The Current State of Liver Transplantation in the United States
Perspective From American Society of Transplant Surgeons (ASTS) Scientific Studies Committee and Endorsed by ASTS Council
Abstract
This article is a review of the salient points and a future prospective based on the 2014 Organ Procurement and Transplantation Network (OPTN)/Scientific Registry of Transplant Recipients (SRTR) liver donation and transplantation data report recently published by the American Journal of Transplantation. Emphasis of our commentary and interpretation is placed on data relating to waitlist dynamics, organ utilization rates, the impact of recent advances in the treatment of hepatitis C, and the increases in end-stage renal disease among liver transplant candidates. Finally, we share our vision on potential areas of innovation that are likely to significantly improve the field of liver transplantation in the near future.
Abbreviations
-
- DAA
-
- direct-acting antiviral agent
-
- DCD
-
- donation after cardiac death
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- KDPI
-
- kidney donor profile index
-
- LDLT
-
- living donor liver transplant
-
- MELD
-
- Model for End-Stage Liver Disease
-
- NASH
-
- nonalcoholic steatohepatitis
-
- OLT
-
- orthotopic liver transplantation
-
- OPTN
-
- Organ Procurement and Transplantation Network
-
- SCS
-
- static cold storage
-
- SRTR
-
- Scientific Registry of Transplant Recipients
Introduction
Every year, the Organ Procurement and Transplantation Network (OPTN) and the Scientific Registry for Transplant Recipients (SRTR) release data about donor and transplant recipient activity in the United States. The 2014 data report spans over a period of 10 years and is based on data collected between 2003 and 2014 1. Liver transplantation represents the second most commonly transplanted organ in the United States. While pediatric liver transplantation trends show a steady decrease of the mortality on the waiting list and improved transplant outcomes, adult liver transplantation continues to be challenged by declining organ quality and increased mortality rates on the waiting list.
The aim of this article is to summarize and highlight what we believe are the most important findings of the 2014 report 1. Emphasis is placed on data relating to waitlist dynamics, organ utilization rates, the use of suboptimal grafts, and optimization of the existing limited donor pool with the intent to recognize trends and areas that could be the object of policy change, intervention, and innovation.
Waitlist Trends
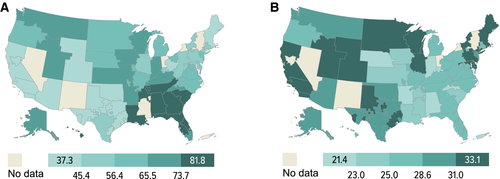
According to the recent OPTN report, as of June 2014, nearly 72 000 adults were living with a functioning liver allograft. In 2014, 6729 liver transplants were performed in adults, 6449 of which were from deceased donors, and 280 from living donors. As of the end of December 2014, 14 632 candidates were registered on the waiting list, of whom 83% (12 204) are active and 17% (2428) inactive. Over the last decade, liver transplant dynamics have been characterized by an annual addition of approximately 10 500 new patients (Figure 1), with a plateau in the number of transplants performed (around 6800/year, Figure 1). The transplants mostly consist of whole liver deceased donor grafts (approximately 95%), with roughly 15 000 patients listed at the end of each year (Figure 1). The annual number of active candidates waiting for a liver transplant has been stable over the past decade (−0.8%) 1. However, the number of patients who entered the list and died without transplant or were removed because of being too sick for transplant has increased 30% over the last decade (n = 2400 in 2004 vs. 3111 in 2014, net increase 711 patients) 2. Since 2009, mortality rates have increased each year from 11.1 per 100 waitlist years to 12.3 in 2014. In 2014, 1821 patients died while waiting for a transplant, while an additional 1290 were removed from the list because they had become too sick to undergo a transplant 1. This increase in overall waitlist mortality could partially be attributed to the higher proportions of waitlist registrants at higher medical urgency status (Model for End-Stage Liver Disease [MELD] >30), lower proportions of candidates at a lower risk of death (MELD <15), and geographic disparity in organ availability (Figure 2).
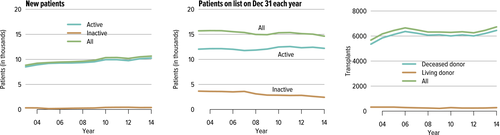
Summary:
- The number of patients in the liver transplant waiting list has plateaued, while mortality during waiting has increased.
- Living donor liver transplantation remains a very small portion of transplanted livers and has failed to increase over the past 10 years.
Current and future directions:
- Share 35 has addressed the waitlist mortality in this group. Now an emerging concern is the waitlist death rate for those patients in the next tier down MELDS >30 to <35 waiting for transplant for prolonged times.
- Strategies are desperately needed to optimize organ use, expedite placement, and improve organ donation rates.
Candidate Selection
Age and chronic renal insufficiency
Over the last decade, the number of candidates who are ≥65 years of age had almost doubled, reaching 20.8% at the end of 2014 compared to 11% in 2004 (n = 3042 and 1724 patients in 2014 and 2004, respectively). In addition, the severity of the liver disease in those patients listed and then transplanted has increased. At the end of 2014, 2.7% of listed patients had MELD ≥30, and 5.8% were listed for simultaneous liver and kidney transplantation, as compared to 0.7% and 1.7%, respectively in 2004. In 2014, 40% of actual adult liver transplant recipients had a MELD score ≥30, and a kidney graft was simultaneously transplanted in 8.7% of the cases, again compared to 20.4% and 4.9%, respectively in 2004 1. These results clearly underscore the ever-increasing observance of liver disease severity and kidney dysfunction.
Obesity epidemics
On the other side of the scale, there is a noticeable worldwide increase in the prevalence of obesity. Over the first decade of this century, a significant increase in the number of orthotopic liver transplants (OLT) performed for nonalcoholic steatohepatitis (NASH) cirrhosis was observed (1.2% in 2001 to 9.7% in 2009). It is projected that 25 million Americans will be diagnosed with NASH by 2025, with 20% progressing to cirrhosis, thus making NASH the leading new indication for liver transplantation 3, 4.
The overall impact of obesity on liver transplant outcomes is currently uncertain. While some studies have demonstrated increased morbidity, life support measures, total hospital and intensive care unit (ICU) length of stay, as well as mortality in obese liver transplant recipients 5, 6, others have reported similar graft and patient survival across different body mass index (BMI) categories 7, 8.
The American Association for the Study of Liver Diseases and the American Society of Transplantation consider a BMI ≥40 kg/m2 as a relative contraindication to transplantation 9. Lifestyle modifications including diet changes and increasing activity could be particularly challenging in the presence of liver disease. Additionally, the role of bariatric surgery is emerging as an effective way to achieve weight loss for obese patients with liver disease, and may even minimize posttransplant metabolic complications 10-14. Studies to better understand obesity in the setting of liver cirrhosis, its impact on transplant outcomes, as well as defining the weight target, are therefore necessary. Outlining a safe, effective method, including the role, timing, and type of bariatric procedures in this subset of patients, is certainly needed 15. This epidemic also further affects the donor pool where more and more donors have some degree of steatosis 16.
Hepatitis C virus (HCV) in the era of direct-acting antiviral agents (DAAs)
To date, HCV-related liver disease remains the single most common diagnosis at the time of transplant 1. Pretransplant attempts to eradicate HCV have traditionally been unsuccessful and poorly tolerated, particularly in patients with decompensated cirrhosis 17, 18. Thus, HCV recurrence has been universal and known to also negatively impact the outcome, with up to 30% of patients progressing to graft cirrhosis 18, 19. Similarly, post OLT HCV treatment has not been effective, with poor tolerability and significant interactions with immunosuppression medications being observed 20. Overall, HCV recurrence has been considered a major cause of graft loss and shortened survival in HCV liver transplant recipients 21.
In 2011, with the approval of the first generation of DAAs (i.e. boceprevir and telaprevir), a new era in HCV management began. Since then, other DAAs are in clinical application, with more expected to come. Patients who are cured of their HCV infection (defined by achieving a sustained virologic response [SVR]) 22, 23 experience numerous health benefits, including a decrease in liver inflammation, reduction of risk of liver cancer (hepatocellular carcinoma [HCC]), and the regression of fibrosis, with some patients demonstrating resolution of cirrhosis with reduction of the risk of liver-related morbidity and mortality 24-28. Achieving SVR could have a paradoxical effect on patient survival because waitlisted responders’ MELD score could decrease, making them less competitive to receive a transplant, particularly for those who depend on their biochemical MELD and do not have an exception MELD 29. In addition, responders may lose the opportunity to use a graft from HCV-positive donors. This restricted access could particularly impact patients whose disease expression is not fully represented by the biological MELD. It has been reported that HCV-positive donors compose up to 40% of donors utilized by some centers 30. Using HCV donors is a practice that has been associated with a decrease in wait time to transplantation without adversely impacting posttransplant survival 31-33. Furthermore, it is expected that there will be an increase in the allocation of grafts from donors who have a documented history of treatment response to DAAs in whom HCVab will remain detected with a negative HCV ribonucleic acid. This is an evolving issue that will need to be addressed both on a program and a national level.
It is important to note that despite the decrease in HCC risk in patients achieving SVR, patients with advanced fibrosis still remain at long-term risk for HCC, and long-term surveillance should be maintained 34-36. Future studies aimed at better identifying those patients with an increased long-term risk for HCC are needed 37.
Thus, while HCV has remained the leading indication for liver transplantation over the past 3 decades, this could soon become a less frequent indication. The greatest demand for OLT due to HCV-associated liver disease will likely be driven by development of HCC, with a projected increase in an aging population among individuals born between 1941 and 1960 38. Potential consideration for nontransplant options for patients who develop HCC after achieving SVR could even be entertained if there has been significant improvement in their liver functions.
In transplant candidates, the interferon-free DAAs regimens have proven to be much more effective in preventing posttransplant HCV recurrence, as well as being better tolerated by pretransplant patients when compared to the traditional therapy with interferon and ribavirin. The risk of post OLT HCV recurrence is inversely related to the number of consecutive days of undetectable HCV RNA prior to transplantation, with virtually no HCV recurrence if HCV RNA was undetectable for 28 days pretransplant 39. Similarly, in the event of post OLT HCV recurrence, 12 weeks of interferon-free all oral combined DAAs with ribavirin were successful to achieve remarkable SVR in up to 96–98% of patients without cirrhosis, or with compensated cirrhosis or mild impairment (Child–Pugh class A). Response rate, however, seems to drop with progression of hepatic impairment to 60–75% of patients with severe hepatic impairment 29. Ribavirin-free DAA regimens were also shown to be effective and well tolerated, with a reported SVR of 90–95% in genotype 1, especially in patients with no or early fibrosis (METAVIR F0-F2) 40, 41. DAAs also appear to be efficacious in patients with fibrosing cholestatic hepatitis 29.
The favorable characteristics of the DAAs allowed for the early initiation of therapy within the first 6 months after transplantation, even for patients with graft cirrhosis and decompensation 29, 40, 41. In view of this, it is predicted that the graft and patient survival of liver transplant for HCV will improve, with an overall decrease in the need for retransplantation related to graft loss secondary to HCV recurrence.
Customarily, in an attempt to avoid rapid progression of fibrosis and graft loss in HCV patients, the recommendations were against using grafts from older donors in HCV recipients 42, 43. It still remains to be determined whether the availability of DAAs will alter this practice.
Summary:
- Simultaneous liver and kidney (SLK) transplant has been low, but the rate is increasing. The impact is removal of a kidney from the kidney-alone list. Furthermore, this is a combination of Acute Kidney Injury in high MELD cases and there is an increase in older-age recipients and patients with chronic kidney disease (CKD) needing a liver transplant 45.
- The obesity epidemic in the donor and recipient are becoming major impediments to successful liver transplantation, while at the same time becoming an increasing indication for transplant for NASH, and on the donor side the leading cause of “turn down” of a donor liver for transplant.
- Hepatitis C remains the main indication for liver transplant. The remarkably successful use of new DAAs may eventually reduce the proportion being listed for initial transplant. Some are projecting that hepatitis C will become a minor listing entity by 2020, being replaced by NASH.
Current and future directions:
- SLK: More “selective” utilization/allocation of a kidney to patients needing a kidney with a liver with establishment of a safety net for those who post liver transplant alone remain in CKD is in policy proposal at United Network for Organ Sharing (UNOS).
- Expediting allocation to high MELD liver patients who have acute renal failure for a specified time frame in an effort to minimize permanent renal injury and need for SLK.
- Obesity: There is significant observed variation in recipient and donor acceptance for patients with a high BMI that needs consensus about best practices. Thus, a multicenter study including pretransplant therapy and a study on steatotic livers leading to best practice is in order.
- Hepatitis C: HCV therapy before or after liver transplantation is being studied, and is expected to have an impact to reduce the numbers on the waitlist. It is also expected that organ acceptance rates for hepatitis C–positive donors will change as will the need for retransplant for those with rapidly reoccurring hepatitis C.
- Most exciting are the recent reports of normothermic reperfusion of extended criteria donor, donation after cardiac death (DCD), and steatotic livers that may allow expansion of liver transplantation, but that await further confirmation in clinical trials.
Organ Utilization Trends
The mean number of all organs transplanted per donor was 3.04 in 2013, a downward trend that has been consistently observed since the highest number of 3.15 was seen in 2003. In 2014, 15% of donors nationally were DCD 1. Additionally, 8594 donors were considered eligible for liver procurement in 2014. Of these, consent was not requested or obtained in 0.9% of cases (72 donors), and only 6383 liver allografts (74.3%) were transplanted. The remaining 25.7% of the liver grafts were divided as follows: recovered for research or hepatocyte isolation 4.2% (357 livers); recovered for transplantation but not transplanted 7.9% (681 livers); organ not recovered 12.8% (1101 livers). The numbers of these “untransplantable” livers (2209) has remained relatively stable over the past decade. The most common reason for not transplanting recovered livers was biopsy findings, whereas the most common reason for not procuring a liver was, “ruled out after evaluation in the operating room” (n = 303), followed by “other” (n = 131), warm “ischemic time too long” (n = 42), “anatomical abnormalities” (n = 68), and “diseased organ” (n = 45). The most common reasons for not recovering an organ were “ruled out after evaluation in the OR” (n =248), “other” (n = 174), “poor organ function” (n = 142), organ refused by all centers (n = 101), and “time constraints” (n = 68) 1.
In 2013 and 2014, respectively, 309 and 363 liver transplants were from DCD 46. This number has remained relatively steady since the peak observed in 2007 47. In 2014, 228 adult living donor liver transplants (LDLTs) were performed, accounting for only 3.4% of all the transplants (6729). This is an overall decrease of 13.6% over a period of 10 years (273 adult LDLTs in 2004), while the number of deceased donor transplants increased by 5.4% during this period 2. However, in the past 3 years we have observed a steady and encouraging increase in adult LDLTs, with an overall increase of about 21% since 2011, when 188 adult LDLTs were performed 2.
Summary:
- Organ donor consent rate, use of DCD livers, and turn down due to biopsy results, poor “visualization,” and time constraints are some of the major barriers leading to a relatively flat rate of organ use. This area is one of the most challenging areas and one in which there are opportunities for improvement.
Current and future directions:
- More study and efforts are needed to understand the gap between true potential donors and actual donors (Figure 3).
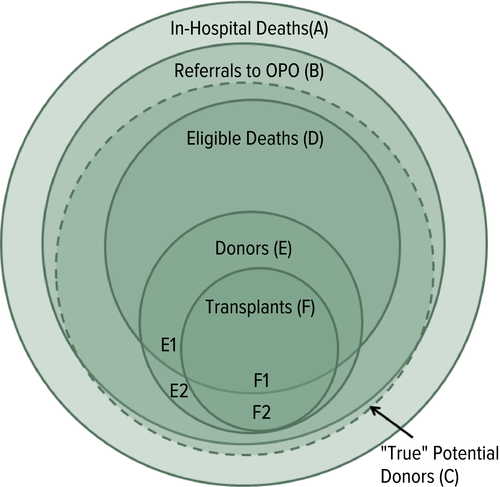 Figure 3Conceptual schematic (Venn diagram) of actual and potential organ donors. (A) Only in rare instances do out-of-hospital deaths result in organ donation. (B) Most in-hospital deaths are reported to the local organ procurement organization. (C) The number of “True” potential donors (dashed circle) is yet to be defined. (D) Eligible Deaths. (E) Actual donors, not all of whom result in transplants. (F) Deceased donor transplants. The transplanted organs that result from eligible and noneligible deaths are labeled F1 and F2, respectively. OPO, organ procurement organization.
Figure 3Conceptual schematic (Venn diagram) of actual and potential organ donors. (A) Only in rare instances do out-of-hospital deaths result in organ donation. (B) Most in-hospital deaths are reported to the local organ procurement organization. (C) The number of “True” potential donors (dashed circle) is yet to be defined. (D) Eligible Deaths. (E) Actual donors, not all of whom result in transplants. (F) Deceased donor transplants. The transplanted organs that result from eligible and noneligible deaths are labeled F1 and F2, respectively. OPO, organ procurement organization. - Organ procurement organizations need to be incentivized to pursue all donors, eliminating financial or metric concerns.
- The use of “nontransplantable” (older, steatotic, and DCD) livers needs further study and solutions implemented (e.g. expedited placement of marginal livers, rollout of best practices for use of steatotic liver, new hypothermia donor management protocols 1, novel preservation techniques [organ pumping and pharmacologic therapy]) in order to modify (resuscitate) the marginal organ or reduce the amount of steatosis that may lead to more useable organs 2. Coupling these therapies with a safety net for poor function or biliary complications allows retransplant. This allowance linked with protection from outcomes review from UNOS or impact on SRTR program results will be paramount.
- Split liver transplantation for two adults remains underutilized. Technical aspects, coordination, and allocation policies are among the issues that needed to be addressed to help expand this practice.
These abovementioned trends offer an invaluable opportunity to identify areas in which to focus future research.
Over the past few years, the total number of organ donors has slowly increased 51; nonetheless, the number of liver donors has not proportionally grown due to a net increase in the number of discarded livers. In 2004, 14.8% of donated livers were not used 52, while this percentage rose to 25.7% in 2014. This dramatic increase is multifactorial. First and foremost, this is a direct consequence of the increasing donor age and prevalence in the general population of obesity, diabetes, and hypertension 52. From 2001 to 2010, the mean donor age increased by 9.3 years (from 34.6 to 43.3), the prevalence of obesity (donor BMI >30) doubled (from 15% to 30.3%), and the prevalence of diabetes and hypertension increased from 3.2% to 12% and from 22.6% to 37.2%, respectively 52. Secondly, as Medicare scrutiny for transplant program outcomes increased, transplant centers became more risk adverse and therefore more reluctant to use expanded criteria grafts 53. Orman et al 16, using a sophisticated statistical forecasting model (Discrete Event Simulation), projected a decrease in the overall liver utilization rate from 77.9% in 2010 to 43.6% in 2030. According to their modeling, 43.7% of the donors will be older than 50 years, 45.7% will be diabetic, and 58.2% will have a BMI >30. These changes in donor quality would result in 2230 fewer liver transplants per year by 2030.
Orman et al 16 modeled the implementation or strategies to counteract this trend and reported that only two significantly affected (>5%) the organ utilization rate: converting 90% of DCD donors to donation after brain death (DBD) and the introduction of a disruptive technology such as ex vivo organ preservation. In spite of the several limitations intrinsic to the statistical forecast model used, this study substantiated the day-to-day perception that each liver transplant professional has about the progressively declining organ quality. Another study from Parikh et al found similar results. They also projected that although the donor population may increase over the next decade, general population growth will exceed donor growth, therefore exacerbating even further donor shortages and mortality on the waiting list.
Over the past decade, expansion of DCD transplantation has remained a controversial issue. While DCD donors represent a precious source of livers and kidneys, there is a concern that the decline in DBD donation may be one of the unexpected consequences of the Organ Donation Breakthrough Collaborative 54. In spite of single center acceptable results 55, 56, DCD liver transplantation remains associated with significantly lower graft and patient outcomes, as shown by the OPTN report. Since such an unfavorable impact is not present in kidney transplant 57, 58, a conundrum has been created.
DCD kidney transplantation has been increasing over the past 10 years; however, every effort should be made in the future to ensure that DCD donation overall does not negatively impact the quality of liver grafts. This could happen if withdrawal of support occurs at an early stage in those donors that are likely to progress to brain death 59. As a consequence the end-stage liver disease patient will be adversely affected while the end-stage renal disease patients will see no negative effect. If Orman and colleagues’ projections hold true, the proportion of DCD donors could reach 36% by 2030 16.
For this reason, in the past decade researchers have placed increasing effort to investigate mechanism of injury during preservation of marginal liver grafts. There is growing evidence that most of the damage occurring during preservation is a direct consequence of static cold storage (SCS) 60, 61, the current (and for the past 4 decades) standard of care in organ preservation. Several groups in the United States, and especially Europe, recently introduced ex vivo machine perfusion in human trials with very promising results 62-65. Data suggest that perfusing livers ex vivo at normothermic temperature, though more challenging and expensive compared to SCS and hypothermic machine perfusion, carries potential key advantages. The most important one is the ability to use the device as an assessment tool for the graft. At physiologic temperature, in fact, a functioning organ is fully metabolically active, as demonstrated by active bile production 66 and the ability to clear lactate 67. This key potential predictive/rehabilitative tool is what has been missing in the liver transplantation field as organ quality has declined. If normothermic machine preservation continues to advance as it has in the past few years, very soon we will be able to test and safely use many of the livers currently discarded. Other advantages of ex vivo perfusion technologies include superior preservation 68, the ability to pharmacologically support the organ during perfusion, and the potential to enhance the physiologic cell repair that follows an ischemic injury 69. The latter would have a central role in the restoration, optimization, and ultimately increased use of DCD grafts.
Liver Transplant Outcome Trends
In 2014, as compared to 2004, patients approached liver transplantation in much worse clinical condition, as shown by both a higher proportion of patients with a MELD score >30 (40.4% vs. 20.4%, respectively), and those hospitalized at the time of transplant (35.7% vs. 28.7%, respectively). Interestingly, thus far, despite the escalation of the severity of liver transplant recipients, graft survival continued to improve (Figure 4). In addition, the incidence of acute rejection continued to decline; this might be related to the increasing use of induction agents, although various other factors may also account for this. The overall 5-year graft survival was 70.2% for patients who underwent a liver transplant in 2009 1.
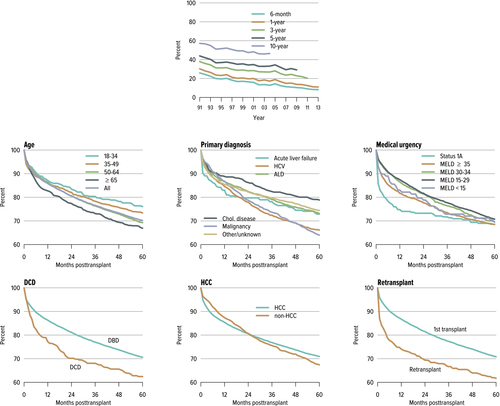
This favorable graft survival, despite higher proportion of patients with high MELD, should be critically evaluated as this could be partially related to transplanting patients with HCC MELD exceptions. In 2014, HCC MELD exceptions were utilized by 25% of transplanted patients, with a portion of them with exception MELD points of ≥35 despite a lower biochemical MELD score. Also, the majority of recipients in 2014 (>50%) had MELD scores of 15–29, which might account for why the overall outcomes are still favorable 1.
However, particular recipient and donor characteristics have been associated with poor outcome. Early survival (1 year) was poorest for status 1A patients, retransplants, and recipients of DCD livers. Intermediate (1–3 years) and long-term survival (>3 years) was poorest for recipients aged ≥65 years of age, or those with HCV infection or malignancy 1 (Figure 4).
Although it is expected that the new antiviral agents will decrease HCV-related graft and patient loss, donor factors (as discussed earlier) and recipient characteristics are likely to worsen. In fact, the liver transplant recipient trends over the last decade demonstrated an alarming change with increase in recipients who are ≥65 years of age 38, as well as a dramatic increased NASH cirrhosis 3, 4, 15. With the current MELD allocation system with the Share 35 and capping of HCC MELD policy it is expected that older, obese patients with complex medical problems requiring significant ICU support and who are physiologically sicker will be prioritized to receive a liver transplant. This highlights the importance of both defining and predicting futility for the increasingly sick patient population.
Posttransplant outcomes: defining and predicting futility
The definition of futility in liver transplantation remains challenging. From the patient's perspective, any potential benefit might exclude futility. From the ethicist's perspective, lack of success of 100 similar cases has been suggested as a working threshold to define futility. From a societal perspective, given the current liver graft scarcity, it is unlikely that survival rates as low as 1% would be considered satisfactory. A minimum acceptable predicted absolute posttransplant survival is needed. Several graft and patient survival thresholds have been considered, but none have suggested graft survival rates less than 50% at 1 year 70, 71.
Predicting futility is certainly another challenge. Currently the MELD score that determines priority measures the degree of sickness from a liver standpoint, but fails to account for other preoperative factors that could be crucial to determine a patient's tolerability and survival of a liver transplant 72, 73. Clinicians have determined candidacy of these sick patients using the “eyeball test,” which currently remains a key element of bedside decision making. There is an urgent need to complement this with more objective parameters. Recently, multiple studies have aimed at defining futility, identifying clinical factors associated with futile transplant, and developing a futility risk model, thus hoping to have a good pretransplant discriminatory ability 74. Furthermore, others have focused on assessing sarcopenia and frailty to predict posttransplant outcomes 75-78.
Summary:
- Transplant outcomes remain good despite older patients and higher MELD score at transplant.
- Futile transplants (i.e. transplanting when a patients is too sick with poor outcomes) is a concern, especially in Donor Service Areas (DSAs) with high MELD at transplant.
Current and future directions:
- Multicenter studies to better define clinical, radiographic, and biochemical factors associated with futile transplantation would lead to better selection of recipients and subsequent reduction in the wastage of an organ on predictably poor outcome.
Allocation
Despite the changes made in the liver transplant allocation system, it is still true that where candidates are listed affects how likely they are to receive a transplant, and therefore their likelihood of death. Multiple proposals to address how to optimize liver distribution and enhance recipient outcomes have been suggested 81, 82. The prediction of their effectiveness, logistical aspects, financial impacts, and influence on patient outcome is currently being studied 83-85. It is expected that results of further studies involving financial implication, true practical applications, and logistic feasibility that includes input from all stakeholders will likely change the current map of organ allocation to reduce geographic disparities.
Candidates receiving exception MELD points for HCC experience higher transplant rates, but have a lower mortality risk than candidates without HCC, and the transplant rates for candidates with HCC were more than twice the rates for candidates without HCC. Recently, revisions to the OPTN liver allocation policy were implemented on October 8, 2015, modifying the maximum value of exception scores for candidates with HCC (capped at 34), and the timing of exception scores assigned to HCC candidates (A 6-month run-in period prior to receiving a score of 28). The effects of this recent change remain to be determined.
Share 35 policy
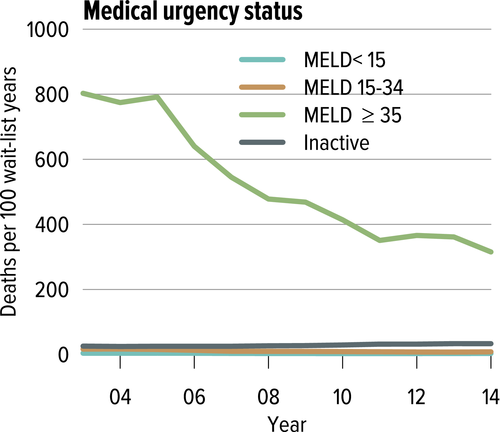
In June 2013, The Share 35 policy was implemented to help address waitlist mortality high MELD patients whose mortality was comparable to status 1A patients. Using the similar status 1A system, this has had a favorable impact to patients with MELD >35. Their median waiting time halved (from 18 days in 2012 to 9 days in 2014); these patients constituted 26.1% of all liver transplants performed in 2014, resulting in a decrease in waitlist mortality among these candidates from 366 per 100 waitlist years in 2012 to 315 per 100 waitlist years in 2014 (−13.9%) (Figure 5). However, in some regions this has been associated with consequences of increased travel costs and coordination efforts, but may be balanced by reduced hospital costs. The latter effect may be due to shortening wait time for hospitalized patients with high true laboratory MELDs. Further investigation of this is necessary to understand the true total impact of these changes to include changes in program behavior.
National and regional geographic disparity
Summary:
- Overall, increasing the number of organ donors should be the primary focus since it remains the cornerstone of increasing the number of transplants.
- The Share 35 policy was implemented to help address regional geographic disparity that was impacting access to organs for high MELD patients. It has had a predicable positive impact (but minor) on high MELD patients receiving a transplant with more lives saved with some downside consequences that were unanticipated. Further improvements and study are needed.
Current and future directions:
- Redistricting into super-regions is being evaluated and may be an additional step toward leveling the geographic disparity; however, other disparities may be accentuated. This controversial issue must be solved in both the patient's best interest and with the most effective use of a scarce national resource in mind. Alternative modeling and proposals are being considered in an effort to optimize organ use, balanced against increased costs with shipping, regional variation in donation rates, and transplant center acceptance rates. In addition, incorporating access to liver transplants with death on the waitlist needs to be modeled as well.
- Additional changes that have been enacted as new UNOS policies include a hold on small HCC, capping all HCCs at MELD 34, and re-evaluating non-HCC exceptions in an effort to level the waitlist death rate to assure equal outcomes for exceptions and high MELD patients.
- A national MELD exception review Board has been proposed to standardize listing acceptances for exceptions.
Discussion
The 2014 OPTN/SRTR report has offered throughout the years an important snapshot of the transplantation activity in the United States. The recently released report on adult liver transplantation shows very clearly that every year the number of patients in need of a liver transplant progressively exceeded the number of transplants performed. This resulted in an increase in the mortality on the waiting list, and increasingly worsening conditions at the time of transplant, with older and more morbid patients approaching surgery. At the same time, the quality of organ donors continued to deteriorate, as shown by the decline of the number of organs transplanted per donor. In spite of the declining recipient and donor quality, liver transplant outcomes continued to improve over time, with 5-year graft survival rates reaching 70.2% 1. With the most recent changes now being implemented for listing and capping of MELD points for HCC and efforts to standardize exception points across the country, it is too early to tell where disparities will exist in the future. Geographic disparity and access to organs will continue to be an important issue.
While UNOS continues to address geographic disparities, there should be considerable focus in the next decade to promote innovation in the area of donor and graft optimization (donor intervention, new preservation solutions, and machine perfusion).
Furthermore, with the approval of human immunodeficiency virus–positive (HIV+) donors for HIV recipients and the cure of HCV, the landscape for the definition of high risk as defined by the Public Health Service will change. An uncharted territory that likely will appear and increase is organs offered (liver and extrahepatic organs) from patients who had SVR after being treated with DAAs with an undetectable HCV via the nucleic acid test, and their allocation and utilization in HCV-naive critical patients. How these new advances will affect who is offered and receives these organs will require further thought, discussion, and ethical consideration. Most importantly, innovation needs to be encouraged to overcome the persistent shortage of organs, but with a provision to exclude innovative practices from Centers for Medicare and Medicaid Services reprisal.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




