Pure laparoscopic living donor hepatectomy: Focus on 55 donors undergoing right hepatectomy
Abstract
Although laparoscopic donor hepatectomy is increasingly common, few centers with substantial experience have reported the results of pure laparoscopic donor right hepatectomy (PLDRH). Here, we report the experiences of 60 consecutive liver donors undergoing pure laparoscopic donor hepatectomy (PLDH), with most undergoing right hepatectomy. None of the 60 donors who underwent PLDH had intraoperative complications and none required transfusions, reoperation, or conversion to open hepatectomy. Forty-five donors who underwent PLDRH between November 2015 and December 2016 were compared with 42 who underwent conventional donor right hepatectomy (CDRH) between May 2013 and February 2014. The total operation time was longer (330.7 vs 280.0 minutes; P < .001) and the percentage with multiple bile duct openings was higher (53.3% vs 26.2%; P = .010) in the PLDRH group. However, the length of postoperative hospital stay (8.4 vs 8.2 days; P = .495) and rate of complications (11.9% vs 8.9%; P = .733) and re-hospitalizations (4.8% vs 4.4%; P = 1.000) were similar in both groups. PLDH, including PLDRH, is feasible when performed by a highly experienced surgeon and transplant team. Further evaluation, including long-term results, may support these preliminary findings of comparative outcomes for donors undergoing PLDRH and CDRH.
Abbreviations
-
- BMI
-
- body mass index
-
- CDRH
-
- conventional donor right hepatectomy
-
- CT
-
- computed tomography
-
- DDLT
-
- deceased donor liver transplantation
-
- ECMO
-
- extracorporeal membrane oxygenation
-
- ICG
-
- indocyanine green
-
- IVC
-
- inferior vena cava
-
- LDLT
-
- living donor liver transplantation
-
- LFT
-
- liver function test
-
- MHV
-
- middle hepatic vein
-
- MRS
-
- magnetic resonance spectroscopy
-
- PLDH
-
- pure laparoscopic donor hepatectomy
-
- PLDRH
-
- pure laparoscopic donor right hepatectomy
-
- RHA
-
- right hepatic artery
-
- RHV
-
- right hepatic vein
-
- RPV
-
- right portal vein
1 INTRODUCTION
The shortage of deceased donor organs has led to the use of living donor liver transplantation (LDLT) as an alternative to deceased donor liver transplantation (DDLT) for patients with end-stage liver disease. Unlike DDLT, LDLT must consider the safety and requirements of the living donor. Pure laparoscopic donor hepatectomy (PLDH) is a new option, which takes into account the donor's cosmetic and functional concerns. Although several studies show that this procedure is safe and reproducible 1-9, they examined relatively small sample sizes, and few compared PLDH with open donor hepatectomy 10-14. Thus, procedures for laparoscopic donor hepatectomy, particularly major right hepatectomy, have not been standardized; therefore, high-level evidence for its efficacy and safety is lacking 10-14.
Since the initiation of our LDLT program in January 1999, more than 1200 LDLTs have been performed in our center. Most of these grafts were right lobe grafts, balancing the demands of the recipient with the safety of the donor. None of the donors died, experienced disabling morbidities, or required transfusions. Following the first 2 hand-assisted laparoscopic living donor right hepatectomies in 2007 15, the laparoscopy-assisted technique was used for a small number of donors who met strict criteria (Figure 1A). The introduction of a flexible 3-dimensional laparoscope for liver surgery in 2015 resulted in more frequent use of the laparoscopy-assisted technique for donor hepatectomy, with the first pure laparoscopic donor right hepatectomy (PLDRH) performed in November 2015 16. This PLDRH has cosmetic benefit (ie, the use of a smaller suprapubic incision, which can be hidden by underwear, for graft retrieval).
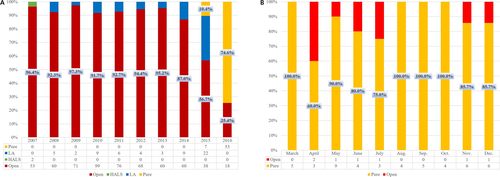
PLDH was performed until February 2016 in selected donors with no anomalies of the bile duct or portal vein. However, the accumulation of experience and the introduction of an indocyanine green (ICG) near-infrared fluorescence camera for real-time demarcation and cholangiography resulted in the use of the pure laparoscopic method, with no selection criteria 16-18, for more than 90% of donor hepatectomies performed since March 2016 (Figure 1B).
The aim of this study is to present the outcomes of our initial 1-year experience with PLDH, mostly PLDRH, and to compare these outcomes with those of donors who underwent conventional open donor right hepatectomy. The ultimate goal was to validate the safety and reproducibility of the pure laparoscopic technique when used for living donor right hepatectomy. To the best of our knowledge, this is the first study to report documented and comparative experiences in a sufficient number of donors without any selection criteria.
2 MATERIALS AND METHODS
2.1 Patients and data
The institutional review board of Seoul National University Hospital approved this study (IRB no. 1703-087-838). The overview of the study design is summarized in Figure 2. From November 2015 to December 2016, 114 live donors underwent donor hepatectomy at Seoul National University Hospital. Of these, 83 donors underwent hepatectomy using the pure laparoscopic method. Donors who underwent surgery by a surgeon other than Kyung-Suk Suh, who performed most donor hepatectomies, were excluded to eliminate operator-dependent bias. Thus, this study included 60 donors who underwent PLDH performed by a single surgeon.
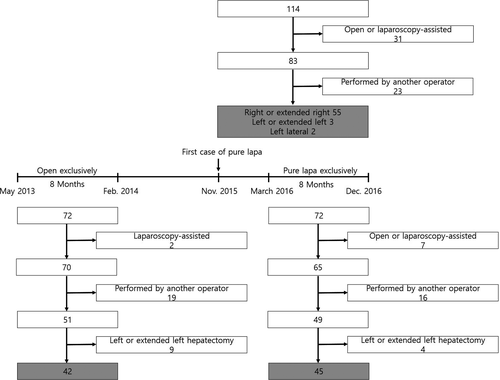
Subgroup comparative analysis was also performed including donors who underwent PLDRH after March 2016, when there were no selection criteria. Seventy-two live donors underwent donor hepatectomy between March 2016 and December 2016; however, the 3-dimensional laparoscopic system was not available on the day of surgery in 4 cases, and 2 donors refused to undergo laparoscopic surgery. One donor, who underwent the first right anterior sectionectomy in the world because of an anatomic variation and graft size issue, was also excluded 19. To overcome possible selection and time bias, 16 donors who underwent surgery performed by a surgeon other than Kyung-Suk Suh, and 4 donors who underwent left or extended left hepatectomy, were excluded. The remaining 45 donors who underwent PLDRH were compared with 42 donors who underwent conventional donor right hepatectomy (CDRH) performed by the same surgeon, between May 2013 and February 2014, the most recent 8-month period during which donors underwent conventional open hepatectomy exclusively. During that period, only 2 young donors, aged 20 and 21 years, underwent laparoscopy-assisted donor hepatectomy because of strong requests and favorable anatomy and size. The medical records of all included donors and their recipients were retrospectively reviewed.
2.2 Donor evaluation process
All donors underwent a complete ethical, medical, and anatomical evaluation, including liver dynamic computed tomography (CT), followed by CT volumetry, and magnetic resonance imaging, including magnetic resonance spectroscopy (MRS) and magnetic resonance cholangiopancreatography (MRCP), which has replaced intraoperative cholangiography in our center since 2009 20. Liver biopsy samples were selectively obtained from potential donors with fat fraction >8%-10%, as determined by MRS, considered together with liver function abnormalities, older age, and higher body mass index (BMI). A short-term weight reduction program, consisting of nutritional support and exercise management, was recommended for donors with fat fraction >8%-10% and when the recipient's condition was acceptable. Donors were informed of the innovative nature of the procedure and the advantages and disadvantages of PLDH. All donors provided written informed consent after choosing the type of operation.
2.3 Surgical procedure
This study will focus on right hepatectomy because most donors underwent this procedure.
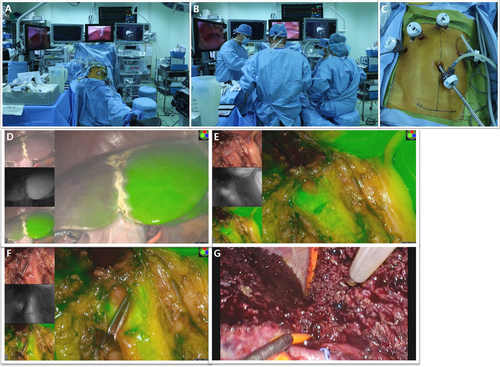
The donor was placed supine, with legs apart, in the reversed Trendelenburg position (Figure 3A). Four monitors (1 showing simultaneous vital signs, 1 showing preoperative MRCP, a laparoscopic monitor for the operator, and a monitor for the ICG near-infrared fluorescence camera) were placed in the middle (Figure 3B). A second laparoscopic view monitor was placed on the right side of the donor for the assistant and the endoscopist. Pneumoperitoneum was established and maintained at 12 mm Hg. Four 12-mm trocars and 1 5-mm trocar were inserted, as shown in Figure 3C. While viewing with the Endoeye Flex 3D laparoscope (Olympus, Tokyo, Japan), the right liver was mobilized by dividing the round, falciform, right coronary, and triangular ligaments with a Thunderbeat (Olympus). The middle hepatic vein (MHV) and right hepatic vein (RHV) were exposed from above. The right part of liver segment I was mobilized to enable dissection of the anterior aspect of the inferior vena cava (IVC) by dividing small venous branches between intracorporeal ties or clips. The right inferior hepatic vein was divided between Hem-O-Lok clips (Weck Closure System, Research Triangle Park, NC) or transected later with the RHV using an endostapler (Echelon Flex Powered Vascular Stapler; Ethicon, Somerville, NJ) if its size was considered large enough for anastomosis. The area between the liver and IVC was carefully dissected as high as possible, followed by insertion of the Goldfinger (Ethicon Endosurgery, Cincinnati, OH) to create a tunnel. A nelaton tube was inserted through the tunnel to lift the cutting area of the liver in the posterior-to-anterior direction. After dividing the cystic artery and duct, the right side of the hilum was exposed and dissected. The right hepatic artery (RHA) and right portal vein (RPV) were identified, and vessel loops were placed around them. The RHA and RPV were temporarily clamped using laparoscopic bulldog clamps. ICG (0.025 mg/kg) was injected intravenously, and the exact midplane of the liver was demarcated using a near-infrared camera (Pinpoint; Novadaq, Ontario, Canada or LuxEndoBright; Korea Electrotechnology Research Institute, Seoul, Korea) (Figure 3D). The liver capsule and superficial parenchyma were transected using a Thunderbeat. Deeper parenchyma was transected along the MHV using a laparoscopic ultrasonic aspirator (CUSA Excel; Valleylab Corp., Boulder, CO). No Pringle maneuver was used. The anterior sections of venous branches were carefully dissected and ligated with Hem-O-Lok clips for possible reconstructions. After further transecting the liver parenchyma, the anterior surface of the hilar plate was exposed. After comparing the results of real-time ICG fluorescence cholangiography and preoperative MRCP imaging, the area around the hilar plate was carefully dissected. The right hepatic duct, the left hepatic duct, and the caudate or aberrant bile duct were clearly visualized by ICG fluorescence cholangiography (Figure 3E). The optimal bile duct division point was determined and clamped with double clips on the remnant side of the bile duct (Figure 3F). After rechecking the patency of each side of the bile ducts and the common hepatic duct, the optimal division point was cut. The caudate lobe was transected, and the nelaton tube was repositioned to the front of the right posterior Glisson pedicle to lift the remnant posterior part of the liver (Figure 3G). After the parenchyma dissection using the hanging maneuver was complete, the right liver was attached only by its vascular structures. A 10-12-cm suprapubic incision was made without opening the peritoneum, and the RHA was divided using a Hem-O-Lok clip and a metal clip. The RPV was transected with an Echelon Flex, taking care that the direction of the stapler did not result in any torsion. The RHV was cut with a unilateral linear stapler (Endo TA; Covidien, Dublin, Ireland). The graft was placed in an endo-bag and retrieved through the suprapubic incision site. The suprapubic incision was closed, and the pneumoperitoneum was re-insufflated to check for hemostasis, anchor the falciform ligament, and insert a drain.
2.4 Statistical analysis
Results were expressed as mean ± standard deviation or as number and percentage. Continuous variables were compared using Student t tests, and categorical variables were compared using the chi-square test or Fisher exact test, as appropriate. Pearson's test was used to test the significance of the correlations. Overall survival rates were estimated by the Kaplan-Meier method and compared by the log-rank test. A 2-tailed P < .05 was considered statistically significant. All statistical analyses were performed using SPSS software (version 22; SPSS Inc., Chicago, IL).
3 RESULTS
3.1 Characteristics and outcomes of subjects undergoing pure laparoscopic liver donation
Between November 2015 and December 2016, 60 donors underwent PLDH; their baseline characteristics, operative outcomes, and postoperative hospital courses are summarized in Table 1. None of these donors required transfusion, conversion to open surgery, or reoperation. Complications were graded according to the classification system proposed by Clavien 21. Two donors (3.3%) were readmitted. One donor with a grade IIIa complication was readmitted due to an abnormal liver function test (LFT). CT revealed a biloma near the bile duct division site, which was treated successfully with endoscopic stent insertion and percutaneous drainage. The other donor with a grade II complication was readmitted 1 day after discharge due to abdominal pain caused by a small amount of biloma, and was treated with antibiotics.
| Variables | PLDH (N = 60) |
|---|---|
| Sex, male:female | 33:27 |
| Age, mean ± SD, y | 33.4 ± 12.9 |
| BMI, mean ± SD, kg/m2 | 23.9 ± 3.2 |
| Preoperative history, n (%) | |
| Alcohol | 30 (50.0) |
| Smoking | 23 (38.3) |
| Abdominal surgery | 6 (10.0) |
| Relationship, n (%) | |
| Son/daughter | 38 (63.3) |
| Father/mother | 3 (5.0) |
| Brother/sister | 7 (11.7) |
| Husband/wife | 11 (18.3) |
| Other | 1 (1.7) |
| Adult to child, n (%) | 2 (3.3) |
| Graft type, n (%) | |
| Right hemigraft | 51 (85.0) |
| Extended right hemigraft | 4 (6.7) |
| Left hemigraft | 2 (3.3) |
| Extended left hemigraft | 1 (1.7) |
| Left lateral graft | 2 (3.3) |
| Estimated remnant volume, mean ± SD, % | 37.0 ± 9.9 |
| Estimated remnant volume <30%, n (%) | 8 (13.3) |
| Estimated GRWR, mean ± SD | 1.4 ± 0.7 |
| Preoperative blood tests, mean ± SD | |
| Hb, g/dL | 14.2 ± 1.5 |
| Total bilirubin, mg/dL | 0.7 ± 0.2 |
| AST, IU/L | 17.8 ± 3.8 |
| ALT, IU/L | 17.8 ± 9.4 |
| GGT, IU/L | 22.6 ± 12.6 |
| Operative time, mean ± SD, min | 340.8 ± 54.9 |
| Time to remove liver, mean ± SD, min | 234.1 ± 81.6 |
| Fatty change, mean ± SD, % | |
| Macrovesicular | 1.9 ± 2.4 |
| Microvesicular | 1.5 ± 1.8 |
| Double portal vein orifices, n (%) | 8 (13.3) |
| Multiple bile duct openings, n (%) | 28 (46.7) |
| Graft weight, mean ± SD, g | 679.4 ± 166.2 |
| GRWR, mean ± SD | 1.2 ± 0.8 |
| Estimated blood loss, mean ± SD, mL | 423.5 ± 199.9 |
| Intraoperative transfusion, n (%) | 0 |
| Postoperative blood tests, mean ± SD | |
| Hb, g/dL | |
| Lowest | 11.9 ± 1.3 |
| Discharge day | 12.8 ± 1.3 |
| POD 3-4 mo | 14.5 ± 1.5 |
| Total bilirubin, mg/dL | |
| Peak | 3.4 ± 1.5 |
| Discharge day | 1.0 ± 0.3 |
| POD 3-4 mo | 0.9 ± 0.4 |
| AST, IU/L | |
| Peak | 223.7 ± 78.7 |
| Discharge d | 44.4 ± 20.4 |
| POD 3-4 mo | 21.6 ± 5.4 |
| ALT, IU/L | |
| Peak | 226.6 ± 90.3 |
| Discharge d | 67.4 ± 36.1 |
| POD 3-4 mo | 19.7 ± 10.4 |
| GGT, IU/L | |
| Peak | 75.3 ± 68.8 |
| Discharge d | 55.2 ± 49.8 |
| POD 3-4 mo | 31.8 ± 22.1 |
| Hospital stay, mean ± SD, d | 8.2 ± 1.2 |
| Postoperative complications, n (%) | 6 (10.0) |
| Grade I | |
| Wound problem | 2 (3.3) |
| Pleural effusion | 1 (1.7) |
| Grade II | |
| Intra-abdominal fluid collection requiring antibiotics | 2 (3.3) |
| Grade IIIa | |
| Biliary leakage requiring endoscopic stenting and percutaneous drainage | 1 (1.7) |
| Rehospitalization, n (%) | 2 (3.3) |
- Complications were graded according to the classification system proposed by Clavien.
- ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GGT, γ-glutamyl transferase; GRWR, graft-to-recipient ratio; Hb, hemoglobin; PLDH, pure laparoscopic donor hepatectomy; POD, postoperative day; SD, standard deviation.
All grafts were successfully transplanted in the standard fashion. Table 2 summarizes recipients’ characteristics and postoperative details. Postoperative major complications (grade III or higher) occurring within 30 days are considered early major complications. The overall 30-day recipient survival rate was 96.7%. One recipient died of heart failure and the other died after self-removal of an extracorporeal membrane oxygenation (ECMO) line.
| Variables | Recipients (N = 60) |
|---|---|
| Sex, male:female | 41:19 |
| Age, mean ± SD, y | 51.5 ± 12.8 |
| BMI, mean ± SD, kg/m2 | 23.4 ± 3.8 |
| Underlying etiology, n (%) | |
| HBV | 41 (68.3) |
| HCV | 4 (6.7) |
| Alcohol | 7 (11.7) |
| Others | 8 (13.3) |
| Hepatocellular carcinoma, n (%) | 41 (68.3) |
| MELD score | 12.5 ± 6.4 |
| Hospital stay, mean ± SD, d | 21.1 ± 16.0 |
| Postoperative complications | |
| Early major complications, n (%)a | 15 (25.0) |
| Intra-abdominal bleedingb | 5 (8.3) |
| Intra-abdominal fluid collectionb | 4 (6.7) |
| Wound problemb | 3 (5.0) |
| Hepatic artery problemb | 2 (3.3) |
| Portal vein or hepatic vein problemb | 5 (8.3) |
| Biliary problemb | 2 (3.3) |
| Cardiac problemb | 2 (3.3) |
| Pulmonary problemb | 2 (3.3) |
| Gastrointestinal problemb | 1 (1.7) |
| Primary nonfunctionb | 1 (1.7) |
| Late major complications, n (%)a | 10 (16.7) |
| Intra-abdominal fluid collectionb | 1 (1.7) |
| Biliary problemb | 10 (16.7) |
| 30-d mortality, n (%) | 2 (3.3) |
| Heart failure | 1 (1.7) |
| Self-removal of an ECMO line | 1 (1.7) |
- BMI, body mass index; ECMO, extracorporeal membrane oxygenation line; HBV, hepatitis B virus; HCV, hepatitis C virus; MELD, Model for End-Stage Liver Disease; PLDH, pure laparoscopic donor hepatectomy; SD, standard deviation.
- a Number of recipients who had complications.
- b Cases of complications.
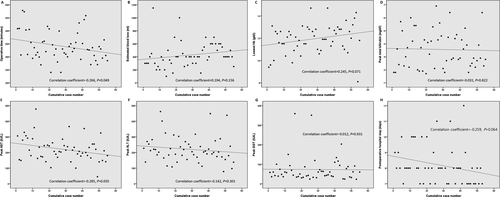
Of the 60 donors who underwent PLDH, 55 (91.7%) underwent right or extended right hepatectomy. Figure 4 shows how operative time, amount of estimated blood loss, and biological data have altered since pure laparoscopic right or extended right hepatectomy was first performed. Operation time (correlation coefficient, −0.266; P = .049) and postoperative peak aspartate aminotransferase (AST) level (correlation coefficient, −0.285; P = .035) decreased significantly from the first to the last donor who underwent right hepatectomy.
3.2 CDRH vs PLDRH
Between March 2016 and December 2016, 42 donors underwent PLDRH without any selection criteria. This group was compared with 45 donors who underwent CDRH between May 2013 and February 2014, a period during which CDRH was performed exclusively. Selection bias and operator-dependent bias were minimized. Table 3 summarizes the demographic characteristics and operative outcomes of both groups. The total operation time (330.7 vs 280.0 minutes; P < .001) was significantly longer in the PLDRH than in the CDRH group. The percentage of grafts with double portal vein orifices was similar in the 2 groups (13.3% vs 19%; P = .469), but the percentage with multiple bile duct openings was significantly higher in the PLDRH group (53.3% vs 26.2%; P = .010). None of the patients in either group required a transfusion during surgery or postoperatively. The ΔHb%, calculated as ΔHb% = [(Preoperative Hb-postoperative Hb)/Preoperative Hb] ×100 was significantly lower in the PLDRH group than in the CDRH group (15.3% vs 18.1%, respectively; P = .020).
| Variables | CDRH (N = 42) | PLDRH (N = 45) | P-value |
|---|---|---|---|
| Sex, male:female | 30:12 | 26:19 | .184 |
| Age, mean ± SD, y | 32.0 ± 11.4 | 32.8 ± 12.6 | .751 |
| BMI, mean ± SD, kg/m2 | 23.4 ± 3.4 | 24.1 ± 3.4 | .358 |
| Preoperative history, n (%) | |||
| Alcohol | 19 (45.2) | 22 (48.9) | .733 |
| Smoking | 16 (38.1) | 20 (44.4) | .548 |
| Abdominal surgery | 4 (9.5) | 4 (8.9) | 1.000 |
| Relationship, n (%) | |||
| Son/daughter | 30 (71.4) | 30 (66.7) | .631 |
| Father/mother | 0 | 1 (2.2) | 1.000 |
| Brother/sister | 6 (14.3) | 6 (13.3) | .898 |
| Husband/wife | 5 (11.9) | 7 (15.6) | .622 |
| Other | 1 (2.4) | 1 (2.2) | 1.000 |
| Adult to child, n (%) | 1 (2.4) | 0 | .483 |
| Graft including middle hepatic vein, n (%) | 0 | 2 (4.4) | .495 |
| Estimated remnant volume, mean ± SD, % | 35.8 ± 6.4 | 34.0 ± 4.1 | .124 |
| Estimated GRWR, mean ± SD | 1.3 ± 0.2 | 1.3 ± 0.3 | .598 |
| Preoperative blood tests, mean ± SD | |||
| Hb, g/dL | 14.5 ± 1.4 | 14.3 ± 1.4 | .501 |
| Total bilirubin, mg/dL | 0.8 ± 0.3 | 0.7 ± 0.2 | .159 |
| AST, IU/L | 18.3 ± 5.8 | 17.8 ± 4.0 | .668 |
| ALT, IU/L | 19.3 ± 11.7 | 17.7 ± 8.2 | .468 |
| GGT, IU/L | 25.0 ± 25.5 | 23.6 ± 13.4 | .750 |
| Operative time, mean ± SD, min | 280.0 ± 39.9 | 330.7 ± 49.5 | <.001 |
| Time to remove liver, mean ± SD, min | 179.5 ± 43.4 | 221.6 ± 87.8 | .006 |
| Warm ischemic timea, mean ± SD, min | 5.4 ± 3.6 | 12.6 ± 4.4 | <.001 |
| Fatty change, mean ± SD, % | |||
| Macrovesicular | 2.8 ± 2.6 | 2.2 ± 2.6 | .257 |
| Microvesicular | 2.7 ± 3.1 | 1.5 ± 1.9 | .037 |
| Double portal vein orifices, n (%) | 8 (19.0) | 6 (13.3) | .469 |
| Multiple bile duct openings, n (%) | 11 (26.2) | 24 (53.3) | .010 |
| Graft weight, mean ± SD, g | 726.1 ± 159.7 | 714.0 ± 145.2 | .719 |
| GRWR, mean ± SD | 1.2 ± 0.3 | 1.1 ± 0.4 | .300 |
| Estimated blood loss, mean ± SD, mL | 338.1 ± 188.2 | 436.0 ± 170.3 | .013 |
| Intraoperative transfusion, n (%) | 0 | 0 | NS |
| Postoperative blood tests, mean ± SD | |||
| Hb, g/dL | |||
| Lowest | 11.9 ± 1.4 | 12.1 ± 1.3 | .443 |
| ΔHb%b | 18.1 ± 5.3 | 15.3 ± 5.8 | .020 |
| Discharge d | 13.6 ± 3.6 | 13.0 ± 1.3 | .263 |
| POD 3-4 mo | 15.0 ± 1.7 | 14.5 ± 1.5 | .180 |
| Total bilirubin, mg/dL | |||
| Peak | 3.9 ± 1.4 | 3.7 ± 1.4 | .363 |
| Discharge d | 1.1 ± 0.5 | 1.0 ± 0.3 | .302 |
| POD 3-4 mo | 1.0 ± 0.5 | 0.9 ± 0.4 | .520 |
| AST, IU/L | |||
| Peak | 133.9 ± 34.7 | 214.4 ± 76.4 | <.001 |
| Discharge d | 50.5 ± 27.1 | 43.2 ± 17.8 | .144 |
| POD 3-4 mo | 25.3 ± 25.0 | 22.2 ± 5.8 | .528 |
| ALT, IU/L | |||
| Peak | 138.0 ± 31.2 | 220.87 ± 90.3 | <.001 |
| Discharge d | 66.4 ± 36.7 | 62.1 ± 28.1 | .544 |
| POD 3-4 mo | 18.0 ± 8.1 | 21.0 ± 11.7 | .232 |
| GGT, IU/L | |||
| Peak | 67.1 ± 70.3 | 76.9 ± 72.1 | .529 |
| Discharge day | 53.3 ± 26.9 | 55.5 ± 49.2 | .803 |
| POD 3-4 mo | 27.1 ± 17.8 | 34.1 ± 25.2 | .213 |
| Hospital stay, mean ± SD, d | 8.4 ± 1.0 | 8.2 ± 1.3 | .495 |
| Postoperative complications, n (%) | 5 (11.9) | 4 (8.9) | .733 |
| Grade I | 2 (4.8) | 1 (2.2) | .608 |
| Ileus | 1 (2.4) | 0 | .483 |
| Wound problem | 1 (2.4) | 1 (2.2) | 1.000 |
| Grade II | 2 (4.8) | 2 (4.4) | 1.000 |
| Intra-abdominal fluid collection requiring antibiotics | 0 | 2 (4.4) | .495 |
| Pneumonia | 1 (2.4) | 0 | .483 |
| Upper respiratory infection | 1 (2.4) | 0 | .483 |
| Grade IIIa | 1 (2.4) | 1 (2.2) | 1.000 |
| Biliary leakage requiring endoscopic stenting and percutaneous drainage | 0 | 1 (2.2) | 1.000 |
| Liver abscess requiring percutaneous drainage | 1 (2.4) | 0 | .483 |
| Rehospitalization, n (%) | 2 (4.8) | 2 (4.4) | 1.000 |
- ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CDRH, conventional donor right hepatectomy; GGT, γ-glutamyl transferase; GRWR, graft-to-recipient ratio; Hb, hemoglobin; PLDRH, pure laparoscopic donor right hepatectomy; POD, postoperative day; SD, standard deviation. Complications were graded according to the classification system proposed by Clavien.
- a Warm ischemic time was considered as time to start when the right hepatic artery was ligated and to time to remove liver.
- b ΔHb% = [(Preoperative Hb-postoperative Hb)/Preoperative Hb]×100.
All liver grafts were successfully transplanted in both groups. Similar percentages of recipients in the 2 groups had early major complications (24.4% vs 26.2%; P = .851) (Table 4). Although not statistically significant, 1 patient (2.2%) in the PLDRH group experienced hepatic artery thrombosis, which required reoperation for hepatic artery anastomosis revision, and 2 patients in this group underwent revision of hepatic artery anastomosis after primary anastomosis because of nonvisible or weak artery flow, detected by intraoperative liver Doppler. The first case that required reoperation showed continuous LFT elevation, and liver Doppler (followed by CT) detected a nonvisible artery flow at postoperative Day 1. During reoperation, an intraluminal partial thrombus was found and removed gently, and revision of hepatic artery anastomosis was performed after infusing urokinase. The latter 2 cases, both of which required revision of the hepatic artery anastomosis, were identified immediately after the primary anastomosis using routine intraoperative liver Doppler. In 1 of these 2 cases, intimal dissection and thrombus was found at the donor side artery; therefore, further resection followed by re-anastomosis under microscopic view was performed after infusing urokinase. The other case showed intimal dissection and thrombus from the recipient side artery to the gastroduodenal bifurcation level; the graft artery was re-anastomosed to the recipient's right gastroepiploic artery. All patients requiring hepatic artery revision recovered completely. None of the patients in the CDRH group required hepatic artery revision.
| Variables | CDRH (N = 42) | PLDRH (N = 45) | P-value |
|---|---|---|---|
| Sex (male:female) | 21:21 | 30:15 | .115 |
| Age, mean ± SD, y | 52.4 ± 8.6 | 52.9 ± 8.8 | .795 |
| BMI, mean ± SD, kg/m2 | 22.5 ± 3.2 | 24.0 ± 3.3 | .031 |
| Underlying etiology, n (%) | |||
| HBV | 24 (57.1) | 33 (73.3) | .112 |
| HCV | 6 (14.3) | 4 (8.9) | .512 |
| Alcohol | 6 (14.3) | 2 (4.4) | .148 |
| Others | 6 (14.3) | 6 (13.3) | .898 |
| Hepatocellular carcinoma, n (%) | 29 (69.0) | 32 (71.1) | .834 |
| MELD score | 14.2 ± 8.0 | 11.4 ± 5.7 | .064 |
| Hospital stay, mean ± SD, d | 23.8 ± 17.9 | 20.7 ± 15.8 | .404 |
| Early major complications, n (%)a | 11 (26.2) | 11 (24.4) | .851 |
| Intra-abdominal bleedingb | 4 (9.5) | 3 (6.7) | .707 |
| Intra-abdominal fluid collectionb | 0 | 4 (8.9) | .117 |
| Wound problemb | 2 (4.8) | 2 (4.4) | 1.000 |
| Hepatic artery problemb | 0 | 1 (2.2) | 1.000 |
| Portal vein or hepatic vein problemb | 4 (9.5) | 4 (8.9) | 1.000 |
| Biliary problemb | 2 (4.8) | 1 (2.2) | .608 |
| Cardiac problemb | 1 (2.4) | 1 (2.2) | 1.000 |
| Pulmonary problemb | 2 (4.8) | 2 (4.4) | 1.000 |
| Gastrointestinal problemb | 1 (2.4) | 1 (2.2) | .608 |
| 30-d mortality, n (%) | 0 | 1 (2.2)c | 1.000 |
- BMI, body mass index; CDRH, conventional donor right hepatectomy; ECMO, extracorporeal membrane oxygenation; HBV, hepatitis B virus; HCV, hepatitis C virus; MELD, Model for End-Stage Liver Disease; PLDRH, pure laparoscopic donor right hepatectomy; SD, standard deviation.
- a Number of recipients who had complications.
- b Cases of complications.
- c One recipient died after self-removal of the ECMO line.
Three recipients in the CDRH group died, 2 from recurrent hepatocellular carcinoma after 34 and 37 months and 1 from septic shock due to pneumonia at 35 months. In the PLDRH group, 1 recipient died after self-removal of the ECMO line. The 1- and 3-year overall survival rates of patients in the CDRH group were 100.0% and 95.1%, respectively, whereas the 1-month overall survival rate in the PLDRH group was 97.8% (P = .334).
4 DISCUSSION
Several factors resulted in the stability, reproducibility, and standardization of our PLDRH technique. First, the surgeon's experience is important. The surgeon in this study has much experience in both liver surgery and LDLT. Since the initiation of our LDLT program in January 1999, he has performed more than 1000 donor hepatectomies, with no donor deaths, disabling morbidities, or transfusions. Since the first 2 successful hand-assisted laparoscopic donor hepatectomies in 2007, he has performed 60 laparoscopy-assisted donor hepatectomies in suitable donors with favorable anatomy. He has also performed more than 200 laparoscopic hepatectomies. Sufficient experience, particularly with open donor hepatectomies and laparoscopic hepatectomies, is important before attempting PLDH, including PLDRH. Second, introduction of the 3-dimensional laparoscope has allowed depth perception, which is not achieved with a conventional laparoscope 16. The flexible scope was able to be manipulated in a small space, which is especially necessary for liver mobilization 22. Third, the real-time ICG near-infrared fluorescence technique was useful in demarcating the exact midplane of the liver and determining the optimal bile duct division point. Since the introduction of this ICG camera in March 2016, our PLDH technique has become standardized with the use of 5 monitors in the operative field, including the ICG camera monitor and preoperative MRCP. The ischemic line on the liver surface in most donors was not clear under laparoscopic view after temporarily clamping the RHA and RPV. However, within 30 seconds of intravenous ICG injection, the demarcation line could be clearly visualized using the ICG camera. Precise dissection of the midplane is important to minimize exposure of small intrahepatic Glisson branches and thus decrease bleeding and bile leakage 20. ICG fluorescence cholangiography also served as an excellent adjunct to preoperative MRCP during bile duct division. Our preliminary study, involving 10 donors, showed the usefulness of ICG fluorescence cholangiography in PLDH 17.
In focusing only on right hepatectomy with or without MHV, we found that the duration of the procedure (correlation coefficient, −0.266; P = .049) and the peak AST concentration (correlation coefficient, −0.285; P = .035) fell significantly over time (from the first to the last donor), reflecting the learning curve for this procedure. The total operation time decreased as the procedure became standardized and simplified by accumulated experience. Transecting the exact midplane of the liver saved time by eliminating the need to ligate or clip the inflow structures in the small intrahepatic Glisson branches. Because bile duct division remains a major issue, ICG near-infrared fluorescence cholangiography may have played a role in saving time, thereby enabling rapid and efficient dissection and increasing the surgeon's confidence with respect to the optimal bile duct division procedure 17. Liver manipulation is a leading cause of hepatocyte injury during liver surgery 23, 24. In addition to transection, liver manipulation itself during perihepatic dissection and mobilization can cause hepatocyte injury, leading to increased levels of markers of liver injury, including AST. This may be more pronounced when using a pure laparoscopic procedure because it results in limited feedback; therefore, it can be regarded as a technique with minimal external exposure but with similar internal invasiveness as the open method. During this initial period, the liver was pressed or pulled by a dissector (wrapped in gauze), making hepatocyte damage unavoidable. Subsequently, the liver was mobilized mainly by lifting and turning the liver while at the same time gently holding the remnant end of the right triangular ligament. This may have minimized the crushing effect on the liver and reduced AST levels postoperatively.
The longer operation time and longer time to liver removal in the PLDRH group was largely due to the minimum time for CDRH achieved by our center. Two recent studies of PLDRH reported mean operation times of 458.7 and 476 minutes, both longer than in the present study 13, 14. The percentage of grafts with multiple bile duct openings was significantly higher in the PLDRH group (53.3% vs 26.2%; P = .010). Despite good quality preoperative MRCP images and real-time ICG fluorescence cholangiography, surgeons may still feel less confident in determining the precise bile duct division point and move to the right side more naturally. Cutting and suturing the remnant donor side of the bile duct may shift the bile division point more to the left side. However, intracorporeal suturing can be cumbersome as it may take more time and can have a higher risk of strictures or leakages in the remnant bile duct. After 1 donor who underwent intracorporeal bile duct suturing experienced biliary leakage, we routinely double clip at the remnant side when dividing the bile duct. Two clips occupy space, which may shift the division point more to the right than intended. To overcome drawbacks resulting from the relatively short bile duct and portal vein obtained with dual clips and a stapler, highly experienced surgeons with excellent skills for recipient side operations are required.
Estimated blood loss was significantly higher in the PLDRH than in the CDRH group (436.0 vs 338.1 mL; P = .013). However, the amount of blood loss was estimated by measuring the amount of liquid in the suction bottle, including water, used for irrigation, thus potentially introducing bias; also, the ΔHb% (which is a more reliable value) was significantly lower in the PLDRH group (15.3% vs 18.1%; P = .020), suggesting less actual blood loss.
Mean recipient BMI was higher (24.0 vs 22.5 kg/m2; P = .031), while Model for End-Stage Liver Disease (MELD) score was lower (11.4 vs 14.2; P = .064), in the PLDRH than in the CDRH group. The reasons for these differences are unclear, but the MELD system for liver allocation was first introduced in Korea in June 2016. Patients with higher MELD scores may have had a greater chance of acquiring a liver from a deceased donor, whereas patients with lower MELD scores and higher nutritional status may have been more likely to undergo LDLT. Although the follow-up period after PLDRH was short, there were no differences in early major complications between the PLDRH and CDRH groups. However, 1 recipient (2.2%) had a postoperative hepatic artery problem, requiring thrombectomy and re-anastomosis due to hepatic artery thrombosis. Another 2 recipients required intraoperative revision of hepatic artery anastomosis due to no or weak arterial flow. Intimal dissection was identified in these 2 recipients, 1 at the graft side and the other at the recipient side. Eventually, 2 cases of hepatic artery thrombosis were related to a graft side hepatic artery problem. These 2 cases of PLDRH were performed in August and September, which was the mid-and-late period of the study. The rate of hepatic artery thrombosis was higher than that reported in a previous systematic review 25 and in our previous report 26. There are several possible reasons for this. Dissection and ligation of small branches of the hepatic artery requires clipping or an intracorporeal tie. However, because the latter lacks tactile feedback during the laparoscopic procedure, excessive force while pulling the thread and spreading the tip of the dissector for dissection can injure the intima of the artery. Second, during caudate transection, CUSA was used while lifting the RHA and RPV with a vessel loop. In some cases, the branch point of the artery sticks to the vessel loop, resulting in excess pulling and injury while lifting the vessel loop. Third, the tip of the CUSA can thermally damage the nearby artery. Although the number of donors was small and the difference between the 2 groups was not significant, we attempted to avoid these 3 factors after experiencing hepatic artery complications, as hepatic artery thrombosis can be a major problem.
Further studies are needed to confirm the positive effects on quality of life and return to work. However, our previous study showed that the patient satisfaction levels were greater with regard to improved cosmetic outcomes (in cases of minimal incision vs cases of conventional incision) 27, and we witnessed subjective satisfaction in donors with regard to the small incision and reduced postoperative pain. Additionally, although not significant, there was a decrease in the length of postoperative hospital stay from the first to the last donor who underwent right hepatectomy (correlation coefficient, -0.259; P = .064). When comparing the last 10 PLDRH cases with the CDRH cohort, the duration of hospital stay was significantly shorter (7.4 vs 8.4 days; P = .009).
This study had several limitations. First, it was a retrospective study, forcing us to rely on the completeness of the medical records for our analysis. Second, the sample size was relatively small and the follow-up period was short, but no larger cohorts of donors have been studied to date.
In conclusion, PLDH, primarily right hepatectomy, is a feasible procedure when performed by a highly experienced surgeon and transplantation team. Compared with the open approach, PLDRH results in less blood loss but a longer operation time and higher postoperative peak AST and alanine aminotransferase concentrations, which are minor problems that can be improved by experience. Further evaluation, including long-term results, is needed to develop a more standardized and safer procedure.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




