Laparoscopic sleeve gastrectomy improves renal transplant candidacy and posttransplant outcomes in morbidly obese patients
Abstract
Morbid obesity is a barrier to kidney transplantation due to inferior outcomes, including higher rates of new-onset diabetes after transplantation (NODAT), delayed graft function (DGF), and graft failure. Laparoscopic sleeve gastrectomy (LSG) increases transplant eligibility by reducing BMI in kidney transplant candidates, but the effect of surgical weight loss on posttransplantation outcomes is unknown. Reviewing single-center medical records, we identified all patients who underwent LSG before kidney transplantation from 2011-2016 (n = 20). Post-LSG kidney recipients were compared with similar-BMI recipients who did not undergo LSG, using 2:1 direct matching for patient factors. McNemar's test and signed-rank test were used to compare groups. Among post-LSG patients, mean BMI ± standard deviation (SD) was 41.5 ± 4.4 kg/m2 at initial encounter, which decreased to 32.3 ± 2.9 kg/m2 prior to transplantation (P < .01). No complications, readmissions, or mortality occurred following LSG. After transplantation, one patient (5%) experienced DGF, and no patients experienced NODAT. Allograft and patient survival at 1-year posttransplantation was 100%. Compared with non-LSG patients, post-LSG recipients had lower rates of DGF (5% vs 20%) and renal dysfunction–related readmissions (10% vs 27.5%) (P < .05 each). Perioperative complications, allograft survival, and patient survival were similar between groups. These data suggest that morbidly obese patients with end-stage renal disease who undergo LSG to improve transplant candidacy, achieve excellent posttransplantation outcomes.
Abbreviations
-
- CrCl
-
- creatinine clearance
-
- DD
-
- deceased donor
-
- DGF
-
- delayed graft function
-
- DM
-
- diabetes mellitus
-
- ESRD
-
- end-stage renal disease
-
- HD
-
- Hemodialysis
-
- KT
-
- kidney transplantation
-
- LOS
-
- length of stay
-
- LSG
-
- laparoscopic sleeve gastrectomy
-
- LD
-
- living donor
-
- NODAT
-
- new-onset diabetes after transplantation
-
- PCKD
-
- polycystic kidney disease
-
- SD
-
- standard deviation
1 INTRODUCTION
Morbid obesity is a barrier to kidney transplantation among patients with end-stage renal disease (ESRD).1, 2 Morbid obesity portends inferior posttransplantation outcomes in this population, including higher rates of new-onset diabetes after transplantation (NODAT), delayed graft function (DGF), graft failure, and death.3-7 Although the survival benefit of kidney transplantation over lifelong dialysis has been well established among obese candidates,8-10 suboptimal outcomes often result in unintended provider bias against transplantation despite its benefits.11 In response, surgical weight loss has become an increasingly popular method for reducing BMI and increasing eligibility for transplantation among morbidly obese patients with ESRD.12
Laparoscopic sleeve gastrectomy (LSG) is an effective method of weight loss for improving transplant candidacy in this population.12 Bariatric surgery has also been shown to ameliorate diabetes mellitus (DM) and alleviate cardiovascular risk factors, further optimizing these patients for surgery.12 The overall impact of LSG on outcomes after transplantation, however, is unknown. In the current study, we examined the effect of LSG on posttransplantation outcomes among morbidly obese kidney transplant recipients. We hypothesized that morbidly obese patients undergoing LSG to meet weight-based criteria for kidney transplantation would have similar or improved posttransplantation outcomes as compared to patients who did not undergo bariatric surgery before transplantation. Primary endpoints were DGF, NODAT, obesity-associated conditions, allograft survival, and patient survival.
2 MATERIALS AND METHODS
2.1 Data collection
A retrospective cohort study was performed for all patients who underwent LSG at our institution between December 1, 2011, and May 1, 2016. Institutional bariatric and transplant surgery datasets were linked using patient identifiers. All patients who underwent both LSG and kidney transplantation during the study period were included, and any patients who underwent transplantation before LSG were removed from the final cohort. The bariatric surgery database included data on patient demographics, perioperative outcomes, baseline BMI, and BMI at all subsequent follow-up visits. All patients included in the bariatric dataset had documented inability to achieve significant weight loss through a medical regimen over 6 months, and met the 1991 National Institutes of Health criteria for bariatric surgery (BMI ≥ 40 kg/m2, or ≥ 35 kg/m2 with two or more obesity-related conditions).13 The renal transplant database included data on etiology of renal failure, donor characteristics, immunosuppression profiles, perioperative outcomes, and long-term outcomes.
Our linked dataset was organized according to date of transplantation. The following patient characteristics were obtained: sex, race, age at LSG, age at kidney transplantation, etiology of kidney disease, and presence of medical comorbidities. Hospital kidney transplantation outcomes analyzed include total hospital length of stay (LOS), perioperative complications, NODAT, DGF, long-term graft survival, 30-day and 1-year readmission rates, and posttransplantation mortality.
2.2 Definitions
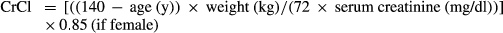
2.3 LSG procedure
All LSG procedures were performed by a single surgeon (TSD) who is fellowship trained in both transplantation and minimally invasive surgery. LSG was performed as described previously.12 Briefly, access was gained into the abdomen through the Hasson technique. After laparoscopic dissection and mobilization of the stomach, we introduced a 38-French bougie and positioned it along the lesser curvature of the stomach. An Endo-GIA stapler (Ethicon Endo-Surgery, Inc., Cincinnati, OH) was used to perform the gastrectomy, with repeated applications from 6 cm proximal to the pylorus to 1.5 cm lateral to the angle of His. The staple line was not reinforced, and the total resected specimen was approximately 60-80% of the total gastric volume. All patients followed our standard postoperative clinical care protocol, which is reported in the literature.17
2.4 Statistical analysis
A 2:1 direct-matched analysis was performed between patients who underwent LSG prior to kidney transplantation, and corresponding non-LSG kidney transplant patients over the study period. Cases and controls were matched for age at kidney transplantation, BMI at transplantation, race, etiology of kidney failure, and allograft type. We aimed to compare patients who underwent LSG to achieve an optimal BMI for transplantation, with non-LSG candidates of a similar BMI. Categorical variables were compared using McNemar's test for correlated proportions and described as percentages (%). Continuous variables were analyzed with Wilcoxon signed-rank test and expressed as mean ± standard deviation (SD) where applicable. Statistical significance was defined as P-values < .05. Data analysis was performed using statistical programs SAS 9.4 and JMP Pro 11 (SAS Institute, Cary, NC). This study was approved by the University of Cincinnati Institutional Review Board (IRB #2013-1761), and conducted in accordance with their criteria.
3 RESULTS
3.1 Patient characteristics
A total of 142 patients with ESRD underwent LSG during the study period, of which 20 subsequently underwent transplantation. These 20 patients represent the first 20 patients to undergo transplantation after LSG. Mean time from LSG to transplantation was 591.4 ± 323.3 days. Patient demographics of the study population are detailed in Table 1. Average age of post-LSG patients undergoing kidney transplantation was 54.0 ± 9.7 years. The majority of patients were male (60%) and Caucasian (65%). Among this cohort, renal failure was predominantly due to DM (45%) and hypertension (35%), followed by polycystic kidney disease (10%). In comparison, the matched non-LSG control group were of similar age (54.0 ± 12.2 years), gender (60% male), and race (67.5% Caucasian) as compared to the post-LSG group (P = NS each). Controls and post-LSG patients did not differ significantly in their etiology of kidney failure. BMI at time of transplantation was equivalent between post-LSG and control groups (32.3 ± 3.1 vs 32.3 ± 2.9 kg/m2, P = NS). These data confirm that the 2:1 direct-matching analysis allowed for proper identification of a control group with regard to patient factors.
| Control KT Mean ± SD, n (%) | Post-LSG KT N/mean (%) | P-value | |
|---|---|---|---|
| Total patients | 40 | 20 | |
| Age at KT (y) | 54.0 ± 12.2 | 54.0 ± 9.7 | NS |
| BMI at KT (kg/m2) | 32.3 ± 3.1 | 32.3 ± 2.9 | NS |
| Gender | |||
| Male | 24 (60.0%) | 12 (60.0%) | NS |
| Female | 16 (40.0%) | 8 (40.0%) | |
| Race | |||
| Caucasian | 27 (67.5%) | 13 (65.0%) | NS |
| African American | 13 (32.5%) | 7 (35.0%) | |
| Etiology of kidney failure | NS | ||
| DM | 14 (35.0%) | 9 (45.0%) | |
| Hypertension | 10 (25.0%) | 7 (35.0%) | |
| PCKD | 4 (10.0%) | 2 (10.0%) | |
| Other | 12 (30.0%) | 2 (10.0%) | |
- BMI, body mass index; DM, diabetes mellitus; KT, kidney transplantation; LSG, laparoscopic sleeve gastrectomy; PCKD, polycystic kidney disease; SD, standard deviation.
3.2 Effects of sleeve gastrectomy on BMI and obesity-associated conditions
Post-LSG outcomes are highlighted in Table 2. This high-risk group did not experience any 30-day readmissions, complications, or mortality following their bariatric procedure. The average hospital LOS was 2.2 ± 0.8 days. Table 3 documents the efficacy of LSG in reducing BMI and ameliorating obesity-associated conditions. At time of initial encounter, average BMI among this cohort was 41.5 ± 4.4 kg/m2, which decreased to 32.3 ± 2.9 kg/m2 before kidney transplantation (P < .01). At most recent follow-up visit, post-LSG patients achieved a sustained durable weight loss as compared to their pretransplantation weight (33.7 ± 4.7 vs 32.3 ± 2.9 kg/m2, P = NS). No patients experienced over 100% excess weight loss.
| Mean ± SD, n (%) | |
|---|---|
| Age at LSG (y) | 52.5 ± 10.2 |
| Hospital LOS (d) | 2.2 ± 0.8 |
| Complications | 0 (0%) |
| Readmission (30-day) | 0 (0%) |
| Mortality | 0 (0%) |
- LOS, length of stay; LSG, laparoscopic sleeve gastrectomy; SD, standard deviation.
| Control KT Mean ± SD, n (%) | Post-LSG KT N/mean (%) | P-value | |
|---|---|---|---|
| BMI (kg/m2) | |||
| Prior to LSG | — | 41.5 ± 4.4 | — |
| Prior to KT | 32.3 ± 3.1 | 32.3 ± 2.9 | NS |
| Most recent | 33.4 ± 5.9 | 33.7 ± 4.7 | NS |
| Hypertension | |||
| Prior to LSG | — | 18 (90.0%) | — |
| Prior to KT | 36 (90.0%) | 12 (60.0%) | .006 |
| Most recent | 33 (82.5%) | 8 (40.0%) | <.001 |
| Midodrine use | 0 (0%) | 3 (15.0%) | .004 |
| DM | |||
| Prior to LSG | — | 12 (60.0%) | — |
| Prior to KT | 22 (55.0%) | 8 (40.0%) | NS |
| Most recent | 21 (52.5%) | 8 (40.0%) | NS |
| NODAT | 3 of 18 (15.8%) | 0 of 12 (0.0%) | NS |
- BMI, body mass index; DM, diabetes mellitus; KT, kidney transplantation; LSG, laparoscopic sleeve gastrectomy; NODAT, new-onset diabetes after transplantation; SD, standard deviation.
The effect of LSG on comorbidities was most pronounced with regard to blood pressure. Among the post-LSG group, 90% were hypertensive prior to their bariatric procedure, which decreased to 60% immediately before kidney transplantation (Figure 1). At most recent follow-up, this percentage further decreased to 40%, and 15% of patients are currently prescribed midodrine for hypotension. Among the control group, a significantly higher proportion of patients were hypertensive before kidney transplantation (90% vs 60%, P < .01). Posttransplantation rates of hypertension were also higher among controls (82.5% vs 40%, P < .01).
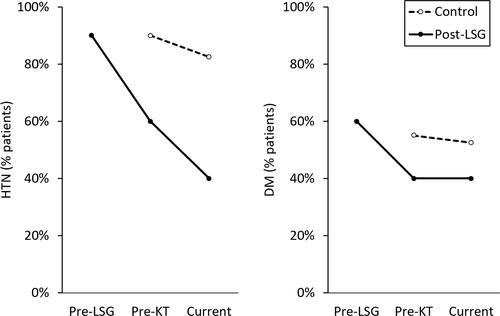
The effects of LSG on glycemic control were less pronounced. In the post-LSG group, the proportion of patients with diabetes decreased from 60% prior to LSG, to 40% at the time of transplantation. As compared to controls, the rates of diabetes mellitus before kidney transplantation (55% vs 40%) and at most recent follow-up visit (52.5% vs 40%) were similar between groups (P = NS each). No patients developed NODAT in the post-LSG group; however, there were no statistically significant difference in rates of NODAT between post-LSG and control patients (15.8% vs 0%, P = NS).
3.3 Effects of sleeve gastrectomy on posttransplantation outcomes
Table 4 highlights perioperative and long-term outcomes after kidney transplantation. Among post-LSG patients, kidney allografts were obtained from both deceased donors (DDs, 70%) and living donors (LDs, 30%). No patients experienced myocardial infarction, cerebrovascular events, wound infections, or other surgical complications. Two patients (10%) developed infectious complications unrelated to their surgical wounds, including urinary tract infection and BK viremia. One patient (5%) experienced DGF, requiring Hemodialysis (HD) within 24 hours after kidney transplantation due to persistent hyperkalemia. There were 3 readmissions (15%) within the first 30 days, and 8 (40%) within the first year. Readmissions in the post-LSG group were due to renal dysfunction, neutropenia, urinary tract infection, abdominal pain, motor vehicle accident, BK viremia, and upper extremity swelling. With regard to long-term outcomes, both kidney allograft survival and patient survival were 100% at 1 year. Two patients (10%) experienced graft loss after 1 year, secondary to severe acute tubular necrosis on day 566 and chronic allograft nephropathy on day 780.
| Control KT Mean ± SD, n (%) | Post-LSG KT Mean ± SD, n (%) | P-value | |
|---|---|---|---|
| Donor type | NS | ||
| DD | 18 (45.0%) | 14 (70.0%) | |
| LD | 22 (55.0%) | 6 (30.0%) | |
| Perioperative complications | |||
| Myocardial infarction | 2 (5.0%) | 0 (0%) | NS |
| CVA/TIA | 0 (0%) | 0 (0%) | NS |
| Postoperative infection | 7 (17.5%) | 2 (10.0%) | NS |
| Surgical complication | 2 (5.0%) | 0 (0%) | NS |
| Readmissions | |||
| 30-day readmission | 4 (10.0%) | 3 (15.0%) | NS |
| 1-year readmission | 16 (40.0%) | 8 (40.0%) | NS |
| Readmission due to renal dysfunction | 11 (27.5%) | 2 (10.0%) | .016 |
| Long-term outcomes | |||
| CMV infection | 1 (2.5%) | 0 (0%) | NS |
| Delayed graft function | 8 (20.0%) | 1 (5.0%) | .046 |
| Graft loss | 6 (15.0%) | 2 (10.0%) | NS |
| Mortality | 1 (2.5%) | 0 (0%) | NS |
| CrCl (mL/min) | 76.8 ± 35.8 | 84.0 ± 32.5 | NS |
| Interval between LSG to KT (d) | — | 591.4 ± 323.3 | — |
| Post-KT follow-up period (d) | 1672.4 ± 358.4 | 616.7 ± 464.4 | <.001 |
- CMV, cytomegalovirus; CrCl, creatinine clearance; CVA, cerebrovascular accident; DD, deceased donor; KT, kidney transplantation; LD, living donor; LOS, length of stay; LSG, laparoscopic sleeve gastrectomy; NODAT, new-onset diabetes after transplantation; SD, standard deviation; TIA, transient ischemic attack.
Control patients had similar rates of perioperative complications and readmissions as compared to post-LSG patients. Hospital readmissions due to renal dysfunction, however, were significantly higher among the control group (27.5% vs 10%, P < .05). Rates of DGF were also higher in the control group (20% vs 5%, P < .05), as determined by need for postoperative dialysis. Both allograft survival (85% vs 90%) and patient survival (97.5% vs 100%) were similar between groups (P = NS each). Renal function, as estimated through CrCl, was also similar between controls and post-LSG patients, although with higher mean clearance in the post-LSG cohort (76.8 ± 35.8 vs 84.0 ± 32.5 ml/min, P = NS). Details regarding posttransplantation immunosuppressive medications are listed in Table 5.
| Control KT Mean ± SD, n (%) | Post-LSG KT Mean ± SD, n (%) | P-value | |
|---|---|---|---|
| Induction therapy | |||
| Thymoglobulin | 38 (95.0%) | 17 (85.0%) | NS |
| Methylprednisolone | 40 (100%) | 20 (100%) | NS |
| Mycophenolate mofetil | 40 (100%) | 20 (100%) | NS |
| Mycophenolic acid | 2 (5.0%) | 0 (0%) | NS |
| Basiliximab | 0 (0%) | 2 (10.0%) | .019 |
| Alemtuzumab | 0 (0%) | 1 (5.0%) | NS |
| Belatacept | 7 (17.5%) | 0 (0%) | .012 |
| Maintenance therapy | |||
| Tacrolimus | 30 (75.0%) | 13 (65.0%) | NS |
| Cyclosporine | 1 (2.5%) | 0 (0%) | NS |
| Azathioprine | 1 (2.5%) | 0 (0%) | NS |
- KT, kidney transplantation; LSG, laparoscopic sleeve gastrectomy; SD, standard deviation.
4 DISCUSSION
To our knowledge, this is the first study to investigate the impact of LSG on posttransplantation outcomes. LSG has previously been shown to effectively reduce BMI among morbidly obese patients with ESRD, thereby increasing the likelihood of meeting weight-based criteria for kidney transplantation.12 The current study demonstrates that the benefits of LSG extend beyond transplant eligibility, and into the posttransplantation phase of care. Whereas morbidly obese candidates have traditionally achieved substandard outcomes after kidney transplantation, our experience indicates that patients who undergo LSG before transplantation achieve improved posttransplantation outcomes, as compared to patients who did not undergo surgical weight loss procedures. These data suggest a larger role for bariatric surgery in the care of morbidly obese patient with ESRD.
Morbid obesity is a de facto barrier to transplantation. Recent studies estimate that the 5-year survival rate for morbidly obese patients with ESRD is 85% after kidney transplantation, compared to 35% when relegated to lifelong dialysis.8-10 Despite this survival benefit, morbid obesity portends a host of poor posttransplantation outcomes, and many institutions have adopted a BMI of 35 kg/m2 as a relative contraindication to kidney transplantation.2 Morbidly obese patients are therefore advised to lose weight to improve transplant candidacy. Although medical weight loss techniques have had unreliable long-term success,18 bariatric surgery has been demonstrated to be both safe and effective in patients with ESRD.12, 19, 20 At our institution, we have adopted a BMI of 38 kg/m2 as a relative contraindication to kidney transplantation, although all patients are reviewed on a case-by-case basis in a multidisciplinary setting to determine candidacy for transplantation. There is no required interval after LSG that precludes eligibility for transplantation.
In the present study, we validate the efficacy of LSG in this high-risk population. These candidates lose a substantial amount of weight, as evidenced by a 9-point decrease in BMI, as well as a significant improvement in obesity-associated conditions before kidney transplantation. Rates of hypertension are significantly lower among post-LSG patients before transplantation, and this benefit extends into the posttransplantation phase. Moreover, we confirm that LSG does not expose these patients to significant harm. None of these high-risk candidates experienced any complications, readmissions, or mortality after their bariatric procedure. In addition, we did not encounter any dehydration postoperatively after LSG. This was due, in part, to our post-LSG clinical management protocol.17 We ensure that all patients can demonstrate adequate oral intake prior to hospital discharge. We also remain in contact with patients’ nephrologists and dialysis centers, to ensure that they are not being overdialyzed and receiving appropriate intravenous hydration when necessary. Therefore, LSG improves transplant candidacy among morbidly obese patients with ESRD in a safe and effective manner.12
The bias against transplanting morbidly obese patients extends further than just the potential technical difficulties in the operating room. These high-risk patients have poor short- and long-term outcomes after transplantation, simplifying the decision to allocate limited organs to lower BMI patients. Three outcomes, in particular, substantiate this bias against morbidly obese candidates. First, morbidly obese recipients have significantly higher readmission rates, resulting in greater resource utilization.21 These patients also have alarmingly high rates of wound complications after kidney transplantation, ranging from 15-44%,3, 22, 23 which further increases posttransplantation readmission rates.24 Our experience indicates that morbidly obese patients who undergo LSG have similar rates of 30-day and 1-year readmissions as compared to non-obese kidney transplant recipients. Postoperative infection rates were also similar between groups. Notably, readmissions secondary to renal dysfunction were lower within the post-LSG group. These results suggest that the advantages of surgical weight loss in morbidly obese patients with ESRD extend into posttransplantation resource expenditure.
Second, pretransplantation morbid obesity is an important risk factor for the development of NODAT.25 The ramifications of NODAT are grave—this metabolic complication significantly increases risks of graft rejection, graft loss, and mortality.25, 26 The reported incidence of NODAT ranges from 9.1% to 21% in the literature, and occurs much more frequently among morbidly obese kidney transplant recipients.27-29 In the present study, the incidence of NODAT in our control group was 15.8%, which is consistent with the reported average. In comparison, none of the post-LSG patients developed NODAT during the follow-up period. Although there was no statistically significant difference in rates of NODAT between the two groups, these trends certainly warrant further investigation.
Third, DGF is a common and costly complication among morbidly obese recipients, occurring in one third of patients.21, 23, 30-34 Comparatively, the reported incidence of DGF among all recipients is estimated at 21%.35 The immediate consequences of DGF are obvious—postoperative dialysis requires the engagement of consult services and prolongs hospital LOS, resulting in greater utilization of healthcare resources. Less evident are the long-term consequences of DGF. Recent studies have demonstrated that DGF can reduce long-term allograft survival by 40%, emphasizing the need to prevent this complication.36, 37 Our rates of DGF in the control group were congruent with the nationally reported average. Morbidly obese patients who underwent LSG prior to kidney transplantation, however, experienced significantly lower rates of DGF compared with both nonmorbidly obese patients and the national experience. Furthermore, perioperative complication rates, allograft survival, and overall survival were similar between post-LSG patients and their non-morbidly obese counterparts. These data suggest that bariatric surgery favorably impacts both short- and long-term posttransplantation outcomes.
Given the current findings, we propose that morbidly obese patients with ESRD may benefit from referral to a specialized transplant/bariatric surgery clinic. The advantages provided to these patients are 3-fold. First, this high-risk population is evaluated from a multidisciplinary approach, including dietary specialists, nephrologists, and bariatric and transplant surgeons. This allows all patients to be optimized from a medical and nutritional standpoint prior to LSG. Second, following bariatric surgery, these patients can be followed on a regular basis to determine when they become eligible for transplantation. Third, previous studies have demonstrated that kidney transplantation in morbidly obese patients causes a 48% reduction in risk of death, despite increased risk of complications. Through pretransplantation LSG, morbidly obese patients can partake in the survival benefits of transplantation without increased complication rates.
Our study has some limitations that must be addressed. First, the retrospective nature of our study exposes it to selection and information biases. Second, the average follow-up period was significantly longer among the control group. Our follow-up period in the post-LSG group was nearly 2 years, which may be considered as long-term, but this interval is shorter than the average 5-year follow-up for our control patients. Third, the study is limited by small sample sizes. Greater numbers may be required to reveal further differences between non-morbidly obese and post-LSG transplantation recipients. Fourth, the optimal control group for the current study may be matched, morbidly obese patients undergoing kidney transplantation. Although this comparison would better highlight the impact of LSG on posttransplantation outcomes, our institutional cutoff for kidney transplantation is 38 kg/m2, and thus there were not enough morbidly obese kidney transplant recipients for matching. Rather than matching with pre-bariatric BMI, we elected instead to match with postbariatric BMI. This allowed us to compare similar patient profiles at time of transplantation, rather than at initial presentation.
5 CONCLUSIONS
This novel study investigates the impact of LSG on posttransplantation outcomes among morbidly obese patients using a direct-matched analysis. Our experience demonstrates that bariatric surgery reduces BMI among morbidly obese patients with ESRD patients, thereby improving their eligibility for transplantation without exposing them to harm. Furthermore, patients who undergo LSG to improve candidacy prior to kidney transplantation achieve excellent short- and long-term posttransplantation outcomes. Postoperative complications frequently experienced among obese recipients, such as DGF, NODAT, and obesity-associated conditions, are favorably impacted by surgical weight loss. These data highlight the many advantages of bariatric surgery in morbidly obese patients with ESRD.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




