Insights on the impact of diet-mediated microbiota alterations on immunity and diseases
Abstract
The intestinal tract is inhabited by a large and diverse community of bacteria collectively referred to as the gut microbiota. The intestinal microbiota is composed by 500-1000 distinct species, and alterations in its composition are associated with a variety of diseases including obesity, diabetes, and inflammatory bowel disease (IBD). Importantly, microbiota transplantation from diseased patients or mice (IBD, metabolic syndrome, etc.) to germ-free mice was found to be sufficient to transfer some aspects of disease phenotypes, indicating that altered microbiota is playing a direct role in those particular conditions. Moreover, it is now well admitted that the intestinal microbiota is involved in shaping and maturating the immune system, with for example the observation that germ-free animals harbor a poorly developed intestinal immune system and that some single bacteria species, such as segmented filamentous bacteria (SFB), are sufficient to induce the expansion of Th17 cells (CD4+ T helper cells producing IL-17). We will present herein an overview of the interactions occurring between the intestinal microbiota and the immune system, and we will discuss how a dietary-induced disruption of the intestinal environment may influence transplantation outcomes.
Abbreviations
-
- APC
-
- antigen presenting cell
-
- CMC
-
- carboxymethylcellulose
-
- DSS
-
- dextran sulfate sodium
-
- GVHD
-
- graft versus host disease
-
- IBD
-
- inflammatory bowel disease
-
- LPS
-
- lipopolysaccharide
-
- M-SHIME
-
- mucosal simulator of the human intestinal microbial ecosystem
-
- NAFLD
-
- non-alcoholic fatty liver disease
-
- P80
-
- polysorbate 80
-
- SCFA
-
- short-chain fatty acid
-
- SFB
-
- segmented filamentous bacteria
-
- SPF
-
- specific pathogen free
-
- TLR
-
- Toll-like receptor
1 IMPACT OF THE INTESTINAL MICROBIOTA ON IMMUNITY
The mammalian intestine is inhabited by a large and diverse community of microbes, referred to as the gut microbiota. The human microbiota consists of approximately 100 trillion (1014) bacteria composed by 6-10 major phyla and about 500-1000 distinct species and weights 1-2 kg. The intestinal microbiota has an overall beneficial impact to its host, as demonstrated in germ-free mice, where the lack of a microbiota induces considerable immune and metabolic defects.1 On the other hand, the observation that most mouse models of intestinal inflammation require a gut microbiota and that its composition is a key determinant of disease, indicate that the microbiota can also dramatically regulate inflammatory processes and disease outcomes in the host. Moreover, while the role of the intestinal microbiota in driving intestinal inflammation is well established, recent studies highlighted that bacterial products can also drive low-grade inflammation and associated metabolic syndrome. This concept was first suggested by Cani et al., who demonstrated that obesity can result in a loss of the intestinal barrier function, an activation of Toll-like receptor 4 (TLR4) by the bacterial product lipopolysaccharide (LPS), and therefore a release of pro-inflammatory cytokines that promotes insulin resistance.2
Accumulating evidence indicates that the intestinal microbiota is playing a central role in directing the development of the mammalian immune system.3 Germ-free mice, harboring a sterile intestine, present many immunological defects.4 Moreover, dysbiosis (imbalanced composition of the microbiota) are observed in patients with irritable bowel disease (IBD) and with other autoimmune diseases, where some specific bacterial species are impacting the differentiation of intestinal immune cells.5-12 Hence, proper interactions between the intestinal microbiota and the host are needed in order to maintain intestinal homeostasis.
In germ-free and newborn mice, immune responses are biased towards Th2 responses when compared to adult mice housed in specific pathogen free (SPF) condition.13, 14 Therefore, early exposure to microbes in the intestine could be a critical factor to modulate the original Th2-biased immune response in order to subsequently induce the differentiation of other T helper cell lineages, such as Th1, Th17, and regulatory T (Treg) cells.15 Treg cells are a principal component of the adaptive immune system that control inflammatory responses in order to maintain homeostasis. Treg cells express the master transcription factor Foxp316, 17 and, intriguingly, specific microbes have been shown to promote intestinal Treg cells18 (Figure 1). A well-documented example of a single microbial member playing a central role in shaping the intestinal immune system is segmented filamentous bacteria (SFB), a gram-positive and spore-forming bacteria that primarily colonize the ileum of mice and rats.19 In animals, SFB promotes the robust differentiation of Th17 cells via MHC class II-dependent pathway,7, 20, 21 which consequently induces CXCR2 dependent recruitment of neutrophils.22 Collectively, the microbiota and derived metabolites are critical components shaping host intestinal immunity, and deciphering the impact of dietary factors-induced alterations of the microbiota on intestinal immunity require further investigations.
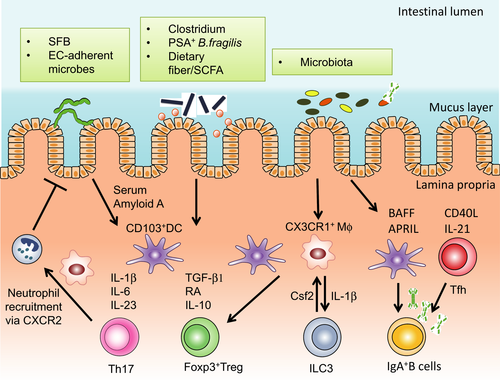
2 DIET–MICROBIOTA INTERACTIONS IN THE INTESTINE
Many factors, including genetic and environment, can impact the intestinal microbiota community leading to detrimental interactions with the host.23 For example, multiple mouse strains with mutations in genes that mediate innate immunity are prone to develop colitis in a microbiota dependent manner.24, 25 In addition, epidemiological observations, such as the dramatic increase in inflammatory diseases during the last 75 years despite relatively constant human genetics, support the concept that environmental factors have altered the way microbiota and host cooperate.26 For example, it has been observed that microbiota in emerging countries is more diverse than the one in developed countries, with the use of antibiotic being incriminated.27, 28
A number of environmental factors, including dietary components, might have detrimentally altered the gut microbiota composition, and/or its interaction with the host. Regimens of modern food production include numerous small molecules that can potentially alter host/microbiota relationship. An illustration of this concept was described in our recent work on food additives carboxymethylcellulose (CMC) and polysorbate 80 (P80).29 CMC and P80 are commonly used synthetic emulsifiers added to a variety of processed foods in order to enhance texture and extend shelf-life. Based on the epidemiology of their consumption, and the notion that their detergent properties could alter mucus layer and promote bacterial translocation, those emulsifiers were hypothesized to increase the incidence of chronic gut inflammatory diseases29-32 (Figure 2). We observed that administration of CMC and P80 to mice resulted in microbiota encroachment into the normally sterile mucus layer, strong alterations in microbiota composition and gene expression, including an increase in pro-inflammatory flagellin and LPS, and development of chronic intestinal inflammation.29, 33 Such inflammation was modest and associated with metabolic syndrome in WT mice, while in interleukin-10 deficient mice, emulsifier consumption lead to an increased incidence/severity of colitis.29 Moreover, we also observed that emulsifying agents consumption was sufficient to increased colitis-associated cancer severity in predisposed animals.34 When using a mucosal simulator of the human intestinal microbial ecosystem (M-SHIME), we observed that both P80 and CMC directly alter the microbiota by increasing its pro-inflammatory potential in a host-independent manner.35 When transplanted to germ-free mice, CMC- and P80-treated in vitro microbiota were able to penetrate the mucus layer, a feature also observed in diabetic patients,36 and were sufficient to promote low-grade inflammation associated phenotypes, indicating that the direct effects of these emulsifiers on the microbiota are sufficient to drive phenotypes previously observed in vivo.35 Other studies have highlighted the importance of the diet/microbiota relationship in metabolic disease, with for example the identification that diet-induced alteration in the microbiota can lead to increased acetate production, altered parasympathetic nervous system, insulin level alterations, hyperphagia and, ultimately, obesity.37 Moreover, similarly to dietary emulsifiers, artificial sweeteners can detrimentally impact the microbiota leading to glucose intolerance in mice and humans.38
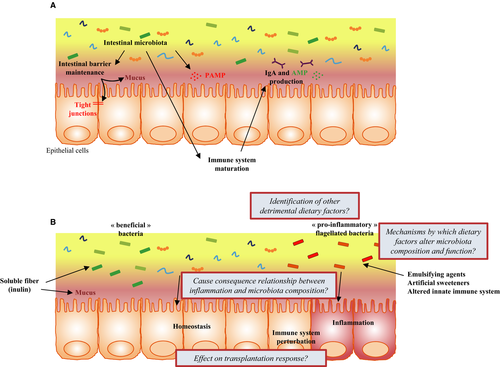
On the other hand, data showing that diet can beneficially impact the microbiota, leading to a healthier state, accumulate. For example, the soluble fiber inulin has been observed to favor the growth of beneficial bacteria such as Bifidobacterium adolescentis and Faecalibacterium prausnitzii,39 to induce short-chain fatty acid (SCFA)40 and mucus production,41 and was associated with a protection against low-grade intestinal inflammation.42 However, while such types of soluble fiber beneficially impacts the intestinal microbiota and immune system, we also recently observed that they have the potential to exacerbate disease severity in response to dextran sulfate sodium (DSS),43 highlighting that recommendations for food supplementation should be taken with a degree of caution.
Hence, the gut microbiota plays a central role in maintaining health, but this complex microbial community must be well controlled in order to protect the host from this potentially highly pro-inflammatory biomass. Failure to properly manage the gut microbiota can result in numerous types of immune-mediated inflammatory disorders, including IBD, metabolic syndrome, and cancer. Moreover, it is now well admitted that the intestinal microbiota can also impact “distant” organs, such as liver. Indeed, microbial products can disseminate from the intestine, via portal vein, to the liver and other tissues that are not normally in contact with bacteria. With such intestinal venous blood stream, it has been proposed that the liver has to serve as a firewall to capture bacteria or their products that breach the intestine.44, 45 This may also be a mean by which aberrant microbiota promote non-alcoholic fatty liver disease (NAFLD).46 Hence, it is clear that an altered intestinal microbiota can impact the inflammatory state of distant organs, and thus may play an important role in transplantation.
3 THE INTESTINAL MICROBIOTA: AN IMPORTANT PLAYER IN TRANSPLANTATION OUTCOMES?
Based on the central role played by the intestinal microbiota on immune cells composition, diet-mediated dysbiosis can impact graft outcome and alloimmunity in various ways. While intrinsic and extrinsic parameters, including host genetics and immune response, diet, medication and infections have been shown to affect the intestinal microbiota, how such microbiota alterations can impact recipients and their response to transplantation (an effective therapy for various end-stage diseases), remains unknown. Irrespective of the transplanted organs, recent studies indicated that the intestinal microbiota is significantly altered in patients between pre- and post-transplantation.47-50 After transplantation, dysbiosis, characterized by an altered microbial community, is usually observed. For example, in humans, the abundance of Bifidobacterium spp., Faecalibacterium prausnitzii and Lactobacillus spp. is significantly reduced after liver transplantation while Enterobacteriaceae and Enterococcus spp. are increased.49 Transplantation induced alteration of the microbiota has also been observed after allogeneic hematopoietic stem cell transplantation.47, 51 In that situation, a low microbiota diversity in post-transplant fecal specimens correlate with the risk of Clostridium difficile infection, graft versus host disease (GVHD), and more importantly survival rate.47 Similarly, an altered microbial diversity in the respiratory tract after lung transplantation associates with an increased risk of infection,52 and an imbalance between Firmicutes and Proteobacteria correlates with rejection rate after small bowel transplantation.53 Taken together, these data highlight that the microbiota has a significant impact not only on short-term outcome after transplantation, but also on prognosis of post-transplant patients.
However, the above referenced studies investigated the impact of the microbiota on response to transplantation, ie, after surgery. The predictive impact of the microbiota on future responses to transplantation is of tremendous interest, as it can help to identify patients with a high rejection risk and to design personalized microbiota modulations in such patients.54 A study exemplifying this concept was published by Oh et al.,53 where the authors described that, in patients receiving small bowel transplantation, the ileal microbiota was significantly altered between non-rejection, pre-rejection and active rejection groups. While these data are of great interest, since there are demonstrating that microbiota composition can be used as a diagnostic biomarker of rejection, it still remains unknown if intestinal microbiota composition in recipients and/or donors can predict future response to transplantation.
With post-transplantation complications being influenced by the host immune system, the intestinal microbiota and its effects on this immune system may play a role in such process. For example, a reduced intestinal microbial diversity was found to correlate with acute rejection of liver graft in rats,55 but the predictive value of the intestinal microbiota on those transplantation outcomes still needs to be investigated. Some approaches have been used in order to modulate the intestinal microbiota in the context of transplantation, such as with antibiotics, prebiotics and probiotics, and the efficacy of such intervention need further investigation.56 In an elegant study, Lei et al.57 recently investigated the role played by the intestinal microbiota as an environmental factor influencing transplant rejection. Using skin graft model, the authors found that antibiotics treatment was sufficient to extend graft survival,57 strengthening the concept that targeting the intestinal microbiota could be a powerful therapeutic approach to improve graft acceptance.
Dietary factors are well known to play a role in transplantation outcomes, with, for example, the findings that high-fat diet and associated hyperlipidemia accelerate graft rejection in mouse models.58, 59 However, such observations could be microbiota dependent or independent, and it also remains needed to investigate if dietary factors with the ability to directly impact microbiota functions, pro-inflammatory potential and localization, such as CMC and P80, are also impacting responses to transplantation.
4 CONCLUSIONS
Data are currently accumulating about the role played by the intestinal microbiota, mainly through its impact on the immune system, on transplantation response, and rejection. However, the predictive value of the intestinal microbiota on transplantation outcomes, and the impact of its modulation in patients undergoing transplantation, need further investigations (Figure 2B). In a not-too-distant future, fecal microbiota transplantation and the use of prebiotics/probiotics may become valuable in patients undergoing organ transplantation. Moreover, with the recently demonstrated direct impact of diet (ie, food additives and dietary fibers) on the intestinal microbial community, dietary intervention prior to transplantation might substantially ameliorate transplantation outcomes.
ACKNOWLEDGMENTS
B.C. is a recipient of the Career Development Award from the Crohn's and Colitis Foundation of America (CCFA). A.H. is a recipient of the Research Fellowship Award from the CCFA. We thank Dr. Emilie Viennois, Dr. Andrew T. Gewirtz, and Dr. Timothy L. Denning (Georgia State University) for helpful discussions.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




