Donor pretreatment with nebulized complement C3a receptor antagonist mitigates brain-death induced immunological injury post–lung transplant
Abstract
Donor brain death (BD) is an inherent part of lung transplantation (LTx) and a key contributor to ischemia-reperfusion injury (IRI). Complement activation occurs as a consequence of BD in other solid organ Tx and exacerbates IRI, but the role of complement in LTx has not been investigated. Here, we investigate the utility of delivering nebulized C3a receptor antagonist (C3aRA) pretransplant to BD donor lungs in order to reduce post-LTx IRI. BD was induced in Balb/c donors, and lungs nebulized with C3aRA or vehicle 30 minutes prior to lung procurement. Lungs were then cold stored for 18 hours before transplantation into C57Bl/6 recipients. Donor lungs from living donors (LD) were removed and similarly stored. At 6 hours and 5 days post-LTx, recipients of BD donor lungs had exacerbated IRI and acute rejection (AR), respectively, compared to recipients receiving LD lungs, as determined by increased histopathological injury, immune cells, and cytokine levels. A single pretransplant nebulized dose of C3aRA to the donor significantly reduced IRI as compared to vehicle-treated BD donors, and returned IRI and AR grades to that seen following LD LTx. These data demonstrate a role for complement inhibition in the amelioration of IRI post-LTx in the context of donor BD.
Abbreviations
-
- AR
-
- acute rejection
-
- BD
-
- brain death
-
- C3aRA
-
- C3a receptor antagonist
-
- C3aR
-
- C3a receptor
-
- C-Inb
-
- complement inhibition
-
- CS
-
- cold storage
-
- DAMPs
-
- damage associated molecular patterns
-
- IRI
-
- ischemia-reperfusion injury
-
- LD
-
- living donors
-
- LTx
-
- lung transplantation
1 INTRODUCTION
Brain dead (BD) donors represent the main source of organs for lung transplantation. Brain death, especially traumatic brain death, has been shown to induce hemodynamic, metabolic, inflammatory, and immunologic derangements, all of which negatively affect peripheral organs.1-6 The lungs are no exception and, considering the prolonged mechanical ventilation which accompanies BD, they are particularly sensitive to this array of insults.7 As a consequence of these insults, only 10-30% of available solid organ donors have lungs acceptable for transplantation,8 which significantly reduces the available donor organ pool. Therefore, a better understanding of the mechanisms induced by BD that injure and prime the donor lung posttransplantation is urgently needed.
While a number of immune mechanisms have been shown to stimulate donor organ injury and predispose grafts to poorer outcomes, activation of the complement system has been shown to play a central role in BD-associated graft injury.9-11 In preclinical and clinical studies of both heart and kidney transplantation, grafts from BD donors were not only associated with more severe ischemia-reperfusion injury (IRI), but posttransplant pathology was also shown to significantly correlate to the level of complement activation.4, 10-13
Given these findings, inhibition of the complement cascade has been studied in the context of BD and transplantation,10, 14-17 and it has been shown that donor therapy or graft-targeted inhibition improves posttransplant outcomes.10, 17 To date, very little is known regarding the role of BD-induced lung injury in LTx and nothing is known in the context of complement activation. A handful of studies have explored the potential for complement inhibition (C-Inb) in LTx exclusive of BD,18-22 showing beneficial impacts on early outcomes and the development of obliterative bronchiolitis when complement inhibitory strategies are administered systemically.22, 23 The lung, unlike other solid organ transplants, is open to the environment, which has two specific implications: (1) it lends itself to localized focused drug delivery via nebulization, and (2) it is constantly exposed to environmental irritants and potential pathogens. Given the latter, systemic or localized C-Inh in the early posttransplant period may well have unique challenges due to the potential for increased risk of infection.
To this end, we sought to study the role of complement in BD and LTx IRI, specifically focusing on C3a, a split-product of the central complement protein C3. C3a, via its interaction with C3a receptor (C3aR), is a potent anaphylatoxin that can drive and enhance both innate and adaptive immune responses.24, 25 We focused on C3a because inhibiting its functions would decrease inflammation while maintaining complement-dependent pulmonary pathogen defense mechanisms, such as complement opsonization.26 Here we show that BD activates an inflammatory response and the complement system, which worsened post–LTx outcomes. Furthermore, therapeutic blockade of C3aR ameliorated BD-associated IRI posttransplantation when delivered directly to the BD donor lung prior to transplantation.
2 METHODS
2.1 Animals
Eight- to 12-week-old BALB/c and C57BL/6 mice weighing 25-30 g were purchased from Charles River Laboratories. Animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences. Three study groups were utilized: (1) living donor control; (2) vehicle-nebulized BD donors; and (3) C3aR antagonist (C3aRA) (Cal Biochem, Darmstadt, Germany) nebulized brain-dead donors (1 mg/mL). Each donor was either nebulized with C3aRA reconstituted in PBS:DMSO solution at a dose of 1 μL/g body weight (n = 6 all groups), which was extrapolated from our previous studies,26, 27 or PBS:DMSO vehicle.
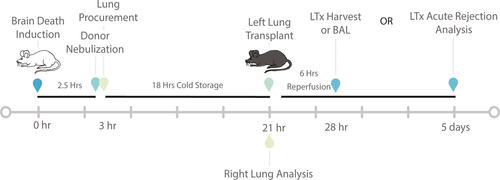
2.2 Rodent surgical procedure
BD was induced in donor mice with a Fogarty catheter balloon as previously described.10 Donor animals with refractory hypotensive episodes, defined as mean arterial pressure <50 mmHg for a duration of longer than 20 mins, were excluded from the studies, as we have previously described.11 Nebulization was performed on BD donor mice 30 minutes prior to harvest using an in-line nebulizer (emka Technologies, Falls Church, VA) attached to the inspiratory tubing of the ventilator (Physiosuite, Kent Scientific, Torrington, CT). Donor left lungs were procured as previously described28, 29 and stored for 18 hours in Perfadex (XVIVO Perfusion Inc., Englewood, CO) at 4°C prior. Donor left lungs were then prepared for transplantation and left orthotopic vascularized lung transplants performed utilizing cuff techniques into C57Bl/6 recipient mice, as previously described.28, 30 Allografts were harvested at 6 hours or 5 days post-LTx for analysis. To determine the degree of donor lung injury at the end of 18 hours of cold storage, lungs were flushed with 1 ml of normal saline, and perfusates assayed for lactate dehydrogenase (Biovision, Milpitas, CA) and HMGB1 (LifeSpan Biosciences, Inc., Seattle, WA) levels via ELISA, according to the manufacturers’ recommendation.
2.3 Lung function
Dual-chamber whole-body plethysmography was performed as described elsewhere31 (emka Technologies). The differential pressure was acquired by an acquisition amplifier at 1 kHz (usbAMP, emka Technologies) and processed in analysis software (IOX Base 2c, IOX 1 PULMO 2c, emka Technologies). Enhanced pause (Penh) was used as a pulmonary function test as it represents a good measure of airway resistance. In the context of whole body, restrained, flow plethysmography (as was used here), Penh has been shown to be an adequate quantitative measure of resistance, particularly when pathology is utilized to corroborate measurements.32, 33
2.4 Histology
Lungs were inflated en-bloc with 10% buffered formaldehyde at either 6 hours or 5 days post-LTx and subsequently paraffin processed, sectioned and stained. Injury was graded in pre- and posttransplant ischemia reperfusion lungs as previously described.34-36 For rejection studies, sections were scored by a lung pathologist (MG) blinded to the experimental conditions using standard criteria (International Society for Heart and Lung Transplantation [ISHLT] A Grade).37
2.5 Immunohistochemistry
Sections were immunostained for complement C3d (BD Pharmingen, San Jose, CA), C3a receptor (Thermofisher, Waltham, MA), neutrophils (NIMP-R14; Abcam, Cambridge, UK) and macrophages (MAC-3; BD Pharmingen) using ImmPress visualization (Vector Laboratories, Burlingame, CA) as previously described.10 All staining was evaluated in a blinded fashion (CA and MG). Controls consisted of isotype controls and omission of primary antibodies.
2.6 Quantitative RT-PCR
Total RNA from lung tissue homogenate was isolated using a RNeasy MiniKit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. cDNA was synthesized using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) and mRNA expression of target genes TNF-α, IL-1β, TGF-β, MCP1 (CCL2), MIP2α (CXCL2), C3, C5, and C3a receptor were determined using MasterCycler RealPlex2 PCR System (Eppendorf, Hamburg, Germany). Primers used are listed in Table S1.
2.7 Bronchoalveolar Lavage (BAL)
Left lung BAL was performed at 6 hours posttransplantation by instilling 3-0.5mL aliquots of ice cold PBS with intermittent aspiration. Fluid was centrifuged, supernatant collected, and ELISA analysis for of albumin (Genway Biotech, San Diego, CA) and HMGB1 performed per manufacturer instructions.
2.8 Statistics
GraphPad Prism version 7.0 for Mac OS X (GraphPad, San Diego, CA) was used for statistical analysis. Except where indicated, differences between various groups were compared by use of one-way ANOVA with the Dunnett's multiple-comparisons test for post hoc analyses. Pairwise comparisons were made to analyze the key questions of the effect of BD on LTx outcomes, and the effect of C3aRA on BD-mediated phenomena. Specifically, comparisons were made between LD and Sham BD pre–cold ischemia, LD and BD pre–cold ischemia, LD and BD post–cold ischemia and again post-LTx, and BD and C3aRA post–cold ischemia and again post-LTx. For histological injury scores, the Kruskal-Wallis test was used, followed by the Dunn multiple-comparisons test for post hoc analyses. P < .05 were considered significant. Values shown are mean ± SEM.
3 RESULTS
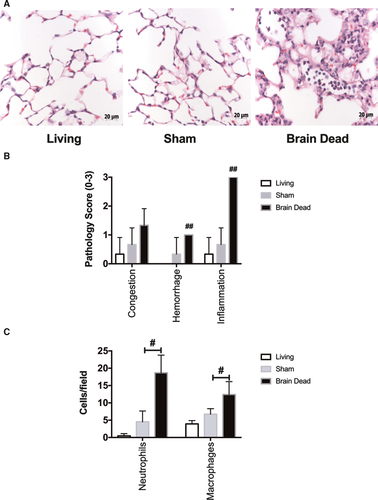
3.1 BD induces donor lung injury
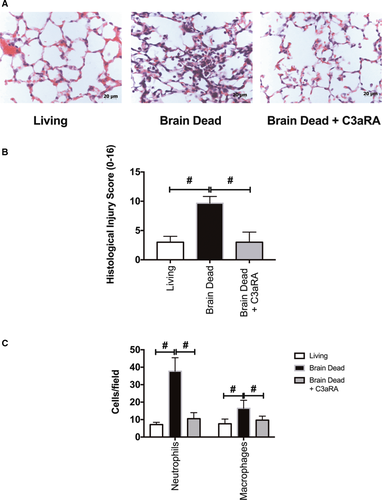
In keeping with previous studies, 3 hours of donor maintenance post–BD induction led to significant donor lung injury.34 BD donor lungs demonstrated increased pathologic injury scores based on histologic analysis when compared to sham and LD lungs (Figure 1A-B). Brain death injury was associated with a significant increase in infiltration of both neutrophils and macrophages in BD lungs compared to LD lungs, as determined by quantification of immunohistochemistry staining (Figure 1C).
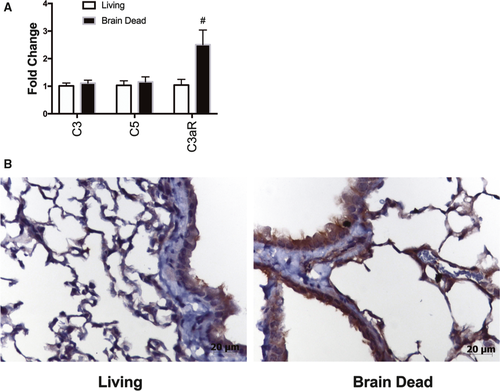
Previous studies have demonstrated that the injurious impact of BD leads to upregulation of complement gene transcription and complement activation in donor organs, as well as increased complement deposition.4, 10 Here we analyzed donor lung complement gene expression using qRT-PCR 3 hours post–BD induction. mRNA levels of the central complement components C3 and C5 were unchanged in the lung in response to BD; however, expression of complement receptor C3aR, a central mediator in complement-driven inflammation,38 was significantly elevated in BD lungs (2.5-fold increase; P < .005; Figure 2A). Consistent with elevated mRNA expression of C3aR, immunohistochemical staining showed increased C3aR protein expression in bronchial and alveolar epithelial cells, and endothelium in BD lungs versus LD (Figure 2B), although no significant difference in C3d was noted (data not shown). Taken together, these findings demonstrate that BD induces donor lung injury, increased immune cell infiltration, and increased C3aR expression.
3.2 Histological changes and inflammatory markers associated with BD induction were not exacerbated during the cold storage period
In vivo LTx studies were carried out as depicted in Figure 3 in order to investigate the impact of BD on allograft IRI. Prior to transplantation, BD and LD donor lungs were harvested and intravascularly flushed with, and subsequently stored in, Perfadex for 18 hours at 4°C. To assess the cumulative injuries incurred by the donor organs prior to transplantation, histologic analysis was performed after the cold storage period. After 18 hours of cold storage (CS), the profiles of injury and inflammation that were seen immediately postprocurement were still present in BD lungs versus LD (Figure 4A). Pathologic scoring of hemorrhage and inflammation in BD lungs was significantly worse than that seen LD lungs (Figure 4B).
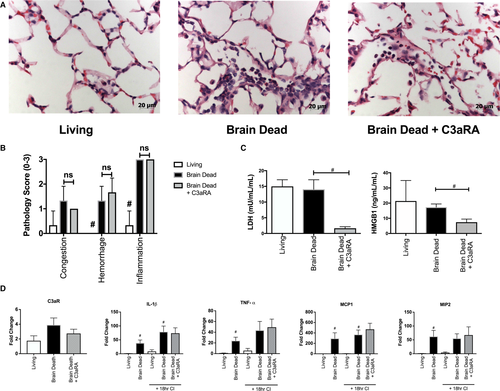
Given our and other's previous findings that BD-induced injury is associated with dysregulation of the complement system and considering the up-regulation of C3aR expression we observed in BD lungs, we investigated whether inhibition of C3a signaling, through nebulization of a C3aR antagonist (C3aRA, 1 mg/mL) delivered to BD donors 30 minutes prior to organ procurement (2.5 hours after BD induction), would improve posttransplant outcomes. When examined at the end of CS, donor lung pretreatment with C3aRA had no impact on lung histopathology (Figure 4A and B) or on gene expression levels of C3aR (Figure 4D).
To determine the impact of BD, cold storage, and C3aRA therapy on cell injury during storage, we assayed lung perfusate for lactate dehydrogenase (LDH) and high-mobility group box-1 (HMGB1) protein. These are markers of cell injury and necrosis that serve well-described roles as damage associated molecular patterns (DAMPs) in the context of brain injury and LTx (particularly HMGB1).39-41 No significant difference in LD versus BD was noted (Figure 4C). However, C3aRA significantly decreased LDH and HMGB1 levels as compared to untreated BD donor lungs (Figure 4C).
Proinflammatory gene expression was examined via qRT-PCR (Figure 4D), and we found a significant increase in proinflammatory gene expression in BD lungs, both pre-CS and post-CS, compared to their respective LD comparators. Specifically, pre-CS, IL-1β, TNF-α, MCP1, and MIP2 were all elevated versus LD (Figure 4D). After 18 hours CS, IL-1β, and MCP1 remained significantly elevated in BD lungs (Figure 4D). Lungs exposed to 18 hours of CS were insignificantly different from their corresponding pre-CS groups. Similar to pathologic injury scoring of these lungs, C3aRA treatment of BD lungs had no effect on inflammatory cytokine expression.
3.3 C3aRA abrogates early post-LTx ischemia-reperfusion injury and inflammation associated with donor BD
Given the susceptibility and sensitivity of lungs to injury, as well as the documented short- and long-term impacts of IRI on allograft outcomes, we procured and analyzed lungs at the early post-LTx time point of 6 hours. Our results, as described above, indicated a significant amount of injury and inflammation associated with BD and CS, therefore we investigated this early time point in an effort to dissect the full effect of these peri-transplant insults on the immediate post-LTx allograft.
Lungs from Balb/c living, BD and BD C3aRA treated donors were transplanted into C57Bl6 recipients. At 6 hours post-LTx, donor BD was associated with significantly worse allograft injury, as determined by histological analysis (Figure 5A-B). Additionally, post-LTx immunohistochemical staining for neutrophils and macrophages revealed significantly increased infiltration of both inflammatory cell types in the BD group (Figure 5C). When C3aRA treatment was delivered to BD donors prior to organ retrieval, the post-LTx injury profile was significantly altered. Treatment decreased histopathological injury and significantly reduced neutrophil and macrophage infiltration (Figure 5A-C). To further investigate IRI-induced damage in our model, BAL fluid was analyzed for albumin content and HMGB1 as markers of cellular injury. Despite documented differences in injury and inflammation by histology, no difference was observed in either total protein (not shown), albumin content, or HMGB1 (Figure 6A) between the LD and BD groups, or between the BD and C3aRA groups.
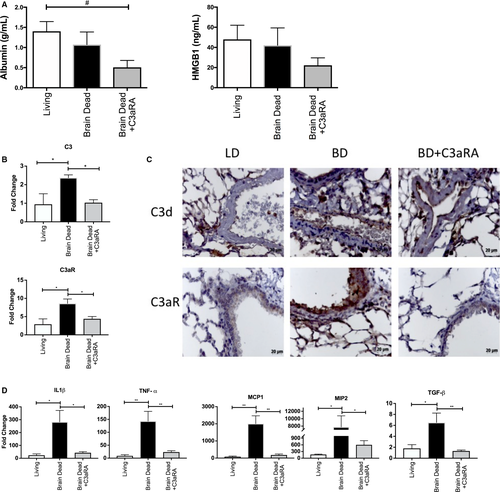
In terms of complement activation, we showed that BD exacerbates C3d deposition when compared to LD, and that C3aRA treatment has no impact on C3d deposition, with an expression pattern and intensity not dissimilar to the BD group (Figure 6B). While C3aRA treatment had no impact on C3d deposition at this early time point, a reduction in C3aR protein expression (Figure 6B) and reduced gene expression of C3, C5, and C3aR posttransplantation (Figure 6C) were seen. In fact, treatment restored complement protein and receptor transcription levels back to that seen in LD lungs (Figure 6C).
Finally, we assessed the impact of BD and C3aRA donor therapy on proinflammatory gene expression post-LTx. TNF-α, IL-1β, MIP2, and MCP1 were all significantly elevated in the BD group versus both the LD and the C3aRA groups (Figure 6D). This pattern was also found for TGF-β expression.
3.4 Donor brain death significantly exacerbates lung allograft rejection
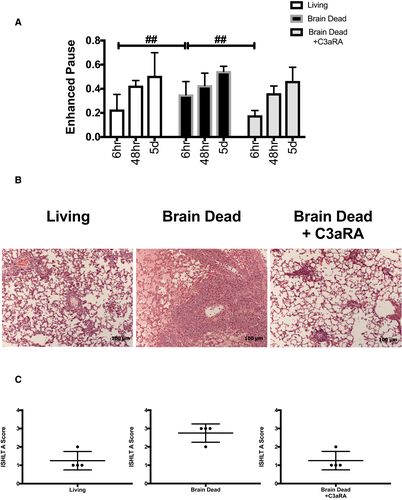
To assess the impact of BD in LTx on acute rejection (AR) and determine the therapeutic potential of BD lung targeted C3aRA in a clinically relevant setting, we performed allograft transplants. The allograft pairing utilized in these studies generates robust grade 3-4 AR at 7 days post-LTx,42 and therefore here given that we hypothesize BD will exacerbate AR, we harvested allografts at 5 days post-LTx. To confirm allograft functionality, we performed serial noninvasive lung function measurements of enhanced pause (Penh) to assess our transplanted lungs at 6 hours, 48 hours, and 5 days posttransplantation. The use of noninvasive lung function allowed for minimal manipulation of mice and repeated measurements prior to tissue harvest and analysis at 5 days. Pretransplant Penh baseline was not significantly different among the three recipient groups. Analysis at the early time point, 6 hours, demonstrated a significant increase in Penh in recipients of BD lungs as compared to recipients of living donor or BD C3aRA treated lungs (Figure 7A), in keeping with our histopathological and inflammatory readouts. Analysis at 48 hours and 5 days demonstrated no significant differences in lung function (Figure 7A). In concordance with the ischemia-reperfusion data above, BD was associated with significant exacerbation of rejection as compared to recipients of living or BD C3aRA treated donor lungs (Figure 7B and C). Pairwise comparisons demonstrated that rejection was significantly reduced among each group when compared to the BD group (P < .01 for all). No significant difference was seen between recipients of living or BD C3aRA donor lungs (Figure 7C).
4 DISCUSSION
Donor organ injury induced by BD and IRI are considered to be important factors in delayed graft function and the accelerated onset of graft rejection following transplantation. Here, we investigated the role of C3a signaling in BD-associated IRI of transplanted lungs. Using our recently described murine model of BD, we show, in accordance with a previous study,35 that donor BD enhances lung injury and inflammation, and additionally show for the first time that BD induces the up-regulation of C3aR and exacerbates posttransplant lung IRI. Further we demonstrate that donor lung nebulized pretreatment with C3aRA 30 minutes before procurement reduces lung injury and inflammation 6 hours posttransplantation, returning the lung to a state more in keeping with a lung transplanted from a living donor lung. Furthermore, we demonstrate that protection at this early time point translates to a reduction in the severity of acute rejection.
Human and animal studies, mainly in heart and kidney transplantation, have demonstrated a multitude of injurious processes and pathways activated by BD.34, 39, 43 However, despite these studies, the BD model has largely been underutilized in LTx studies. Studies in rodents confirm our findings that BD induces the development of a proinflammatory state44 that has the potential to adversely affect posttransplant outcomes. As a result, the mechanisms that contribute to donor organ injury, how these pretransplant injuries modulate posttransplant outcomes, and more importantly how these insults can be combated have been underinvestigated.
In our lung BD studies, we corroborated previous findings that BD is associated with a time dependent increase in pulmonary inflammation, particularly neutrophilia, and endothelial activation.34 Studies have shown that BD induces complement activation in donor hearts and kidneys, increases systemic complement activity, and exacerbates posttransplant IRI and rejection.4, 10, 15 In contrast, we did not detect any differences in C3d or complement gene expression between LD and BD donor lungs, albeit our analysis was at an earlier time point. However, in accordance with previous heart and kidney transplant studies, we did demonstrate a significant increase in C3d deposition and C3 gene transcription post-LTx in recipients of BD donor organs.
Complement proteins are largely regarded as systemically originating proteins, but there is a growing appreciation that complement produced within the local microenvironment may affect pathological outcomes. Therefore, the differences in complement deposition and time to expression may be organ specific and related to local organ synthesis. Interestingly, while we observed no difference in C3/C5 deposition or gene transcription between LD and BD, we noted a significant elevation in C3aR expression. Traditionally, C3aR has been identified on macrophages, dendritic cells, epithelial and endothelial cells.37,45–47 C3a signaling is associated with the production of proinflammatory cytokines, activation of innate immune cells, promotion of immune cell infiltration, and priming of adaptive immune responses. Given these mechanistic pathways, we hypothesized that protection from C3a mediated lung inflammation via the pretransplant nebulization of a C3aR competitive inhibitor (C3aRA) would ameliorate injury and inflammation. C3aRA has been shown to have therapeutic potential in a variety of disease models, such as IRI,48, 49 immune complex diseases,50,51 and experimental lung disease.47,52 The nebulization of C3aRA for administration served multiple purposes. First and foremost, it enabled us to direct therapy exclusively to the lung without impacting other organ systems. Secondly, it directed therapy to the epithelial compartment and alveolar macrophages, which are key players in the ischemic induced proinflammatory signaling cascade. Thirdly, given the ease and ubiquity of nebulization in hospitals around the world, the accessibility and translatability of these studies becomes immediately relevant.
While a lung targeted delivery strategy was efficacious, we acknowledge that this is not the only possible route of application of C3aRA therapy to the donor lung. For example, C3aRA could be added to the perfusion/storage solution, although this would likely only impact the vascular compartment and would require the antagonist to function at cold temperatures. Cold storage by design reduces cellular metabolism to less than 5%,53 and thus organ pretreatment using therapeutics added to the perfusate may be limited by reduced metabolic uptake or reduced activity of downstream mechanisms and cellular gene machinery required for drug functionality. Normothermic delivery, as used here not only delivers C3aRA directly to the lung during active ventilation, but also provides time for “organ treatment” at physiological temperatures, fluid environment, and aeration, and is an approach that has been explored with success by others.54-56
To remain consistent with the relevant clinical scenario, we incorporated cold storage in addition to BD in our paradigm. C3aRA donor therapy just prior to lung harvest had no impact on lung histopathology, injury scores, or proinflammatory gene transcription at the time of donor lung explant. However, following transplantation, C3aRA treatment appeared to reverse the detrimental changes associated with BD. The transplantation of BD lungs, in keeping with other BD organs,1,10,17,35 was associated with increased lung injury, inflammation, complement deposition, proinflammatory gene expression, and increased airway resistance as compared to LD, while treatment of BD donors with C3aRA returned donor lungs to a profile more in keeping with LD injury profiles. These trends were present not only in the early post-operative time period generally associated with IRI, but also translated into improved acute rejection pathology in the BD C3aRA versus BD alone.
We and others have previously demonstrated a similar phenomenon in heart and kidney transplant models, where systemic or graft targeted C-Inh improved BD organ graft survival.10,17 The use of C3aRA, as opposed to broad spectrum C3 inhibitors used by others, enables C3 opsonic functions to remain intact, and accordingly we show no significant difference in C3d deposition between BD and BD C3aRA groups. Further, our previous studies have shown that C3a blockade can serve as an inflammation-limiting strategy in the treatment of acute lung injury induced by viral infections, with inhibition associated with improved viral clearance.26 While complement deposition was not significantly reduced, C3aRA treatment did reduce complement gene transcription. Whether C3aRA reduced gene activity during the posttransplant period directly via a C3a feedback loop, or secondarily as a result of decreased inflammation and cellular injury, remains to be elucidated.
There are a number of potential reasons for the therapeutic effect of C3aRA. Our findings indicate that at least part of the conferred protection is a result of preserved cellular integrity and decreased cellular necrosis. Intracellular factors such as LDH and HMGB1 are released during cell necrosis, and levels of these molecules were reduced in perfusate solutions following cold storage in response to donor C3aRA nebulization, suggesting an additive protective role for C3aRA. Danger associated molecular patterns, such as HMGB1, can activate the innate immune system via conserved receptors such as TLR4, and so the propagation of necrotic injury can be further reduced. It should also be noted that HMGB1 and C3a share the alternative receptor RAGE (receptor for advanced glycation end-products), which has been implicated in a number of disease processes including TBI-associated inflammation and pulmonary dysfunction, with effects on post-LTx outcomes.39, 57 Somewhat surprisingly, we could not demonstrate any difference in post-LTx injury markers, LDH and HMGB1, between recipients of LD and BD donor organs. We had anticipated that recipients of BD donors would have increased markers of injury early post-LTx as compared to LD, however, only treatment of BD donors with C3aRA had any protective effect. A previous study has shown that complement activity persists during CS and can negatively impact cell survival.58 Therefore, it is possible that impairment of C3a signaling during CS may result in the reduced early graft injury markers seen post-reperfusion, as compared to LD and BD.
Proinflammatory cytokines have been shown to be associated with the development of IRI and PGD. Here we show a broad spectrum of injurious cytokines are exacerbated in BD versus LD donors and reduced by C3aRA donor therapy. Of note, TGF-β expression has been shown to be a positive predictor of PGD and the later development of chronic lung allograft dysfunction (CLAD), and we show here reduced TGF-β gene expression associated with donor C3aRA treatment.
Multiple questions still remain regarding the potential use of this therapy, and donor nebulization in general. The longevity of C3aR antagonism needs to be addressed, and in this context subsequent additional posttransplant doses may further protect the allograft from injury. In addition to the impact on inflammation and lung injury, C3aR blockade can also affect the functional interaction of dendritic cells and T cells in the adaptive immune response. While we demonstrated that BD donor C3aRA reduces the severity of rejection seen 5 days posttransplantation, whether this is due to inhibition of IRI, modulation of adaptive immunity, or both requires more detailed investigation in future studies.
A single human clinical trial has explored short-term perioperative C-Inh in LTx. Administration of TP10, a C3 convertase inhibitor, led to early extubation in a significantly higher proportion of LTx patients in a small randomized control trial.22 Whether this led to any long-term benefits has yet to be reported, but taken together, these clinical and experimental data lend support for a rationale to utilize C-Inh as a means to ameliorate IRI- and BD-induced IRI. The inclusion of nebulized complement therapeutics as part of the lung donor management protocol, or within ex vivo lung perfusion, may well enable the utilization of marginal donor lungs by mitigating the more severe IRI associated with the use of these extended criteria donors.
ACKNOWLEDGMENTS
These studies were supported by grants from the NIH (NHLBI 1R01090144 to CA), NIH (NIBIB K08EB19495 to SN), Lee Patterson Allen Foundation Award (CA and SN), NIH Institutional Postdoctoral Training Grant, NIH-HL-007260 (KP), and South Carolina Clinical & Translational Research Institute, Medical University of South Carolina's CTSA, NIH/NCATS Grant Number UL1TR000062.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




