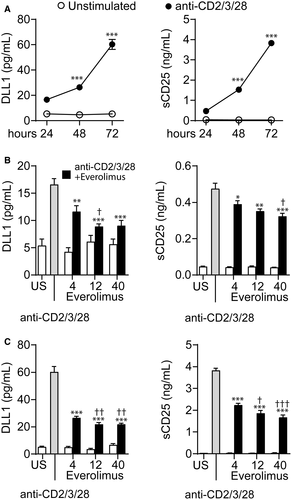
3.7 EVR suppresses DLL1 in activated T cells
Activated T cells are involved in the pathogenesis of CAV,35 and Notch and mTOR signaling are crucial contributors in T cell–mediated immune responses. We therefore evaluated the effect of different concentrations of EVR (4, 12, and 40 ng/mL) on DLL1 release. Activation of T cells was associated with a time-dependent increase in DLL1 and the T cell activation marker soluble (s)CD25 as compared to unstimulated cells (Figure 5A). EVR attenuated DLL1 and sCD25 release in a time- and dose-dependent manner, but with only modest effects in further decreasing DLL1 and sCD25 release at the highest doses (Figure 5B,C). Using even higher doses (100 and 500 ng/mL) had limited effects (Figure S1A). A similar dose–response pattern has previously been reported of rapamycin on activated T cell production of Th1/Th2 cytokines,36 and it is important to note that the lower doses are relevant to targeted concentrations of EVR in the HTx patients (6-10 ng/mL in the SCHEDULE and 3-8 ng/mL in the NOCTET study). Stimulation of activated T cells with the mTOR inhibitor sirolimus, but not the CNI CsA, dampened the DLL1 and sCD25 release in a pattern similar to EVR, whereas CNI reduced sCD25 release dose dependently at 72 hours (Figure S2). These results suggest that the observed effects on circulating DLL1 in the EVR group may be mediated by T cells, attributable to EVR exposure rather than freedom from CNI. Moreover, in activated T cells, EVR attenuated mRNA expression of the mTOR-responsive gene UTP15 (Figure S1B) in a pattern similar to DLL1 and sCD25, supporting a link between mTOR complex (mTORC) signaling and DLL1 release. Contrary to the effects on DLL1, the effect of EVR on mRNA levels of Notch receptors 1 and 2 was more complex, with a reduction of NOTCH1 in un-activated T cells, and a dose-dependent increase in NOTCH1 in activated T cells at 72 hours (Figure S3).
3.8 ECs and VSMCs may be sources of circulating DLL1
DLL1 may be released from cells other than T cells, potentially contributing to circulating DLL1 in HTx recipients. To elucidate this, we examined the release of DLL1 from VSMCs, human aortic and umbilical vein ECs, and cardiac fibroblasts. These experiments revealed (Figures S4/S5/S6): (1) VSMCs and ECs, but not cardiac fibroblasts, secrete DLL1 in the conditioned medium and could be potential sources for circulating DLL1 in addition to T cells; (2) activation of VSMCs with the prototypical upstream inflammatory cytokine interleukin 1β enhanced DLL1 secretion, whereas the release of DLL1 upon tumor necrosis factor (TNF)–mediated EC activation, another prototypical upstream inflammatory cytokine, was more modest; and (3) EVR attenuated DLL1 release.
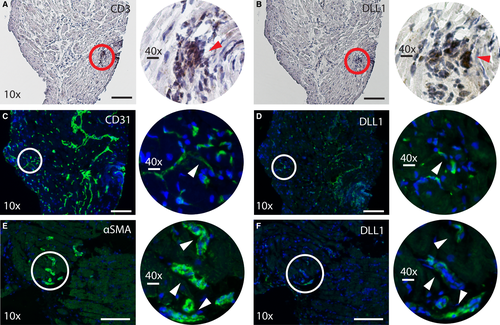
3.9 Expression of DLL1 in myocardial tissue
Our findings so far show that DLL1 mRNA is expressed in myocardial tissue following HTx. Our in vitro studies suggest that T cells, ECs, and VSMCs could be important cellular sources of DLL1. To further elucidate these findings, we performed immunohistochemistry analysis of myocardial specimens from 2HTx recipients with high MIT (0.90 and 0.81 mm), indicative of the presence of CAV. The biopsies were obtained at the first annual post-HTx visit for participants in the SCHEDULE trial (Figure 6). DLL1 protein was expressed in myocardial tissue and exhibited a distribution pattern corresponding to the presence of T cells, ECs, and VSMCs. Although we were unable to firmly establish the proximity to CAV in our specimens, the strong staining of the EC marker CD31, shown to the left in Figure 6C, could reflect endothelial activation, a common feature of CAV.37
4 DISCUSSION
We studied circulating Notch ligands DLL1, POSTN, and DLK1 and CAV in HTx recipients receiving standard or EVR-based, CNI-free immunosuppression and in maintenance HTx recipients. Our main findings were the following: (1) In HTx recipients, plasma DLL1 and POSTN were elevated compared to healthy controls; (2) use of EVR-based, CNI-free immunosuppression was associated with decreased plasma DLL1 levels; (3) during long-term follow-up, changes in plasma DLL1 in de novo HTx recipients correlated with changes in markers of CAV (MIT and PAV); (4) in myocardial tissue from de novo HTx recipients, the DLL1 protein was expressed in a distribution pattern similar to that of T cells, ECs, and VSMCs, and DLL1 mRNA levels were attenuated in the EVR group and correlated with CAV (MIT and PAV); (5) in vitro, activation of T cells increased DLL1 secretion, and EVR and sirolimus, but not CsA, attenuated this release, and (6) ECs and VSMCs released DLL1, with attenuating effects of EVR in EC. These findings suggest a link between CAV development and DLL1 involving a complex interaction between T cells, VSMCs, and ECs, also within the heart allograft.
As in chronic HF, circulating DLL1 levels at baseline in de novo HTx recipients are correlated with kidney function.38 More importantly, enhanced DLL1 levels compared to healthy controls persisted after adjustment for creatinine. DLL1 was correlated positively with systemic inflammation, as reflected by hsCRP and in particular uric acid, which is markedly elevated early after HTx, potentially reflecting increased metabolic and oxidative stress.39 The persistently elevated DLL1 levels, suggesting enhanced Notch pathway activity, could mean that DLL1 is a marker of these processes, but could also indicate that Notch signaling and DLL1 contribute to inflammation, endothelial activation, and metabolic and oxidative stress in HTx recipients. Furthermore, DLL1 is central in T cell–mediated responses,40 and differentiation and activation of T cells require DLL1-mediated Notch signaling.41, 40 Although T cell activation may be important for maintaining adaptive immune responses, dysregulated T cell activation may cause maladaptive immune response characterized by extensive production of inflammatory cytokines. The release of DLL1 from activated T cells in our in vitro study may propose DLL1 to be a part in a pathogenic loop leading to enhanced T cell activation in HTx recipients and potentially maladaptive T cell responses.
A major finding was that EVR downregulated DLL1 in vivo. Our in vitro findings show a similar effect of sirolimus but not the CNI CsA, suggestive of mTORC involvement but the underlying mechanisms are not clear. mTORC regulates several pathways relevant for T cell activation such as nuclear factor (NF)-κB, and is activated by the Akt pathway, which links T cell activation to mTORC.42 Because the pattern of DLL1 release mimics that of sCD25, it is tempting to hypothesize that DLL1 could be directly regulated through mTORC. TNF, the prototypical activator of NF-κB, has been shown to increase DLL1 expression in fibroblast-like cells.43 Furthermore, another mTORC inhibitor, Torin-1, decreased the expression of DLL1 in vivo in colorectal cancer xenografts.44 Moreover, the release of DSL ligands including DLL1 is achieved by shedding induced by ADAM9/10/12,45-47 and, of interest, rapamycin (ie, sirolimus) has been shown to inhibit ADAM1048 and ADAM12.49, 50 Thus, both direct mTOR-mediated effects as well as protease activity that is also influenced by the mTOR pathway could contribute to DLL1 release.
Neointimal proliferation and infiltration by macrophages are principal to CAV development and likely driven by several processes including upstream cytokine production from activated T cells. Indeed, T cells dominate among the immune cells accumulating in the thickened intima,51 and the beneficial effect of EVR on CAV development as reported in the SCHEDULE population5 may mirror attenuation of T cell activation. However, the molecular mechanism for the beneficial effect of EVR on CAV is not clear. Our previous attempts to identify associations between the effect of EVR on CAV and effects on a wide range of inflammatory markers, including CRP, have failed. Our present findings that EVR downregulates DLL1 both in plasma and the myocardium, and that DLL1 correlates with MIT and PAV, reflective of CAV development, may suggest that the beneficial effects of EVR on CAV could involve downregulation of DLL1 and Notch signaling. Of interest in experimental HTx, blockade of transcriptional activation downstream of all Notch receptors in T cells eliminated the effects of canonical Notch signaling and improved graft survival.52 EC injury from immunologic and nonimmunologic factors is central to CAV development,3 and Notch signaling is involved in EC dysfunction.11 Herein we show that ECs release DLL1, attenuated by EVR. Thus it is tempting to hypothesize that the beneficial effect of EVR in CAV could involve attenuated Notch signaling with inhibitory effects on DLL1 release from T cells and ECs. Although we lack data on the effects of EVR on DLL1 protein levels in the myocardium, the immunostaining showing co-localization of DLL1 to markers of T cells and ECs within the myocardium in CAV patients, may suggest that such mechanisms also could be operating in vivo within the myocardium in HTx recipients with CAV.
In vivo, EVR decreased DLL1 in plasma and myocardium from HTx recipients. In contrast to circulating DLL1, where the temporal change in DLL1 and not the level at a specific timepoint correlated with change in MIT and PAV, myocardial DLL1 correlated with MIT and PAV in biopsies taken 1 year post-HTx. This “disassociation” between plasma and myocardial DLL1 may suggest that circulating DLL1 at a single timepoint may reflect the contribution of multiple cell types in the body, some of which may not be involved in CAV progression, whereas the change in circulating DLL1 over time to a larger extent may mirror concerted long-term changes during CAV progression. On the other hand, myocardial mRNA levels of DLL1 presumably mainly reflect DLL1 in ECs, VSMCs, and immune cells in proximity to CAV.
Limitations to the present study include the relatively small sample sizes and the homogenous Scandinavian population. Thus, the SCHEDULE and NOCTET study populations may differ from the global HTx recipient population, which may constrain the extrapolation of our results. Moreover, lack of blood samples obtained before HTx limits the ability to interpret the pattern of DLL1 levels after HTx. Furthermore, associations do not necessarily imply any causal relationship, and we lack data on Notch signaling within the CAV lesions and relevant cells (ie, T cells and ECs), and analysis of their DLL1 mRNA and protein expression could illuminate our findings. Mechanistic data on the effects of mTOR inhibition on ligand-receptor interactions would have strengthened our findings. For our experiment on the effect of EVR on NOTCH1/2 mRNA expression in activated T cells, it might have been informative to quantify in parallel the corresponding receptor proteins and DLL1, as others have reported an upregulation of NOTCH1 proteins after anti-CD3/28 stimulation of T cells.53 Whereas plasma DLL1 performs technically well and displays good coefficients of variation and no diurnal or postprandial variation,54 less is known for POSTN. To illuminate the robustness of our findings, the stability of DLL1 in samples that were thawed more than once could have been measured. Unfortunately, we have no data on freeze/thaw stability for DLL1 for the present study. Finally, other Notch ligands, such as DLL3/4 and Jagged 1/2 could also be involved in CAV development. Future studies should address the limiting issues of the present trial.
We conclude that in HTx recipients, DLL1 is increased and downregulated by EVR-based immunosuppression and that change in DLL1 is correlated with CAV progression. Our findings may suggest a role for Notch signaling and DLL1 in CAV progression.
ACKNOWLEDGMENTS
HMN thanks the Raagholt and Kardimmun Foundations for grants for the present work.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Dag Solbu is an employee of Novartis Scandinavia. The SCHEDULE and NOCTET studies were supported by Novartis Scandinavia. The other authors have no conflicts of interest to disclose.




