Myeloid-derived suppressor cells increase and inhibit donor-reactive T cell responses to graft intestinal epithelium in intestinal transplant patients
Abstract
Recent advances in immunosuppressive regimens have decreased acute cellular rejection (ACR) rates and improved intestinal and multivisceral transplant (ITx) recipient survival. We investigated the role of myeloid-derived suppressor cells (MDSCs) in ITx. We identified MDSCs as CD33+CD11b+ lineage(CD3/CD56/CD19)−HLA-DR−/low cells with 3 subsets, CD14−CD15− (e-MDSCs), CD14+CD15− (M-MDSCs), and CD14−CD15+ (PMN-MDSCs), in peripheral blood mononuclear cells (PBMCs) and mononuclear cells in the grafted intestinal mucosa. Total MDSC numbers increased in PBMCs after ITx; among MDSC subsets, M-MDSC numbers were maintained at a high level after 2 months post ITx. The MDSC numbers decreased in ITx recipients with ACR. MDSC numbers were positively correlated with serum interleukin (IL)-6 levels and the glucocorticoid administration index. IL-6 and methylprednisolone enhanced the differentiation of bone marrow cells to MDSCs in vitro. M-MDSCs and e-MDSCs expressed CCR1, -2, and -3; e-MDSCs and PMN-MDSCs expressed CXCR2; and intestinal grafts expressed the corresponding chemokine ligands after ITx. Of note, the percentage of MDSCs among intestinal mucosal CD45+ cells increased after ITx. A novel in vitro assay demonstrated that MDSCs suppressed donor-reactive T cell–mediated destruction of donor intestinal epithelial organoids. Taken together, our results suggest that MDSCs accumulate in the recipient PBMCs and the grafted intestinal mucosa in ITx, and may regulate ACR.
Abbreviations
-
- 7-aad
-
- 7-Amino-Actinomycin D
-
- ACR
-
- acute cellular rejection
-
- BMC
-
- bone marrow cell
-
- e-MDSC, CD14−CD15−
-
- myeloid-derived suppressor cell
-
- FSC
-
- forward scatter
-
- FSC-A
-
- FSC area
-
- FSC-H
-
- FSC height
-
- FSC-W
-
- FSC width
-
- G-CSF
-
- granulocyte colony-stimulating factor
-
- GM-CSF
-
- granulocyte/macrophage colony-stimulating factor
-
- IFN
-
- interferon
-
- IL
-
- interleukin
-
- ITx
-
- intestinal and multivisceral transplantation/transplant
-
- LPC
-
- lamina propria component
-
- MDSC
-
- myeloid-derived suppressor cell
-
- M-MDSC, CD14+CD15−
-
- myeloid-derived suppressor cell
-
- MP
-
- methylprednisolone
-
- PBMC
-
- peripheral blood mononuclear cell
-
- PMN-MDSC, CD14−CD15+
-
- myeloid-derived suppressor cell
-
- SSC
-
- side scatter
-
- SSC-A
-
- SSC area
-
- SSC-H
-
- SSC height
-
- TNF
-
- tumor necrosis factor
-
- Treg
-
- Foxp3+ regulatory CD4 T cell
-
- vs
-
- versus
1 INTRODUCTION
Intestinal and multivisceral transplantation (ITx) has evolved as the standard treatment for short bowel syndrome and gastrointestinal failure due to congenital and acquired diseases.1, 2 The intestinal allograft presents a formidable challenge for controlling the alloimmune response. Although the requirement for acute and chronic high-dose immunosuppression had resulted in high patient morbidity and mortality of the patients in the initial era of ITx, the introduction of induction therapy with lymphocyte-depleting agents, such as antithymocyte globulin and alemtuzumab, has markedly improved short-term graft survival and decreased the incidence and severity of acute cellular rejection (ACR).3, 4 It has remained unclear whether the positive effects of induction therapy are simply due to decreases in T cell and/or B cell numbers or to the appearance of regulatory components that attenuate recipient responses to the graft.5 Elucidation of these mechanisms may contribute to the development of new strategies to induce donor-specific immunological tolerance or hyporesponsiveness and improve long-term graft outcomes in ITx.
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of cells originally characterized in rodent cancer models and patients with cancer that inhibit the immune response to tumors.6, 7 MDSCs suppress both the proliferation and effector functions of T cells, B cells, and natural killer cells and induce regulatory T cells via various mechanisms.5 During chronic inflammatory conditions, MDSCs develop from immature myeloid cells and accumulate in peripheral tissues and secondary lymphoid organs.7 Factors inducing MDSC development include cyclooxygenase 2, prostaglandins, stem cell factor, macrophage colony-stimulating factor, interleukin (IL)-6, granulocyte/macrophage colony-stimulating factor (GM-CSF), and vascular endothelial growth factor.7 MDSCs play a critical role in tolerance induction in rodent transplant models, and increases in MDSCs have been reported in human renal transplant patients.8-11 However, the mechanisms promoting the development of MDSCs and their impact on alloimmune responses in clinical transplantation remain unclear. It is also unclear as to how chronic immunosuppressants administered to human transplant patients affect the differentiation of MDSCs.
We here investigated the identity and accumulation and the role of MDSCs in ITx.
2 MATERIALS AND METHODS
2.1 Patients and patient characteristics
Thirty-seven patients who underwent ITx at the Cleveland Clinic Transplant Center from 2009 to 2016 (n = 36) or at the Intestinal Rehabilitation and Transplant Center, University of Pittsburg Medical Center in 2008 and visited the Intensive Care Unit at Cleveland Clinic (n = 1) were enrolled in this study. The clinical study was approved by the regional ethic committee (IRB no. 11-966 and no. 17-471). All participating patients provided informed consent. Additional information is available in Supplementary Materials.
3 RESULTS
3.1 MDSCs increase in peripheral blood mononuclear cells (PBMCs) of patients after ITx
We first investigated whether myeloid cells expressing the MDSC phenotype were present and increased in PBMCs of patients after ITx without histopathologic evidence of ACR. The flow cytometry gating strategy excluded doublets and dead cells, and MDSCs were identified as lineage−HLADR−/lowCD33+CD11b+–expressing cells (Figure 1A), consistent with other reports.7, 10, 12-14 Within the MDSC population, 3 subsets were identified based on CD14 and CD15 expression: CD14+CD15− monocytic MDSCs (M-MDSCs), CD14−CD15+ polymorphonuclear MDSCs (PMN-MDSCs), and CD14−CD15− early-stage MDSCs (e-MDSCs) (Figure 1A).13 As reported in previous studies,12, 13, 15-17 M-MDSCs had kidney-shaped nuclei and PMN-MDSCs had lobulated nuclei; these MDSCs were relatively smaller than conventional monocytes and granulocytes, respectively (Figure 1B). In contrast, e-MDSCs contained a mixture of cells with small round, lobulated, and kidney-shaped nuclei, and the cell size was smaller than that of the other MDSC subsets (Figure 1B).
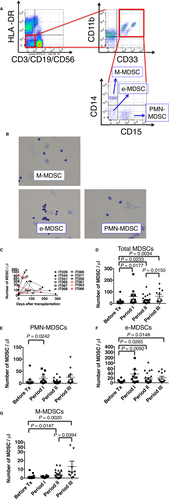
Although small numbers of cells expressing the MDSC phenotype were detected in PBMCs before transplant, the total MDSC numbers in PBMCs were significantly increased by 2 months after ITx and maintained past 1 year posttransplant (Figure 1C, D). Although levels of each of the MDSC subset were increased after ITx, they showed different patterns. Peripheral blood PMN-MDSC and e-MDSC numbers were significantly increased from pretransplant levels within 2 months after ITx (Figure 1E, F), and e-MDSC numbers were maintained throughout the study period (Figure 1F). However, M-MDSC numbers were not significantly increased until 2 months after ITx and then increased after 2 months following ITx (Figure 1G). As part of the induction therapy at the time of transplant, 32 (84%) of the 37 patients were treated with alemtuzumab (Campath-1H), a humanized anti-CD52 mAb, known to be a depleting antibody, which has rapid and long-lasting effects.18 Therefore, to investigate whether alemtuzumab affect the numbers of MDSC subsets, we assessed CD52 expression on MDSCs. We consistently observed high CD52 expression on M-MDSCs, low-absent expression on PMN-MDSCs, and heterogeneous expression on e-MDSCs across the patient samples (Figure S1). Consistent with the CD52 expression, M-MDSCs were detected 4 weeks after transplant in peripheral blood of ITx patients not treated with alemtsuzumab; however, M-MDSCs were not detected in those patients treated with alemtuzumab (Figure S2A, B). In serial samples from ITx patients treated with alemtsuzumab, M-MDSCs were detected > 4 months after ITx when other CD52+lineage(CD3, CD19, and CD56)+ cells were detected but not within a month after ITx (Figure S2C). Overall, these findings suggest that the 3 MDSC subsets increase in the peripheral blood of ITx patients after transplant and that alemtuzumab induction therapy affects the number of M-MDSCs.
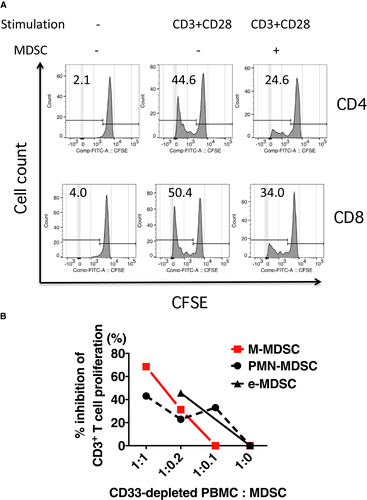
Next, the MDSC-phenotype cells isolated from the peripheral blood of ITx patients were tested for the ability to suppress T cell activation. CD33-depleted cells from pretransplant PBMC samples were used as a source of responder T cells and cultured in anti-CD3/CD28 mAb–coated wells, with autologous MDSCs enriched from posttransplant PBMC samples of the same recipient. The MDSCs inhibited both CD4 and CD8 T cell proliferation in response to anti-CD3/CD28 stimulation (Figure 2A) and interferon (IFN)γ production (Table S2). To examine the suppressive activity of each MDSC subset, each subset was sorted on a flow cytometer. Each MDSC subset suppressed T cell proliferation in a dose-dependent manner (Figure 2B).
3.2 Exogenous steroid hormone enhances expansion of MDSCs from human bone marrow cells (BMCs)
Because GM-CSF and proinflammatory cytokines, such as tumor necrosis factor (TNF)-α and IL-6, induce MDSC expansion in tumor rodent models,7 we investigated whether these factors contribute to MDSC accumulation in ITx patients and determined the plasma levels of IL-6, TNF-α, and GM-CSF and their relationship to peripheral blood MDSC numbers. Plasma IL-6 levels, but not those of TNF-α and GM-CSF, were correlated significantly with MDSC numbers in PBMCs (Figure 3A; r = 0.6888, P = .0278 vs 0.3638 and 0.1942, respectively). In addition, we investigated the correlations of MDSC numbers in PBMCs with the days after ITx, tacrolimus trough levels and administered doses of exogenous steroids per body weight, which was normalized to a value based on the potency of each steroid drug (Table S3). MDSC numbers were positively correlated with the exogenous steroid dose (P = .0013) and negatively with tacrolimus trough levels (P < .0001). In contrast to the significant increases in MDSC numbers in peripheral blood before versus after transplantation (Figure 1D), no significant correlation was observed between MDSC numbers and the days after ITx (Figure 3B).

These results led us to hypothesize that serum IL-6 and exogenous steroid induce MDSC expansion. Whereas PBMCs from healthy volunteers cultured with IL-6 and methylprednisolone (MP) did not result in MDSC expansion (Figure S3), IL-6 and MP enhanced the numbers of MDSC-phenotype cells when BMCs from healthy volunteers were cultured in basic medium supplemented with G-CSF and GM-CSF (Figure 3C, Figure S4). MP was more potent than IL-6 in expanding the MDSCs (Figure 3C), and the effect of MP was dose dependent (Figure 3D). Mifepristone, a glucocorticoid receptor antagonist, abrogated the effect of MP (Figure 3E). MDSCs generated from BMCs grown with IL-6 and MP suppressed both autologous CD4 and CD8 T cell proliferation in cultures with allogeneic B cells (Figure 3F, G) and IFNγ production (Figure 3H).
3.3 Positive correlation between MDSCs and T regulatory cells in ITx recipients
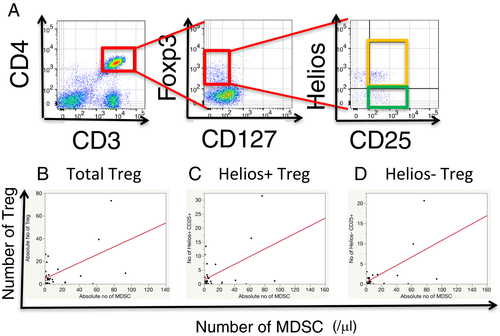
In kidney transplant recipients, MDSCs induce FoxP3+CD4+ T cell proliferation.19 Therefore, we investigated the relation between peripheral blood MDSCs and total regulatory CD3+CD4+CD25+CD127−Foxp3+ T cell (Treg) numbers (Figure 4A). In this study, we also dissected subsets of Tregs based on Helios expression, which was preferably expressed in naturally occurring regulatory CD4 T cells,20-23 and examined the relations between MDSCs and the numbers of cells in subsets of Treg (Figure 4A). The numbers of total, Helios+, and Helios− Treg cells in PBMCs of ITx patients were correlated with MDSC numbers (Figure 4B: r = 0.5584, P = .0056; 4C: r = 0.4622, P = .0263; and 4D: r = 0.6029, P = . 0023, respectively).
3.4 MDSCs increase in the allograft intestinal mucosa
To investigate whether MDSCs infiltrate into transplanted grafts, mononuclear cells from lamina propria components (LPCs) were analyzed by using flow cytometry. MDSC levels were low or undetectable in the allograft mucosa before implantation, but they significantly increased in percentage among CD45+ cells after ITx (Figure S5, Figure 5A). For each MDSC subset, the percentages of each of the 3 MDSC subsets increased in the graft biopsies in the first 12-month period after ITx; however, similar to the PBMCs of the recipients, a minimal increase in M-MDSCs within the first 2 months after ITx was evident compared with the graft infiltration of the other 2 subsets (Figure 5B-D).
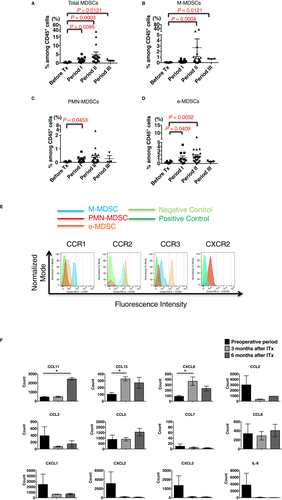
To investigate potential mechanisms directing MDSCs into transplanted intestinal grafts, comprehensive chemokine RNA levels were assessed on the NanoStrings® platform. Because distinct MDSC populations were detectable in the intestinal mucosa of grafts after ITx, we focused on chemokines upregulated after ITx. We detected 2 clusters of chemokine RNA expression levels, including CCL25, CCL15, CXCL6, CCL28, CXCL16, and CCL11, upregulated after ITx (Figure S6). Therefore, MDSC subsets in PBMCs were labeled with antibodies to the corresponding chemokine receptors, including CCR1, CCR2, CCR3, CCR7, CCR9, CXCR2, CXCR6, and β7 integrin, and analyzed by using flow cytometry (Figure 5E). M-MDSCs showed strong expression of CCR1 and CCR2 and weak expression of CCR3, e-MDSCs showed weak expression of CCR1 but strong expression of CCR2 and CCR3, and PMN-MDSCs did not express these chemokine receptors. In contrast, PMN-MDSCs and some e-MDSCs showed strong expression of CXCR2, whereas M-MDSCs did not. None of the MDSC subsets expressed CXCR6, CCR7, CCR9, or β7 integrin. Graft expression of the CCR1 and CCR3 ligand CCL15 increased approximately 3-fold at 3 months posttransplant, and this expression level was maintained through 6 months posttransplant (Figure 5F). Expression of the CCR3 ligand CCL11 was low at the time of transplant and at 3 months posttransplant; however, it was markedly increased at 6 months posttransplant (Figure 5F). In contrast, expression levels of other CCR1, CCR2, and CCR3 ligands were as follows: CCL7, CCL16, and CCL26 levels were low in allografts before and after transplant; CCL2, CCL3, and CCL23 levels were low in allografts after transplant; and there was no significant increase in the expression levels of CCL5, CCL8, CCL13, CCL14, CCL24, and CCL28 after transplant (Figure 5F, Figure S6). Graft expression of CXCR2 ligands CXCL1, CXCL2, CXCL3, and IL-8 decreased to near absent levels after transplant when tested at 3 and 6 months posttransplant. However, graft expression of the CXCR2 ligand CXCL6 was low at the time of transplant and increased by > 3-fold by 3 months posttransplant (Figure 5F, Figure S6). Expression of the IFNγ inducible chemokines CXCL9, CXCL10, and CXCL11 that direct recruitment of alloantigen-primed T cells did not increase after the transplant (Figure S6). Overall, these results support a role for CCL11 and CCL15 in directing the recruitment of CCR1- and CCR3-expressing M-MDSCs and e-MDSCs and a role for CXCL6 in directing the recruitment of CXCR2-expressing PMN-MDSCs and e-MDSCs into the intestinal grafts after transplant.
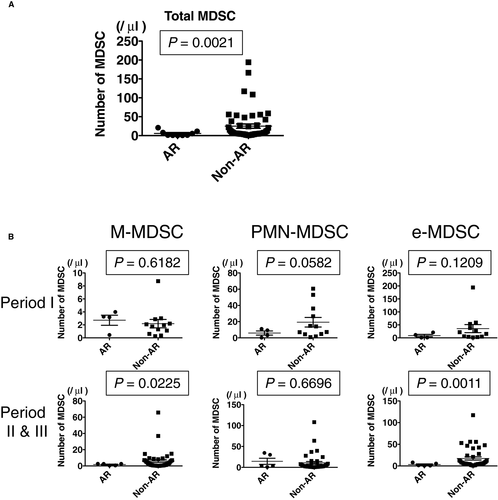
3.5 Reduced peripheral blood MDSCs during ACR
The relationship between peripheral blood MDSC numbers and occurrence of ACR was investigated in samples from 9 ITx patients with biopsy-proven ACR. The total numbers of MDSCs in PBMCs from ITx recipients with ACR was significantly lower than the numbers in patients without ACR (Figure 6A). ACR occurring within the first 2 months after transplant had no significant effect on the numbers of MDSCs in the peripheral blood; however, the numbers of PMN-MDSCs during ACR tended to be lower than the numbers in patients without ACR (Figure 6B). In addition, the numbers of M-MDSCs and e-MDSCs, but not PMN-MDSCs, significantly decreased in PBMCs from ITx patients experiencing ACR 2 months or more after transplant compared with the numbers in patients without ACR (Figure 6B). In terms of clinical characteristics and demographics of the ITx patients, while de novo DSAs were detected at higher rates in patients with ACR than in patients without ACR, there was no significant correlation between patients with ACR and without ACR in the other factors (Table S4).
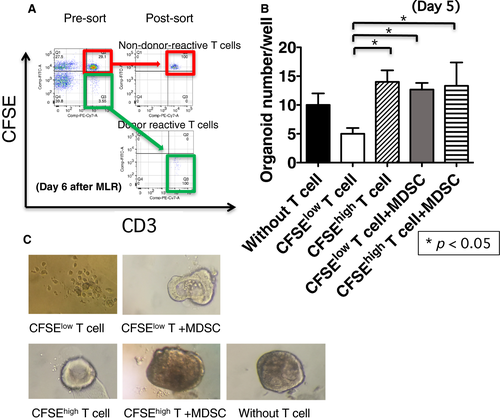
3.6 MDSCs suppress donor-specific T cell–mediated damage of intestinal epithelial organoids
A critical histologic criterion for the diagnosis of ACR is > 6 apoptotic bodies/10 sequential crypts,24, 25 which is proposed to reflect donor-reactive T cell–mediated epithelial damage.26 To directly investigate the role of MDSCs in ACR, we established an ex vivo coculture system, called “organoid formation assay,” using donor-reactive T cells and intestinal epithelial organoids from donor intestinal grafts. Donor-reactive T cells (CFSElow T cells) and non–donor-reactive T cells (CFSEhigh T cells) were sorted after 6 days of coculture with irradiated donor B cells (Figure 7A). Subsequently, epithelial organoids derived from donor intestinal grafts, were cocultured with CFSElow T cells or CFSEhigh T cells in the presence or absence of the sorted recipient MDSCs for 5 days. The number of viable epithelial organoids was significantly less in the presence of donor-reactive T cells compared with that in the presence of non–donor-reactive T cells (Figure 7B, C), suggesting that the epithelial damage was mediated by donor-specific T cell response. It is noteworthy that the number of viable organoids in the cocultures with MDSCs and donor-reactive T cells was comparable to that of organoids without T cells, suggesting that MDSCs suppress the damage to organoids by donor-reactive T cells (Figure 7B, C). These findings suggest that accumulated MDSCs suppress donor-reactive T cell response to the donor intestinal epithelium.
4 DISCUSSION
In this study, we first demonstrated the key features of MDSCs in clinical ITx: (1) the 3 distinct populations of MDSCs began to increase in the recipient peripheral blood early after ITx, and each subpopulation had the ability to suppress T cell activation. (2) Although PMN-MDSC numbers were increased within 2 months after ITx, M-MDSC and e-MDSC numbers were increased and maintained at high levels in PBMCs after 2 months after ITx; the differences in the number of each MDSC subset during the early period after ITx could be affected by the administration of alemtuzumab, as M-MDSCs, but not PMN-MDSCs, expressed CD52 and M-MDSCs were rarely detected in PBMCs of patients, who received alemtuzumab, during the early period after ITx. (3) The number of MDSCs was significantly correlated with plasma IL-6 levels and administered exogenous glucocorticoid doses, and MP markedly enhanced propagation of MDSCs in BMC cultures. (4) The numbers of Treg cells, including both inducible and naturally occurring Treg cells, was significantly correlated with MDSC numbers. (5) The frequency of MDSCs among CD45+ cells was increased in the intestinal allograft mucosa; the percentages of PMN-MDSCs and e-MDSCs were increased during the first 2 months after ITx, and those of M-MDSCs and e-MDSCs were increased thereafter. In addition, these graft-infiltrating MDSCs expressed chemokine receptors responsive to chemokines expressed by the allograft mucosa. (6) ACR episodes were accompanied by decreases in MDSC levels. (7) Using a novel ex vivo ACR assay, MDSCs suppressed the ability of donor-specific T cells to mediate donor intestinal epithelial organoid damage. Overall, these results suggest that exogenous glucocorticoid promote MDSC differentiation from BMCs and their accumulation in the recipient peripheral blood and donor graft intestinal mucosa to regulate the characteristic T cell–mediated destruction of the graft epithelium. Among MDSC subpopulations, M-MDSCs may play more important roles in control of ACR of intestinal grafts, especially in the late period after ITx, than the others.
Numerous factors that contribute to the differentiation and expansion of MDSCs have been reported.7, 27-30 The MDSCs may have an almost entirely recipient-derived origin, as macrochimerism of myeloid cells is hard to detect in intestinal transplantation,31, 32 which we also confirmed in some of the recipients (data not shown). In this study, we demonstrated that MP expand MDSCs from human BMCs but not from PBMCs. Notably, previous rodent models have demonstrated that glucocorticoids enhance the accumulation of MDSCs33, 34; however, this study is the first to demonstrate that glucocorticoids enhance the expansion of human MDSCs in a dose-dependent manner by glucocorticoid receptor signaling in BMC cultures. Maximal numbers of MDSCs in the BMC culture were observed with treatment of 1 μmol/L MP (Figure 3D), which corresponds to the peak level in a 70-kg patient given an intravenous dose of 50 mg of MP (0.756 mg/kg) or to the 24-hour trough level following a 30-mg/kg intravenous dose.35, 36 The latter dose is similar to that used in MP-pulse treatment in ITx. Moreover, on day 3 after MP-pulse treatment for ACR in an ITx patient, the number of MDSCs in PBMCs increased to 6 times the levels observed before treatment (data not shown). Therefore, the use of glucocorticoid drugs to maintain immunosuppression and treat ACR may drive MDSC accumulation in patients after ITx, in addition to the known effects, such as downregulation of effector molecules and induction of effector cell apoptosis, especially in T cells.36 In terms of calcineurin inhibitors, although the trough levels were negatively correlated with MDSC numbers, addition of FK506 to the BMC culture did not affect the numbers of MDSCs (Figure S7). Wang et al reported that a calcineurin inhibitor, cyclosporine, enhanced infiltration of MDSCs in skin grafts and apoptosis,37 so inhibition of the calcineurin–nuclear factor of activated T cells (NFAT) axis may promote MDSC migration toward intestinal grafts rather than MDSC differentiation from bone marrow stem cells, resulting in decreased numbers of MDSCs in PBMCs.
MDSCs migrate not only to secondary lymphoid organs but also to effector sites, such as transplanted grafts and tumors, creating an immunosuppressive environment in rodent models.7, 9, 14, 38-40 This MDSC migratory potential is essential for tolerance induction in a heart transplantation model.9 While there is growing evidence for chemokine signaling in MDSC recruitment to tumor sites,40 the mechanisms of MDSC migration in infection, autoimmune disease, and transplantation remain unclear. Recently, Pallett et al reported that PMN-MDSCs potentially use signaling through CCR2, CXCR1, and CXCR3 to infiltrate liver tissue after hepatitis B virus infection to regulate metabolism in the liver, although they did not determine the expression of the respective chemokine ligands in the liver tissue during this recruitment.41 We demonstrated that all MDSC subsets were detected in intestinal grafts after transplantation and that the percentages of MDSCs among CD45+ cells significantly increased compared to those in pretransplant grafts. This study is the first to demonstrate accumulation of MDSCs in the transplanted organ of human patients. In intestinal graft protocol biopsies, etiology-unknown neutrophilic infiltration in the intestinal mucosa of transplanted grafts is often observed, and the infiltrating neutrophil-like cells are smaller than conventional neutrophils. Our results imply that infiltrating PMN-MDSCs may be detected as neutrophilic infiltration in some cases. Of note, unique chemokine ligand profiles were observed in intestinal grafts after ITx (Figure 5F) and that MDSCs expressed inflammatory chemokine receptors binding these mediators, including CCR1, CCR2, CCR3, and CXCR2 (Figure 5E).
Thus far, it has been unclear whether MDSCs regulate immune responses within effector sites. The lack of clinically relevant model systems for investigation in vivo immune responses is a major obstacle toward understanding MDSC function in peripheral nonlymphoid organs in transplantation. Our findings that MDSC levels increase in PBMCs and intestinal grafts in patients without rejection coupled with the low number of MDSCs observed during ACR led us to hypothesize that MDSCs may suppress donor-reactive T cell responses to intestinal grafts. The recent development of new 3-dimensional organoid culture methods42-46 allows us to evaluate MDSC function in the transplanted intestinal mucosa. Consistent with our initial hypothesis, our ex vivo rejection model revealed that MDSCs suppressed donor-reactive T cell–mediated damages of donor-derived intestinal epithelial organoids. Our findings suggest that balance between donor-reactive T cell responses and their suppression by MDSCs may affect the occurrence of ACR; during ACR, MDSC activities may be overwhelmed by donor-reactive T cell responses, or decreased number of MDSCs may allow donor-reactive T cells to induce ACR. This hypothesis warrants further study to determine the molecular mechanisms underlying MDSC-mediated immunomodulation within ITx grafts. Furthermore, it will be of interest to investigate the role of other components, such as stromal cells, secreted molecules, or extracellular matrix, that may affect MDSC function.
Our study has certain methodologic limitations. Although monocytes and M-MDSCs can be distinguished on the basis of expression of MHC class II molecules, there remains difficulty in separating PMN-MDSCs from neutrophils based on surface markers. Therefore, density separation or functional studies are needed to distinguish between PMN-MDSCs and neutrophils.12, 17 Although we obtained PBMCs using Ficoll-separation medium, small numbers of mononuclear cells from biopsy samples prevented us from performing the density separation. It was also difficult to obtain sufficient quantities of peripheral PBMCs and graft-infiltrating cells of ITx patients to investigate MDSC functions in multiple replicate of samples or in all the patients, as the patients were usually subjected to heavy immunosuppressive conditions, especially during the early time period after transplantation. Next, the sample size is relatively small, and we need to confirm our findings using a multicenter study. Finally, it was also difficult to investigate the spatiotemporal distribution of MDSCs in the body of each patient. Rodent intestinal transplantation models may be useful to identify critical chemokines and chemokine receptors in future experiments.
Finally, follow-up histologic studies of graft biopsies from ITx patients without clinically evident ACR demonstrated variable levels of apoptosis, epithelial cell regeneration, and mononuclear cell infiltration. Therefore, it is expected that subclinical ACR responses may intermittently or continuously emerge even after induction therapy for ITx. MDSCs may be crucial in controlling the subclinical ACR and preventing the occurrence of clinically evident ACR within the graft mucosa through mechanisms facilitating MDSC differentiation from BMCs and MDSC recruitment to intestinal grafts via distinct chemokine ligand–chemokine receptor interactions in ITx. Garcia et al suggest that MDSCs play a critical role in suppressing the T cell response to donor antigens in the graft sites before the establishment of immunologic tolerance to donor grafts.9 In ITx, donor-specific T cell hyporesponsiveness is frequently observed in intestinal transplant patients without rejection after a long lapse of ITx with induction therapy (data not shown).47 Overall, our findings support the hypothesis that MDSCs play an important role in bridging to donor-specific T cell hyporesponsive states in clinical ITx, as observed in rodent models,9 and provide strong evidence that MDSCs can function to suppress pathogenic T cell responses to the epithelium in the context of ITx.
ACKNOWLEDGMENTS
We thank Mansour A Parsi, MD, Gregory Zuccaro Jr, MD, Menon K.V. Narayanan, MD, Ibrahim Hanouneh, Robert S.O. Shea, Amit Bhatt, MD, Abdullah S. Shatnawei, MD, Bradley D. Confer, MD, Carlos Romero-Marrero, MD, Prashanthi Thota, MD, and all the staff in the Department of Gastroenterology and Hepatology for sampling of intestinal biopsies. We thank Ana Bennett, MD, Lisa Yerian, MD, Deepa Patil, MD, and all the gastrointestinal pathology staff at the Department of Pathology. We thank Bookie Min, DVM, PhD, and Jeong-su Do, PhD, for assistance with flow cytometry. We thank Nina Dvorina, MD, BS, for technical assistance with Giemsa staining and Kewal Asosingh, PhD, Joseph Gerow, BS, and Eric Schultz, BS, for assistance with analysis of flow cytometry and flow sorting in the flow cytometry core. We thank Raw Lisa, MSN, Natasha Raush, BSN, Christine Wagner, RNC, Shatina Roddy, RN, Cindy Shovary, RN, BSN, Anita Barowski, RN, MS, Neha Parekh, MS, RD, LD, CNSC, Suzanne Himes, RN, BSN, and Elizabeth Lennon, MS, PA-C, RD, for assistance as transplant coordinators and mid-level practitioners. We thank Daniel Zwick and Sreedevi Goparaju for proofreading and editing of the figures, respectively. This work was supported by a grant from the Research Program Committee, Cleveland Clinic RPC 2011-1015 (K.H.) and NIH R01-AI40459 (R.L.F).
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose, as described by the American Journal of Transplantation.




