Clinical outcomes of hepatitis C treatment before and after kidney transplantation and its impact on time to transplant: A multicenter study
See also: Kiberd et al, Kadatz et al, and Sawinski
Abstract
Waitlist time for kidney transplantation is long but may be shortened with the utilization of hepatitis C positive allografts. We retrospectively reviewed the course of 36 hepatitis C positive patients awaiting kidney transplantation at 2 large centers within the same health system, with near-identical care delivery models with the exception of timing of hepatitis C treatment, to determine the impact of timing of hepatitis C treatment on access to transplant, waitlist time, and treatment efficacy and tolerability. The majority of patients had hepatitis C genotype 1a or 1b, and all received direct acting antiviral therapy with 100% treatment response. One patient underwent transplantation in the pretransplant treatment group. The 1-year transplantation rate was 12.5% vs 67.9% (P = .0013) in those treated posttransplantation. The median waitlist time in the posttransplant group was 122 (interquartile range [IQR] 21.5, 531.0) days, which was significantly shorter than the center’s regional and national wait time. Pathologic review revealed no difference in allograft quality. Overall treatment related adverse events were not different between the 2 groups. A strategy of posttransplant hepatitis C treatment increased access to transplant and reduced waitlist time. Delaying treatment until after transplant did not appear to adversely affect recipients’ kidney allograft or overall survival.
Abbreviations
-
- AE
-
- adverse events
-
- ALT
-
- alanine aminotransferase
-
- CNI
-
- calcineurin inhibitor
-
- DAA
-
- direct acting antiviral therapies
-
- DBD
-
- donation after brain death
-
- DCD
-
- donation after cardiac death
-
- ESRD
-
- end-stage renal disease
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- IQR
-
- interquartile range
-
- KDPI
-
- Kidney Donor Profile Index
-
- KT
-
- kidney transplantation
-
- MELD-Na
-
- Model for End-Stage Liver Disease - sodium
-
- SVR
-
- sustained virologic response
-
- UNOS
-
- United Network for Organ Sharing
-
- WLT
-
- waitlist time
1 INTRODUCTION
Kidney transplantation (KT) decreases healthcare costs and increases available life years for patients.1, 2 However, significant organ shortage exists.3 The annual incidence of hepatitis C virus (HCV) infection in end-stage renal disease (ESRD) patients awaiting KT ranges from 1.4% to2.1%, with prevalence approaching 12%-23% in the United States, which is approximately 100–1000 times higher than the general population.4-8 However, less than one-third of patients with HCV will receive a HCV-positive kidney, and HCV-positive kidneys are 2.6 times more likely to be discarded.9 Nearly two-thirds of HCV-positive kidneys, accounting for thousands of potentially usable organs over a 10-year period, were discarded.10
Underutilization of HCV-positive organs in HCV-positive recipients is likely multifactorial. Receipt of HCV-positive organs was shown to increase the risk of death, though risk is delayed for approximately 2 years.11 Direct acting antiviral therapies (DAA) boast HCV-cure rates in excess of 95%. However, renal failure poses a unique challenge as many DAA-based regimens are not currently approved for use in ESRD.
Although there is concern that DAA therapy may temporarily cause allograft dysfunction and lead to the need for closer immunosuppression monitoring, most data suggest DAA therapy is well tolerated and effective with treatment responses of 96%-100%.12-16 Patients may benefit from being left viremic going into transplant because of substantial reduction in waitlist time (WLT) from years to ≤ 2 months.9, 15 However, untreated HCV post-KT could have a more severe course because of the permissive effects of chronic immunosuppression on HCV viral replication, which could lead to progression of liver disease.17, 18 Optimal timing of HCV treatment remains unknown. Given this uncertainty, we compared the impact of HCV treatment pre- vs post-KT on organ access as judged by WLT between 2 large-volume KT centers within the same health system with identical KT management protocols with the exception of timing of HCV treatment.
2 MATERIALS AND METHODS
2.1 Patient population
All patients with HCV evaluated and actively listed for KT between 2012 and 2017 were considered for study inclusion. Patients aged ≥ 18 years, with confirmed HCV infection with quantifiable HCV RNA levels regardless of genotype and treated with DAA therapy, were included. Exclusion criteria included patients ineligible or not listed for KT, patients treated with regimens other than DAAs, patients undergoing living-donor or simultaneous liver-kidney transplantation, and those transplanted at other centers.
2.2 Study design
We compared DAA therapy response, presence of adverse events, and WLT. This study was conducted retrospectively from chart review and was approved by the institutional review board at Mayo Clinic, conforming to the ethical standards as set forth in the Declaration of Helsinki. All patients were treated within the same health system at 2 institutions that have consensus protocols for the pre-KT evaluation, peri- and postoperative management, and immunosuppression. The only significant practice difference between the 2 centers is timing of HCV treatment. Antithymocyte globulin or basiliximab induction was given based on recipient characteristics. Thereafter, triple immunosuppression with tacrolimus, mycophenolic acid preparations, and steroid taper was used. Trough tacrolimus levels were considered therapeutic if in the range of 10-12 ng/mL in the first month, 8-10 ng/mL from 1 month to 4 months, and 6-8 ng/mL beyond 4 months post-KT. Posttransplant infectious prophylaxis differed slightly because of center geographic location. Specifically, center B’s practice was to use fluconazole prophylaxis because of the risk of endemic regional fungal infections.
2.3 Data collection
Demographics included gender, race, ethnicity, age, blood type, HCV genotype, presence of cirrhosis, HIV status, duration of hemodialysis, and history of prior KT. Laboratory measures included HCV RNA levels before, during, and after conclusion of therapy. Baseline markers of renal function included serum creatinine, cystatin C, and iothalamate testing as available.
Previous regimens, current regimen, and duration of HCV treatment were collected. Treatment response parameters included achievement of sustained virologic response (SVR) at 12 weeks posttreatment. Adverse events of HCV treatment including increase in alanine aminotransferase (ALT) > 5 times the upper limit of normal, fatigue, headache, nausea, myalgia, insomnia, and hypertension were recorded.
KT WLT was calculated from the date of United Network for Organ Sharing (UNOS) listing to the date of transplant. The quality of kidney allografts was measured by obtaining the Kidney Donor Profile Index (KDPI) score. Donor allograft HCV status was recorded as positive if either HCV antibody or nucleic acid amplification testing was positive. Donation after brain (DBD) or cardiac death (DCD) allograft type was recorded. All post-KT biopsies were processed with standard protocols. Banff scores for allograft pathology were reviewed.19 The presence of posttransplant delayed graft function was collected. The date of last follow-up, need for repeated transplantation, episodes of allograft rejection, and status of the graft were recorded.
Assessment of liver disease was made based on the presence of cirrhosis, with diagnosis relying on liver biopsy or transient elastography. Parameters included baseline liver transaminases and Model for End-Stage Liver Disease - sodium (MELD-Na) in cirrhotic patients.
2.4 Statistical analyses
Demographics and clinical characteristics were compared using chi-square test, Fisher’s exact test, 2 sample t test, or Wilcoxon rank sum test as appropriate. Log-rank test compared transplant Kaplan-Meier (KM) curves. Wilcoxon rank sum test compared WLT, and Fisher’s exact test compared survival status and adverse events. Data were analyzed using SAS Studio version 9.3.
3 RESULTS
3.1 Demographics
Fifty-five ESRD patients with HCV were considered for KT and received DAA-based interferon-free regimens between 2012 and 2017. Exclusion of patients not meeting inclusion criteria left 36 patients eligible for analysis. The median age of the entire cohort at start of HCV treatment was 59 (IQR 53, 62) years. 75% were hemodialysis dependent. In transplant center A, 8 listed patients were treated pre-KT; 100% were male, 63% African American, 25% Caucasian, and 12.5% Hispanic/Latino. Median age at start of HCV treatment was 62 (IQR 56, 66) years. Cirrhosis was present in 12.5% of center A’s patients.
In transplant center B, 28 patients received HCV-treatment post-KT. The majority were male (82%) and Caucasian (75%); 29% identified as Hispanic/Latino. The median age was 61 (IQR 53, 63) years. Twenty (71%) were dialysis dependent. Cirrhosis was present in 36% of center B’s patients. Demographic and clinical characteristics from both transplant centers are presented in Table 1. Characteristics of kidney allografts are shown in Table 2. There was no difference in age (P = .25), gender (P = .57), HCV genotype (P = .62), viral load (P = .60), blood type (P = .47), chronic kidney disease stage (P = .40), presence of cirrhosis (P = .21), or MELD-Na (P = .27).
| Transplant center A HCV treatment before transplant (N = 8) | Transplant center B HCV treatment after transplant (N = 28) | Total (N = 36) | P value | |
|---|---|---|---|---|
| Age at HCV treatment start, mean (SD) | 62.0 (8.2) | 58.2 (7.8) | 59.1 (7.9) | .253 |
| Gender, male | 8 (100.0%) | 23 (82.1%) | 31 (86.1%) | .566 |
| Race | .002 | |||
| Caucasian | 2 (25.0%) | 21 (75.0%) | 23 (63.9%) | |
| American Indian or Alaska Native | 0 (0.0%) | 2 (7.1%) | 2 (5.6%) | |
| Asian | 5 (62.5%) | 1 (3.6%) | 6 (16.7%) | |
| Black or African American | 0 (0.0%) | 2 (7.1%) | 2 (5.6%) | |
| Native Hawaiian or other Pacific Islander | 1 (12.5%) | 2 (7.1%) | 3 (8.3%) | |
| Other | 2 (25.0%) | 21 (75.0%) | 23 (63.9%) | |
| Hispanic or Latino ethnicity | 1 (12.5%) | 8 (28.6%) | 9 (25.0%) | .648 |
| Blood type | .470 | |||
| A | 3 (37.5%) | 15 (53.6%) | 18 (50.0%) | |
| AB | 0 (0.0%) | 1 (3.6%) | 1 (2.8%) | |
| B | 2 (25.0%) | 2 (7.1%) | 4 (11.1%) | |
| O | 3 (37.5%) | 10 (35.7%) | 13 (36.1%) | |
| Chronic kidney Disease stage | .397 | |||
| 4 | 1 (12.5%) | 9 (32.1%) | 10 (27.8%) | |
| 5 | 7 (87.5%) | 19 (67.9%) | 26 (72.2%) | |
| Hemodialysis | 7 (87.5%) | 20 (71.4%) | 27 (75.0%) | .648 |
| Duration of hemodialysis days (IQR) | 1525 (770, 1932) | 992 (444, 2381) | 1235 (604, 2343) | .4892 |
| Prior kidney transplant history | 1 (12.5%) | 3 (10.7%) | 4 (11.1%) | 1.000 |
| HCV genotype | .617 | |||
| 1a | 7 (87.5%) | 15 (53.6%) | 22 (61.1%) | |
| 1b | 1 (12.5%) | 8 (28.6%) | 9 (25.0%) | |
| 2 | 0 (0.0%) | 1 (3.6%) | 1 (2.8%) | |
| 3 | 0 (0.0%) | 3 (10.7%) | 3 (8.3%) | |
| High baseline viral load (> 600 000) | 6 (75.0%) | 24 (85.7%) | 30 (83.3%) | .596 |
| HCV treatment experienced | 2 (25.0%) | 7 (25.0%) | 9 (25.0%) | 1.000 |
| Cirrhosis | 1 (12.5%) | 10 (35.7%) | 11 (30.6%) | .209 |
| MELD-Na, Mean (SD, range) | 21.0 | 13.8 (9.7, 6.0-40.0) | 14.5 (9.4, 6.0-40.0) | .267 |
| Transplant center A HCV treatment before transplant (N = 8) | Transplant center B HCV treatment after transplant (N = 28) | Total (N = 36) | |
|---|---|---|---|
| Current kidney transplant status | |||
| Pretransplant | 7 (87.5) | 0 (0) | 7 (19.4) |
| Posttransplant | 1 (12.5) | 28 (100) | 29 (80.6) |
| Median age (years) at time of transplant (IQR) | 70 (70, 70) | 59 (53, 62) | 59 (54, 62) |
| KDPI % (IQR) | 51 (51, 51) | 47 (36, 63) | 49 (36, 63) |
| Received DBD allograft (%) | 1 (100) | 22 (78.6) | 23 (79.3) |
| Received DCD allograft (%) | 0 (0) | 6 (22.2) | 6 (21.4) |
| Received HCV-positive allograft (%) | 0 (0) | 19 (67.9) | 19 (66) |
| Presence of delayed graft function (%) | 1 (100) | 11 (39) | 12 (41) |
| Suffered allograft rejection (%) | 1 (100) | 2 (7.1) | 3 (10.3) |
| Median length (days) of follow-up (IQR) | 229 (76, 275) | 413 (360, 561) | 364 (260, 512) |
| Survival status (%) | 8 (100) | 27 (96) | 35 (97) |
3.2 Hepatitis C treatment regimens and efficacy
HCV treatment regimens are shown in Table 3. Genotypes 1a, 1b, 2, and 3 were identified within the cohort with HCV genotype 1a (61%) being most common. All patients in the pre-KT group were genotype 1a or 1b. Seventy-five percent (27) were treatment naïve (P = 1.00). Thirty (83%) had a starting viral RNA greater than 600 000 IU/mL. All pre-KT patients received 12 weeks of elbasvir/grazoprevir. A variety of sofosbuvir containing regimens were used in post-KT patients. The most common regimen was ledipasvir/sofosbuvir for 12 weeks (42%). All patients achieved SVR. Median time from transplant to initiation of HCV treatment was 77 (IQR 51, 186) days.
| Current HCV treatment duration | Regimen | Transplant center A HCV treatment before transplant (N = 8) | Transplant center B HCV treatment after transplant (N = 28) | Total (N = 36) |
|---|---|---|---|---|
| 12 weeks | Elbasvir/grazoprevir | 8 (100.0%) | 0 (0.0%) | 8 (22.2%) |
| Ledipasvir/sofosbuvir | 0 (0.0%) | 15 (53.6%) | 15 (41.7%) | |
| Ledipasvir/sofosbuvir + ribavirin | 0 (0.0%) | 2 (7.1%) | 2 (5.6%) | |
| Daclatasvir/sofosbuvir + ribavirin | 0 (0.0%) | 1 (3.6%) | 1 (2.8%) | |
| Velpatasvir/sofosbuvir | 0 (0.0%) | 4 (14.3%) | 4 (11.1%) | |
| 24 weeks | Ledipasvir/sofosbuvir | 0 (0.0%) | 5 (17.9%) | 5 (13.9%) |
| Sofosbuvir + ribavirin | 0 (0.0%) | 1 (3.6%) | 1 (2.8%) |
3.3 Impact on access to transplant and waitlist time
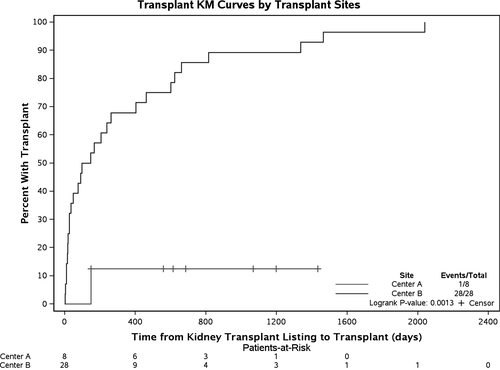
At center A, 8 patients were listed for KT and 1 underwent KT. The WLT was 148 days in the transplanted patient. The median (IQR) WLT as calculated from the date of listing to the date of transplant or last follow-up was 650 (354; 1133) days. In 28 patients at center B who underwent transplant, median WLT was 122 (IQR 22; 531) days (Figure 1). Significant difference was noted between the centers with respect to WLT (P = .02). The 1-year transplantation rate was significantly different: 12.5% at center A and 67.9% at center B (P = .0013).
3.4 Renal allograft quality and outcome
No difference in blood type distribution existed between the centers (P = .47). Blood type O was most common. Nineteen (68%) patients received a HCV-positive allograft. Three patients did not have a donor KDPI, as it was introduced during the study period. Median (IQR) KDPI was 47 (36, 63) in those treated post-KT vs 51 in those treated pre-KT (Table 2). At center B, 22% of patients received a DCD allograft. Time zero biopsy was performed on 14 transplanted organs. Overall organ quality was excellent with 11 showing no pathologic abnormalities (defined as Banff scores of 0 for ct, ci, cv, and ah). No organs had greater than mild abnormalities (defined as Banff scores for ct, ci, cv, and ah greater than 1). Delayed graft function occurred in 41% of the cohort, including the patient treated pre-KT. Three patients had allograft rejection, including a patient at center A. At center B, one allograft rejection (Banff i1, t2) occurred in the first week of HCV-treatment with ledipasvir/sofosbuvir, despite therapeutic tacrolimus troughs; the other (Banff i1, t2) occurred prior to HCV treatment. In both cases, patients had borderline changes suspicious for acute T cell–mediated rejection and were treated with pulsed steroids.
All allografts were considered successful and functioning as determined by a subsequent transplant nephrology clinic visit. Twenty-seven of 28 post-KT patients were alive at last follow-up. Median follow-up (IQR) at center A was 229 (76, 275) days. At center B, median follow-up was 413 (360, 561) days. One patient in the post-KT treatment group died after KT and SVR due to cholangiocarcinoma. Follow-up protocol kidney biopsies were obtained in 19 patients at 4 and 12 months posttransplant. Two patients had moderate hyaline arteriolosclerosis at 4 months that was not identified on either the time zero biopsy or the 1-year biopsy. Only 1 patient showed progression of Banff scores to moderate interstitial fibrosis and tubular atrophy (ct2 and ci2) at 1 year. The remaining 1-year biopsies had mild or no significant interstitial fibrosis and tubular atrophy.
3.5 Adverse events during hepatitis C treatment
Adverse events (AE) were reported in about half of patients undergoing HCV treatment (Table 4). More patients undergoing treatment post-KT had symptoms (P < .01). Fatigue was more common in the post-KT group (P = .01). None had elevated ALT or myalgia. No serious AEs were reported. No AEs resulted in treatment interruption.
| Adverse events | Transplant center A HCV treatment before transplant (N = 8) | Transplant center B HCV treatment after transplant (N = 28) | Total (N = 36) | P value |
|---|---|---|---|---|
| Fatigue (%) | 0 (0) | 15 (53.6) | 15 (41.7) | .011 |
| Headache (%) | 0 (0) | 7 (25.0) | 7 (19.4) | .309 |
| Nausea (%) | 0 (0) | 2 (7.1) | 2 (5.6) | 1.000 |
| Insomnia (%) | 0 (0) | 2 (7.1) | 2 (5.6) | 1.000 |
| Hypertension (%) | 1 (12.5) | 0 (0) | 1 (2.8) | .222 |
| Other (%) | 0 (0.0) | 2 (7.1) | 2 (5.6) | 1.000 |
| Reported any adverse event (%) | 1 (12.5) | 19 (67.9) | 20 (55.6) | .012 |
4 DISCUSSION
KT remains a treatment modality for ESRD and offers benefits with regard to morbidity and mortality.20 In a large multinational cohort, approximately 10% of patients on HD were infected with HCV.21 In patients considered otherwise eligible for KT, optimal timing of HCV treatment is unknown. In our study we have compared 2 approaches to HCV eradication. Both resulted in eradication of HCV. However, deferring HCV treatment until post-KT resulted in significant decreases in transplant WLT without influencing safety or efficacy of HCV DAA-based therapy.
According to UNOS, nearly 97 000 people await KT in the United States. Approximately 19 000 received a transplant in 2016. Five-hundred kidneys are discarded annually because of HCV infection.10 Currently, transplantation of HCV-positive grafts into negative recipients is limited to controlled clinical trials. Our study highlights that there does not appear to be additional risk to the patient or graft in transplanting a HCV-positive kidney into a positive recipient. Added benefits include decreased WLT, higher transplant rate, and potentially avoidance of discarding organs that could be used in lifesaving transplantation.
Liver transplant data show that leaving patients HCV-viremic entering transplant decreases WLT and increases access.22 Data show that transplantation of HCV-positive kidneys into negative recipients decreased WLT to a median of 58 days.15, 23 Median WLT in our study was higher at 122 days. However, either strategy shortens WLT compared with national and regional averages. For comparison, at the time of our study, national WLT was 701 days, with center A’s regional WLT time at 726 days and center B’s regional WLT at 915 days. It should be noted in December 2014, which fell during the study period, WLT was redefined as initiation date of dialysis among other factors. Patients with long dialysis times were moved up the list. Time on dialysis is associated with increased risk of HCV.24 We believe this change should have affected our centers similarly given length on dialysis was not different and all patients had HCV. However, we would recommend caution in comparing the WLT regionally and nationally with our cohort. Ongoing study to determine the exact impact of leaving patients HCV viremic pre-KT is needed.
By transplanting HCV-positive kidneys into positive recipients, no patient was newly infected with HCV. The costs of infecting a naive patient with HCV are not fully elucidated. Additionally, the possibility of transmitting a resistant infection may become a reality.
Response to HCV treatment was favorable in the KT population both pre- and posttransplant. Previously reported eradication rates exceed 90%; 100% achieved SVR in our study. Given the lack of approval of sofosbuvir, the backbone of most HCV treatment regimens, in ESRD, treatment is limited to elbasvir/grazoprevir or glecaprevir/pibrentasvir. However, treatment options targeting all genotypes are expanded with improved renal function post-KT. Therapy appears well tolerated pre- and post-KT. It is unclear what drives the increase in side effects post-KT, and whether factors such as post-KT immunosuppression may play a role; reporting bias could have affected the results. Headache and fatigue were the most common reported adverse events. Headache is commonly associated with calcineurin inhibitor (CNI) use.
A risk of waiting until post-KT is that HCV therapy may be delayed, resulting in graft and liver damage due to enhanced viral replication in the setting of immunosuppression. However, median time to initiation of HCV treatment was 77 days, likely an insufficient amount of time to cause significant permanent renal or hepatic injury. With the advent of more DAA regimens with improved safety profiles and lower drug–drug interaction rates, duration from transplant to treatment further may be reduced. Data have previously demonstrated that sofosbuvir-containing regimens are largely safe and effective in the post-KT setting. HCV treatment may affect CNI levels.14 All patients in our study were on a combination of therapy with CNI, mycophenolic acid, and prednisone. Only 1 patient suffered an episode of suspected rejection while on treatment, which did not result in graft loss. Therapeutic CNI trough levels argued against an HCV-treatment induced rejection. Closer monitoring of CNI-troughs during HCV treatment is warranted.
Concern over kidney allograft quality may contribute to underutilization of HCV-positive allografts. The KDPI is used to assess allograft quality.25 The KDPI is reported as a percentage representing the graft’s risk of failure compared to other allografts. For example, a KDPI of 40% indicates that the particular allograft has a risk of graft failure greater than 40% of all allografts. A lower KDPI indicates a more optimal organ. The score is derived from several donor factors including age, height/weight, ethnicity, history of hypertension, diabetes, cause of death, serum creatinine, DCD status, and notably HCV status. High-risk organs are those with a KDPI in excess of 85%. There is bias that higher KDPI scores may result in increased likelihood of discarding an organ.26 Our study shows that median donor KDPI score is 47%. Additionally, pathologic review shows that these kidneys are of comparable quality to those that are not infected with HCV. This is likely because of the exclusion of suboptimal grafts during procurement assessment, perhaps with greater scrutiny, knowing they are exposed to HCV. It is possible that with prompt treatment posttransplant that a patient is receiving an organ that is of higher quality than what KDPI reflects, given that within a few months post-KT the allograft is rid of HCV and if KDPI were performed on identical donors would be substantially lower if HCV-negative. However, long-term outcomes with the use of HCV-positive kidneys are not as yet fully elucidated.
Our study has several strengths in that it evaluates 2 very large transplant centers with a large cohort of HCV-positive patients and compares practices between 2 otherwise similar institutions within the same health system, using identical pre-and post-KT management and immunosuppression protocols. The only notable practice divergence is timing of HCV treatment. Additionally, we report allograft pathology for HCV-positive kidneys. Limitations lie in the small overall number of patients transplanted in one center compared with the other, and retrospective nature of the study.
In conclusion, in a multicenter study between 2 nearly identical transplant centers, we show that a strategy of deferring HCV treatment in patients with ESRD until post-KT increases access to transplant and decreases WLT. HCV treatment efficacy and tolerability is similar regardless of treatment timing. Additionally, kidney allograft quality does not appear to be jeopardized by leaving patients viremic.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




