Failure of Calcineurin Inhibitor (Tacrolimus) Weaning Randomized Trial in Long-Term Stable Kidney Transplant Recipients
Abstract
Long-term renal transplant outcome is limited by side effects of immunosuppressive drugs, particularly calcineurin inhibitor (CNI). We assumed that some patients selected for a “low immunological risk of rejection” could be eligible and benefit from a CNI weaning strategy. We designed a prospective, randomized, multicenter, double-blind placebo-controlled clinical study (Eudract: 2010-019574-33) to analyze the benefit–risk ratio of tacrolimus weaning on highly selected patients (≥4 years of transplantation, normal histology, stable graft function, no anti-HLA immunization). The primary endpoint was improvement of renal function. Fifty-two patients were scheduled in each treatment arm, placebo compared to the CNI maintenance arm. Only 10 patients were eligible and randomized. Five patients were assigned to the placebo arm and five were assigned to the tacrolimus maintenance arm. In the tacrolimus maintenance arm, all patients maintained stable graft function and no immunological events occurred. Contrastingly, in the placebo arm, all five patients had to reintroduce a full dose of tacrolimus since three of them presented an acute rejection episode (one humoral, one mixed, and one borderline) and two displayed anti-HLA antibodies without histological lesion (one donor-specific antibodies [DSA] and one non-DSA). Clearly, tacrolimus withdrawal must be avoided even in long-term highly selective stable kidney recipients.
Abbreviations
-
- ALS
-
- anti-lymphocyte serum
-
- AUC
-
- area under the curve
-
- CNI
-
- calcineurin inhibitor
-
- DSA
-
- donor-specific antibodies
-
- HES
-
- hematoxylin eosin safran
-
- IvIg
-
- intravenous immunoglobulin
-
- MMF
-
- mycophenolate mofetil
-
- PAS
-
- periodic acid–Schiff
-
- SAE
-
- serious adverse events
-
- TAC
-
- tacrolimus
Introduction
In kidney transplantation, the routine use of calcineurin inhibitor (CNI) has contributed to controlling acute rejection rates and has changed transplantation and patient outcomes in the short term 1. However, chronic exposure of patients to CNI is responsible for an increased risk of infections and malignancies 2-8. Most attempts to decrease or discontinue CNI prescriptions were generally performed on unselected patients and were associated with an increased risk of rejection 9-14 and in “real life,” patients who experienced CNI noncompliance increased their risk of graft dysfunction 15.
Recent, more cautious trials have thus involved patients defined as “at low risk of rejection” or “low immunological risk” 16-19, suggesting that some patients may be eligible for such strategies.
Based on these observations, our working hypothesis was that strict screening of patients for clinical and biological stability for more than 4 years should facilitate investigation of gradual CNI weaning. We proposed a prospective, randomized, multicenter, double-blind and placebo-controlled clinical study (Eudract: 2010-019574-33) (http://www.fondation-centaure.fr/) to analyze the benefit–risk ratio of tacrolimus (TAC) weaning in highly selected patients. Following an extremely stringent selection process, only 10 of the 1500 prescreened patients (0.66%) were randomized. We thus showed that CNI weaning is associated with an unexpected risk of rejection even in patients with a very low immunological risk. These findings are discussed in the context of designing an immunosuppression minimization protocol following kidney transplantation and avoiding future failed trials mainly due to the repetition of the same errors worldwide because of the lack of publication.
Methods
Endpoints
Primary endpoint: Improvement in renal function 1 year after complete TAC weaning evaluated by the GFR up to 7.5 mL/min calculated by cystatin C measurement according to Le Bricon's equation. GFR was compared between day 0 (d0: start of the weaning procedure) and day 480 (d480: 1 year after the end of weaning). Secondary endpoints: biopsy-proven acute and chronic rejection, occurrence of or worsening interstitial fibrosis and tubular atrophy lesions (IFTA), de novo anti-HLA immunization, daily proteinuria, incidence of cancers, infections, cardiovascular and/or metabolic events, and assessment of the quality of life and graft loss.
Patient recruitment
Prescreen criteria: Between 18 and 80 years old, first kidney transplantation from a heart beating or living donor, transplanted between 4 and 10 years before the study onset, daily proteinuria <0.5 g/day, no significant changes (±25%) in mean creatinemia levels during the year preceding enrollment. Inclusion criteria: No subclinical rejection on baseline histology, cystatin C measured GFR (mGFR) ≥40 mL/min, and patients under TAC (Prograf; Astellas, Le vallois-Perret, France) with a C0 trough level between 5 and 10 ng/mL combined with mycophenolate mofetil (MMF; Cellcept, Roche, France) with a MMF area under the 0 to 12 h concentration–time curve (AUC12) >30 mg·h/L, with or without steroids as maintenance therapy; no anti-HLA/DSA (donor-specific antibody) antibodies using Luminex HD technology. Noninclusion criteria: Suspicion of noncompliance. Patients who met the inclusion criteria signed the informed consent form and were randomized 1:1 (CNI weaning/CNI maintaining arm with double-blind status).
Description of treatments
Tacrolimus and MMF doses were first adjusted to enter into the defined range before randomization. Patients were randomized double blind to two treatment arms: either the tacrolimus weaning (placebo so-called NO TAC) arm or the maintenance (TAC) arm. In the NO TAC arm, TAC was progressively tapered by one third of the initial dose every 2 months and replaced by a placebo for complete weaning 6 months after inclusion. Patients still received MMF during the trial and afterwards. In the TAC arm, all patients received tacrolimus and mycophenolate mofetil (MMF). In both arms, patients who received low-dose steroids before inclusion continued to receive the same dose of steroids.
Treatment preparation and data collection
Astellas Pharma provided the PROGRAF® and a placebo formulation for PROGRAF®. Both were delivered in bulk at Nantes University Hospital, which carried out blister packaging, labeling, and delivery. The placebo was made with empty caps entered into a verum cap. Routine laboratory tests (creatinemia, daily proteinuria, and eGFR using the MDRD formula) and physical examination were followed up, respectively, every 15 and 30 days during the 480-day trial period. Anti-HLA/DSA antibody monitoring and identification, MMF AUC, and mGFR (Cystatin C) were assessed at day 30, d120, d300, and d480.
Sample size
One hundred six patients (53 patients in each arm) were estimated for inclusion in order to achieve a 5% alpha risk (α) and 80% test power (1−β) for a unilateral test. We assumed an improvement in the GFR of 7.5 mL/min/1.73 m2 and a GFR level in patients in the maintaining arm at 65.5 mL/min (±5.5).
Supervisory and Scientific Committee
A Supervisory Committee was responsible for ensuring trial safety and could decide at any time to stop the study. A Scientific Committee ensured that the trial was conducted in accordance with the protocol and evaluated all ethical aspects and reserved the right to decide to suspend or continue the trial in accordance with the Supervisory Committee.
Anti-HLA immunization monitoring
A three-step procedure was designed (see 3 section). For the first phase of the protocol, the presence of pregraft anti-HLA class I and II immunization was determined by a Luminex screening using LABScreenT mixed technology (LAT-M; One Lambda, Canoga Park, CA). When serum was positive with LABScreenT mixed, a LABScreenT single Antigen assay (Labscreen Single Antigen; One Lambda) was performed. A LABScreenT single Antigen assay allows identification of the anti-HLA antibodies using several types of beads coated with only one antigen per bead. The positive threshold was fixed at 2000 for mean fluorescence intensity (MFI). For the second phase, LABScreenT single Antigen assay was performed retrospectively (already enrolled patients) and prospectively for all the sera and the positive threshold was fixed at 2000 for the MFI. In the third phase, the MFI threshold was decreased to 1000.
Histological analysis
Graft biopsies were performed on day -30 and -480 to eliminate subclinical acute or chronic humoral and/or cellular rejection before inclusion and to diagnose rejection or IFTA progression. Supplementary graft biopsies were performed when graft rejection was suspected or DSA (MFI > 2000) identified. Serial deparaffinized 4-μm sections stained with hematoxylin eosin safran (HES), periodic acid–Schiff (PAS), Masson's trichrome, and Jones’ colorations were scored by two pathologists. C4d deposition was analyzed.
Results
Description of the enrollment process
Among 1500 prescreened patients, 166 met the preinclusion criteria among which 12% were not included because of nonacceptance, 40.5% due to the presence of anti-HLA antibodies, 2.5% due to proteinuria >0.5 g/day, 12% due to eGFR (using MDRD) <40 mL/min, and 23.5% for other criteria (cancer, infection). Following this screening, 16 patients out of the 166 (9.5%) prescreened patients were retained and only 10 patients were eligible and randomized between May 2011 and July 2014 (Figure 1). At the end of July 2014, the Scientific Committee decided to interrupt the protocol on the advice of the Supervisory Committee, given the number and severity of the side effects in the TAC withdrawal arm and the enrollment difficulties. Since we had to stop the study prematurely, it was not possible to statistically assess the effect of CNI weaning on the primary endpoint, namely, to compare patient characteristics in both treatment arms and the improvement in renal function 1 year after complete TAC withdrawal.
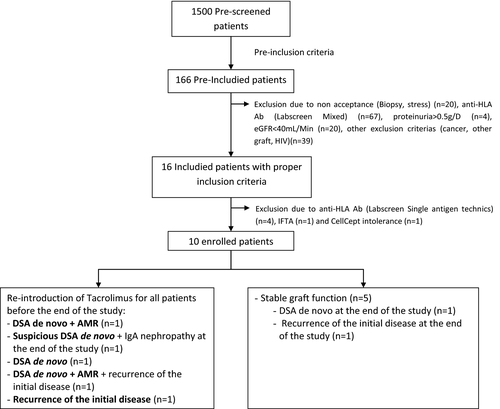
Characteristics of the patients at baseline
The demographics and clinical characteristics of patients are described in Table 1. All patients received a kidney graft from a deceased donor and had induction protocols (anti-thymocyte globulin [ATG] or Simulect; Novartis, Boulogne Billancourt, France). The time postgraft medians at baseline were 6 years including at least 4 years with very stable graft function (median: 6 years ± 1.27 for TAC and 6 years ± 2.05 for NO TAC). The median donor age was 50 ± 7.26 years for the TAC arm and 34 years ± 11.28 in the NO TAC arm. The median age of the recipients at transplantation time was 55 years ± 7.89 for TAC versus 40 years ± 12.12 for NO TAC and 61 years ± 8.09 for TAC versus 45 years ± 13.95 for the NO TAC arm at the time of enrollment. One patient in the NO TAC arm had a past history of acute rejection episode before inclusion. All patients had normal histology at d30. At baseline, all patients were anti-HLA negative according to the LABScreenT mixed Luminex analysis (Table S1). All patients were taking at least 0.75 g/day of MMF and at least 3 mg/day of TAC at inclusion.
| Demographic and clinical characteristics | Patients in the TAC arm | Patients in the NO TAC arm |
|---|---|---|
| n = 5 | n = 5 | |
| Time postgraft at inclusion (years) |
Median 6 Range (4–7) |
Median 6 Range (5–10) |
| Donor gender M/F | 3/2 | 3/2 |
| Recipient gender M/F | 4/1 | 4/1 |
| Donor age (years) |
Median 50 Range (42–62) |
Median 34 Range (21–50) |
| Recipient age at transplantation time (years) |
Median 55 Range (38–58) |
Median 40 Range (29–60) |
| Recipient age at inclusion time (years) |
Median 61 Range (43–62) |
Median 45 Range (35–70) |
| First graft | 5/5 | 5/5 |
| Induction therapy | 5 (2 ALS-3 Simulect) | 5 (1 ALS-4 Simulect) |
| Blood transfusion (Y/N) | 2/3 | 3/2 |
| Cold ischemia (min) |
Median 2172 Range (857–2376) |
Median 927 Range (437–2620) |
| Recipient positive for CMV | 3 | 0 |
| Recipient positive for EBV | 5 | 5 |
| Acute rejection episode | 0/5 | 1/5 |
| HLA A-B-DR incompatibilities | 3-1-2-2-2 | 1-3-3-3-1 |
| HLA A incompatibilities | 0-1-1-1-0 | 1-1-1-2-0 |
| HLA B incompatibilities | 2-0-1-0-0 | 0-1-1-0-1 |
| HLA DR incompatibilities | 1-0-0-1-2 | 0-1-1-1-0 |
| Pregraft immunization | 0/5 | 2/5 |
| Postgraft anti-HLA immunization after starting protocol | 0/5 | 4/5 |
| Postgraft DSA immunization after starting protocol | 1/5 | 3/5 |
| Treatment before starting protocol |
Cellcept (Range: 0.75–1.5 g/day) Prograf (Range: 3.5–6 mg/day or 0.041–0.075 mg/kg/day) |
Cellcept (Range: 1–2.5 g/day) Prograf (Range: 3–6 mg/day or 0.041–0.096 mg/kg/day) |
- ALS, antilymphocyte serum; CMV, cytomegalovirus; DSA, donor-specific antibodies; EBV, Epstein-Barr virus; TAC, tacrolimus.
Clinical outcome of the patients
Of the 10 patients enrolled, 5 patients were randomized in the TAC arm and 5 in the NO TAC arm. No death or graft loss occurred. Patient follow-up and outcome are described in detail in Supplementary Data S1, Figure S1.
In the TAC arm, all patients maintained stable graft function and no immunological events occurred. Contrastingly, in the NO TAC arm, all patients had to reintroduce a full dose of TAC because of serious adverse events (SAE) leading to interruption of the trial (Figure 1): (1) appearance of de novo anti DR53 (MFI 2092) DSA (Patient 8), (2) non-DSA anti-HLA and IFTA grade 1 on biopsy performed at the end of the study (Patient 7), (3) a mixed acute graft rejection with anti-DQ 7 (MFI 8237) and DQ-9 DSA (MFI 5513) and a positive CDC cross-match on B cells treated with plasma exchanges, intravenous immunoglobulin (IvIg) and two rituximab infusions and steroid boluses (Patient 6), (4) acute humoral rejection with anti-DQ5 DSA (MFI 11 452) and non-DSA anti-HLA DQ6 (MFI 9603) treated with rituximab, IvIg, and plasma exchanges followed by the recurrence of IgA nephropathy on the final biopsy (Patient 9), and (5) borderline rejection associated with a recurrence of IgA nephropathy (Patient 10). Among the five patients in the NO TAC arm, three completed the total TAC weaning (d120). The two other patients only achieved two thirds of TAC weaning (continued to receive one third of the initial TAC dose [d90]) (Table S2).
De novo and/or pre-existing DSA
Three patients in the NO TAC arm tested positive for DSA using the LABScreenT single Antigen assay after randomization, without clinical evidence of graft dysfunction. In one patient, TAC was re-administered although anti-HLA was not accompanied by rejection. In the two other patients, anti-class 2 DSA were detected on day 180 after randomization (DQ7 and DQ9 for Patient 6, DR53 for Patient 8, and DR14, DQ5, DP09:01 for Patient 9) (Table S1). In the TAC arm, one patient tested positive for anti-class 1 DSA at the end of the trial (d480). Overall, DSA were detected in 4 out of 10 patients: 1/5 in the TAC arm and 3/5 in the NO TAC arm. Since all patients tested negative for anti-HLA antibodies using the LABScreen mixed assay at the time of inclusion, the Supervisory Committee decided to test all patients for antibodies retrospectively at baseline using the highly sensitive LABScreenT single antigen assay (positivity >1000). This analysis revealed that DSA were already present at baseline in one patient of the TAC arm (Cw16 = 2621) despite good graft function during the trial and normal histology. In the NO TAC arm, two out of the five patients had pre-existing DSA and one patient developed DSA de novo (Patient 9). One patient with pre-existing DSA and one patient with de novo DSA developed antibody-mediated rejection (AMR) in this arm.
Graft histology
All patients presented a normal histology on routine baseline biopsy. Among the five patients in the NO TAC arm, three biopsies were performed because of DSA identification on days 60 (Patient 7) and 180 (Patient 6 and 9) showing a normal histology (Patient 7) and two acute rejections (Patient 6 and 9), and one was performed due to a graft dysfunction episode on day 90 (Patient 10) with evidence of borderline lesions associated with recurrent IgA nephropathy. At the end of the trial, histology was normal for all patients in the NO TAC arm. In the TAC arm, IgA nephropathy recurred in one patient at the end of the trial (Patient 2). Overall, three of five of NO TAC patients developed signs of rejection (two AMR, one borderline rejection) and no patients in the TAC arm. Two had recurring IgA nephropathy: one in each arm, both detected at the end of the trial.
Graft function during the trial
Creatinemia and eGFR levels were similar in the two treatment arms from d60 until the end of the trial. We did not observe any differences in the treatment arms either before or after TAC withdrawal. There was no obvious clinical evidence of a difference between creatinemia, proteinuria, and MDRD eGFR levels in the four patients who had pre-existing or de novo DSA (one in the TAC arm and three in the NO TAC arm).
Safety
No SAE was observed in TAC patients during the trial. After the trial, we observed initial disease recurrence in patient 2. In the NO TAC arm, one patient had pneumonia and severe agranulocytosis following the rejection treatment, one experienced initial disease recurrence, two presented facial squamous cell carcinoma, and one had a transient cerebral ischemic attack during the protocol.
Discussion
We hypothesized that safe gradual weaning from TAC could be successful if restricted to patients with highly stable graft function and normal histology for at least 4 years and a low immunological risk profile (no anti-HLA immunization). Our aim was to establish secure procedures for immunosuppression-weaning protocols. The rationale of this hypothesis has been built on by years of observations of minimization procedures 9, 11, 14, 16, 20-23 and a focus on operational tolerance 24, 25. Most of the prior trials of CNI weaning were conducted 1 year or less after transplantation, based on the assumption that CNI-associated toxicity mainly occurred in the first year following transplantation at a time when lesions were irreversible. However, several studies also show a sizeable increase in GFR, even when CNI weaning is initiated at a later stage 10. Thus, the literature suggests that selecting “low-risk” patients (absence of acute rejection and panel-reactive antibody) is fundamental but also that the CNI weaning procedure should be initiated later after transplant and that MMF treatment, the only immunosuppressive treatment remaining, may represent a key factor to reduce the risk of graft rejection.
Recent studies performed on “better selected” patients led to disappointing conclusions. Hricik et al reported on a prospective study of nonsensitized primary recipients of living donor kidney transplants receiving ATG, TAC, MMF, and prednisone 26. Six months posttransplantation, patients without de novo DSA, acute rejection, or inflammation at protocol biopsy were randomized to wean off or remain on TAC. As for our own study, it was terminated prematurely because of unacceptable rates of acute rejection and/or de novo DSAs in the TAC withdrawal arm. Despite the fact that we selected patients with longer posttransplantation times and tightly monitored the AUC of MMF, our assay leads to the same conclusions.
Our trial had to be stopped because all of the patients of the NO TAC arm were restarted on a full dose of TAC before the end of the trial for serious immunological events. Moreover, the rigorous patient selection process renders patient recruitment impossible since only 10 patients were enrolled in accordance with our selection criteria. Even ancillary studies could not be informative in these 10 patients since the clinician had to reintroduce the drugs immediately, which could prevent the development of some regulatory mechanisms. However, such regulation processes are possible only following the decrease in immunosuppressive drugs that create beneficial conditions for some small cell populations to expand 27. Regarding this point, investigations on other immunosuppressive drugs using different mechanisms from the CNIs led to the development of “second generation” drugs, such as belatacept 28, 29, which may prove a better “alternative” than CNI withdrawal.
Thus, based on what has been achieved over the past decade, it is unclear whether significant progress has been made to date. CNI withdrawal in kidney transplantation appears to be very difficult. Which cells are involved? Analysis of the T, B, and NK cells compartment during the trial did not revealed any difference between the two groups or during the TAC withdrawal period, probably due to the too-short break in TAC administration (data not shown). Do we have to take more risk and/or do we need to rethink our protocol strategies? This is the instrumental question and it seems logical to conclude with Hricik et al that TAC withdrawal has to be avoided at this stage and in such conditions. As reported by Matas et al 30, there is now an evident need for biomarkers, and the explosion of such research is promising, but “clinical care responsibilities, regulatory oversight, and limited research funding” are still brakes to the success of such projects.
Acknowledgments
This work has been carried out with the support of the Labex IGO project (No. ANR-11-LABX-0016-01) and the LABEX TRANSPLANEX (ANR-11-LABX-0070_TRANSPLANTEX), funded by the “Investissementsd'Avenir” French Government program, managed by the French National Research Agency (ANR) and the EU consortium BIO-DrIM (www.biodrim.eu). This work was carried out within the scope of a CENTAURE foundation grant and under the auspices of the IHU-Cesti project, which received French Government financial support managed by the National Research Agency via the “Investment into the Future” program ANR-10-IBHU-005. We wish to thank the Supervisory Committee in charge of trial safety and the Scientific Committee, S. Garde (Nantes University Hospital, Nantes, France), Dr. A. Chiffoleau (Nantes University Hospital, Nantes, France), Dr. L. Flet (Nantes University Hospital, Nantes, France), Pr. C. Legendre (Necker University Hospital, Paris, France), Pr. E. Morelon (Descartes University Hospital, Lyon, France), Pr. P. Landais (Necker University Hospital, Paris, France), Pr. E. Thervet (Georges Pompidou University Hospital, Paris, France), and Pr. E. Cozzi (Azienda University Hospital, Padova, Italy), who ensured that the trial was conducted in accordance with the protocol, evaluated all related ethical aspects, and decided on any appropriate changes to be made to the protocol in order for the trial to continue.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




