Interferon Gamma and Contact-dependent Cytotoxicity Are Each Rate Limiting for Natural Killer Cell–Mediated Antibody-dependent Chronic Rejection
Abstract
Natural killer (NK) cells are key components of the innate immune system. In murine cardiac transplant models, donor-specific antibodies (DSA), in concert with NK cells, are sufficient to inflict chronic allograft vasculopathy independently of T and B cells. In this study, we aimed to determine the effector mechanism(s) required by NK cells to trigger chronic allograft vasculopathy during antibody-mediated rejection. Specifically, we tested the relative contribution of the proinflammatory cytokine interferon gamma (IFN-γ) versus the contact-dependent cytotoxic mediators of perforin and the CD95/CD95L (Fas/Fas ligand [FasL]) pathway for triggering these lesions. C3H/HeJ cardiac allografts were transplanted into immune-deficient C57BL/6 rag−/−γc−/− recipients, who also received monoclonal anti–major histocompatibility complex (MHC) class I DSA. The combination of DSA and wild-type NK cell transfer triggered aggressive chronic allograft vasculopathy. However, transfer of IFN-γ–deficient NK cells or host IFN-γ neutralization led to amelioration of these lesions. Use of either perforin-deficient NK cells or CD95 (Fas)–deficient donors alone did not alter development of vasculopathy, but simultaneous disruption of NK cell–derived perforin and allograft Fas expression resulted in prevention of these abnormalities. Therefore, both NK cell IFN-γ production and contact-dependent cytotoxic activity are rate-limiting effector pathways that contribute to this form of antibody-induced chronic allograft vasculopathy.
Abbreviations
-
- CAV
-
- chronic allograft vasculopathy
-
- DSA
-
- donor-specific antibodies
-
- FasL
-
- Fas ligand
-
- IFN-γ
-
- interferon gamma
-
- MHC
-
- major histocompatibility complex
-
- NK
-
- natural killer
Introduction
Solid organ transplantation is an important therapy for patients with end-stage organ dysfunction. One-year adjusted graft survival rates have steadily increased within the last 10 years and are now >80% for all solid organ recipients 1-5. Despite this improvement in early success rates, long-term graft outcomes have not improved significantly in the last 20 years 6, 7, and the immunological mechanisms that drive chronic allograft dysfunction remain poorly understood. Donor-specific antibodies (DSA) have recently been shown to be associated with this process 6 and, clinically, the de novo development of DSA is associated with decreased survival in kidney, heart and lung transplant recipients 8-13. Using a murine heterotopic heart transplant model, Hirohashi et al (14) determined that natural killer (NK) cells interact with DSA through a crystallizable fragment (Fc) receptor–dependent mechanism to trigger chronic allograft vasculopathy (CAV) . Once generated, this route of CAV development occurs independently of T cells or host complement components 14. However, it is unclear which effector pathways utilized by NK cells contribute to this pathogenic process.
NK cells make up approximately 10–15% of the circulating lymphocyte population and are an important component of the innate immune system. When NK cells are “armed” with specific antibodies by binding of the antibody Fc region, they provide an antigen-specific means of directing NK cell reactivity, effectively linking the innate and adaptive immune response. Specifically, NK cell activation results in expression of key inflammatory mediators, notably interferon gamma (IFN-γ) 15. In addition, NK cell activation can trigger two independent contact-dependent cytolytic pathways: one involving secretion of the pore-forming protein perforin and the second involving CD95L (Fas ligand [FasL]) expression leading to target cell apoptosis through an interaction with CD95 (Fas) on these cells 15-18. Prior studies of acute allograft rejection mediated by CD4+ or CD8+ T cells have indicated that both IFN-γ and a combination of perforin and the Fas/FasL pathway are essential for allograft injury 19-22. In the present study, we sought to determine whether NK cells contributing to antibody-dependent chronic rejection require similar pathways to those utilized by cytotoxic T cells during acute rejection.
Materials and Methods
Mice
C3H/HeJ (C3H, H-2k), C3.MRL-Faslpr/J (C3H.lpr, H-2k), C57BL/6 (B6, H-2b), B6.129S7-Rag1tm1Mom/J (B6.rag−/−, H-2b), B6.129S7-Ifngtm1Ts/J (B6.IFNγ−/−, H-2b) and C57BL/6-Prf1tm1Sdz/J (B6.pfp−/−, H-2b) mice aged 8–10 weeks were obtained from the Jackson Laboratory (Bar Harbor, ME). B10;B6-Rag2tm1Fwa II2rgtm1Wjl (B6.rag−/−γc−/−, H-2b) mice were obtained from Taconic Biosciences (Hudson, NY) and bred in our facility according to a material transfer agreement. All mice were maintained under pathogen-free conditions throughout the experiments and were cared for according to methods approved by the American Association for the Accreditation of Laboratory Animal Care. All live animal experiments and procedures were performed with the approval of the Institutional Animal Care and Use Committee at the University of Colorado, Denver, Anschutz Medical Campus.
Heterotopic heart transplantation
Cardiac allografts from C3H or C3H.lpr donor mice (10–20 weeks old) were transplanted heterotopically into either T cell/B cell–deficient B6.rag−/− or T cell/B cell/NK cell–deficient B6.rag−/−γc−/− recipients (10–20 weeks old) as previously described according to standard microsurgical techniques 23, 24. Briefly, the donor aorta and pulmonary artery were anastomosed to the recipient abdominal aorta and inferior vena cava, respectively. The transplanted hearts were removed on day 30, and the allografts were cross-sectioned into three parts (base, middle, and apex). All parts were fixed in 10% formalin and embedded in paraffin. Sections were stained using Verhoeff's method 25 for elastic fibers to evaluate and quantify the severity of coronary artery lesions.
Administration of monoclonal antibodies
Anti-H2k IgG2a (clone 36-7-5) monoclonal antibody (mAb) was obtained from BioXCell (Lebanon, NH). Noted B6.rag−/− or B6.rag−/−γc−/− mice were given intraperitoneal (IP) injections of mAb at a dose of 30 μg in 100 μL 0.9% normal saline beginning the day after transplantation and twice weekly (eight doses total) as previously described 14; the last dose was administered 2 days prior to allograft recovery (day 28). Anti-IFN-γ antibody (clone XMG1.2) mAb was recovered and quantified from in vivo hybridoma ascites production. Noted B6.rag−/− recipients were given IP injections of 1 mg in 200 μL 0.9% normal saline that were administered beginning the day of transplantation (day 0) and subsequently on days 3, 6, 9 and 15 posttransplantation (five doses total).
NK cell isolation and adoptive transfer
Splenocytes from 8- to 12-week-old B6, B6.pfp−/− and B6.IFN-γ−/− mice were utilized as the source of adoptively transferred NK cells. Briefly, T cells were depleted from donors in vivo by administration of anti-CD4+ (clone GK1.5, BioXCell) and anti-CD8+ (clone 2.43, BioXCell) antibodies (dose of 10 mg/kg) 6 days before spleen recovery to minimize contamination from these cells. NK cells were then enriched from this whole splenocyte preparation by negative selection with an NK cell isolation kit (Miltenyi Biotec, San Diego, CA) used according to the manufacturer's instructions. Isolation resulted in NK populations that ranged in purity from 65% to 85% (CD3ε− CD122+ NK1.1+) as determined by flow cytometry. This cell preparation was also analyzed for the presence of CD4+ (CD3ε+ CD45.2+ CD4+) and CD8+ T cells (CD3ε+ CD45.2+ CD8a+). Enriched NK cells (1.5 × 106) were adoptively transferred intravenously via retro-orbital injection on day 1 posttransplantation. All B6.rag−/−γc−/− recipients that received adoptively transferred cells were treated with additional doses of anti-CD4+ and anti-CD8+ mAb (dose of 10 mg/kg on day 1 posttransplantation) to further ensure that any potentially contaminating T cells would not participate in a subsequent response.
Histological techniques and morphometric analysis
Morphometric analysis was performed on images of coronary arteries from the three tissue sections of the explanted cardiac allografts. An image of all vessels larger than 85 μm in diameter was captured digitally by light microscopy at 10× magnification. Image processing and analysis with ImageJ software (NIH, Bethesda, MD) were used to manually demarcate the borders of the lumen and the intima of the artery. The software then quantitated the luminal and intimal areas; from these area values, the neointimal index (NI) was defined as the neointimal area divided by the neointimal area plus the luminal area multiplied by 100, as previously described 26. This quantity was calculated for each vessel, with the NI reported for each recipient representing the average of the individual values over the three cross sections obtained per recipient.
Flow cytometry
Flow cytometric analysis was used to assess the purity of adoptively transferred NK cells prior to transplantation. Cells obtained after NK isolation (see previous sections) were incubated for 20 min at 4°C with CD3ε−PerCP/Cy5.5 (clone 145.2C11, BioLegend, San Diego, CA), CD122-FITC (clone TM- β1, BD Pharmingen, Franklin Lakes, NJ) and NK1.1-APC (clone PK136, eBioscience, San Diego, CA). To detect the possible presence of CD4+ and CD8+ T cells, a separate cell preparation was also stained with CD45.2-APC (clone 104, eBioscience), CD3ε−PerCP/Cy5.5, CD4-Pacific Blue (clone RM4-5, BD Biosciences) and CD8a-PerCPCy5.5 (clone 53-6.7, BD Biosciences, San Jose, CA). The cells were then washed once with phosphate-buffered saline (PBS), 1% bovine serum albumin (BSA) and 0.1% sodium azide (fluorescence-activated cell sorting (FACs) buffer); data were collected using a BD LSR II flow cytometer (BD Biosciences) and analyzed with FlowJo software (Ashland, OR). This same analysis was also used to detect the presence of these adoptively transferred cells in recipients posttransplantation. Here, the spleen was recovered from recipient animals on day 30 and depleted of erythrocytes via use of a red blood cell lysis buffer (Sigma-Aldrich, St. Louis, MO) and resuspended in the FACS buffer. Cells were incubated with the same antibodies and analyzed as described previously.
Statistical methods
The NI is expressed as the median ± interquartile range. The Mann–Whitney U test was performed to compare the NIs between two groups. Cell population numbers posttransplantation are reported as the mean ± the standard error of the mean (SEM). For cell population numbers posttransplantation, a Student's t-test was performed to compare two groups, and a one-way analysis of variance (ANOVA) was used to compare multiple groups. A p-value of <0.05 was considered significant. GraphPad Prism (La Jolla, CA) and Microsoft Excel statistical software (Seattle, WA) were used for statistical analysis.
Results
Donor-specific antibody in conjunction with adoptively transferred NK cells can induce CAV
As previously described by Hirohashi et al 14, we found that administration of DSA (α-H2k IgG2a mAb) into NK cell–replete B6.rag−/− recipients bearing C3H allografts induced pronounced CAV at 30 days posttransplantation (NI 18.4% ± 18.1%) (Table 1; Figures 1A and B and 2). These vascular lesions were heterogeneous by nature (exemplified in Figure 1A), as shown in prior studies 27. However, allografts of untreated NK cell–replete B6.rag−/− recipients showed little CAV (NI 1.86% ± 1.45%) (Figures 1C and 2), indicating that NK cells alone are insufficient to mediate injury. In B6.rag−/−γc−/− recipients that received only DSA, there was again no significant CAV (NI 1.99% ± 1.99%), suggesting that DSA alone is insufficient to induce vascular changes. Furthermore, there was no significant difference between these latter two groups and B6.rag−/−γc−/− recipients that received neither DSA nor NK cells, who demonstrated little CAV and a low NI (1.97% ± 1.71%) (Figures 1D and 2).
| Group | Donor | Recipient | Treatment | Frequency of CAV |
|---|---|---|---|---|
| 1 | C3H | B6rag−/− | No Treatment | 0/5 |
| 2 | a-H2k | 5/6 | ||
| 3 | C3H | B6.rag−/−γc−/− | No Treatment | 0/5 |
| 4 | a-H2k | 1/5 | ||
| 5 | α-H2k + B6 wild-type NK cells | 6/6 |
- CAV, chronic allograft vasculopathy; DSA, donor-specific antibodies; NK, natural killer.
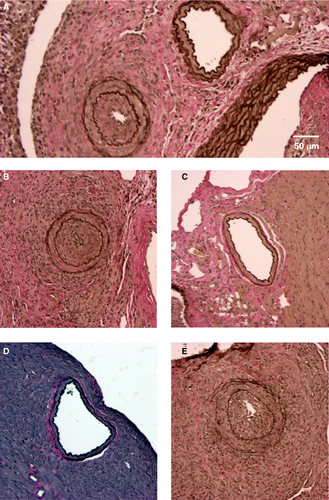
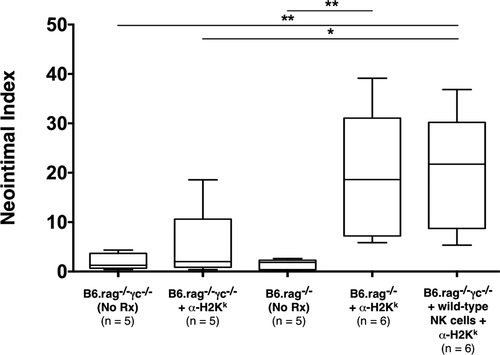
To test the role of specific NK cell–derived effector pathways contributing to CAV, we next developed a model utilizing NK cell adoptive transfer into B6.rag−/−γc−/− recipients, which are deficient in the common gamma chain and thus lack mature NK cells 28. B6.rag−/−γc−/− recipients bearing C3H allografts that received DSA in conjunction with adoptively transferred B6 wild-type NK cells showed marked vascular abnormalities, with 100% of animals (6/6) demonstrating CAV (Table 1). In addition, histological examination showed prominent neointimal formation in the coronary arteries of these cardiac allografts (Figure 1E). Importantly, there was no significant difference between the degree of CAV in this group and NK cell–replete B6.rag−/− recipients that received DSA (NI 18.4% ± 18.1% vs. 21.5% ± 20.1%, respectively; p = 0.937) (Figure 2). This effectively demonstrated that CAV induced by DSA in B6.rag−/− mice could be replicated via adoptive transfer of NK cells into B6.rag−/−γc−/− mice.
NK cell–mediated CAV is IFN-γ dependent
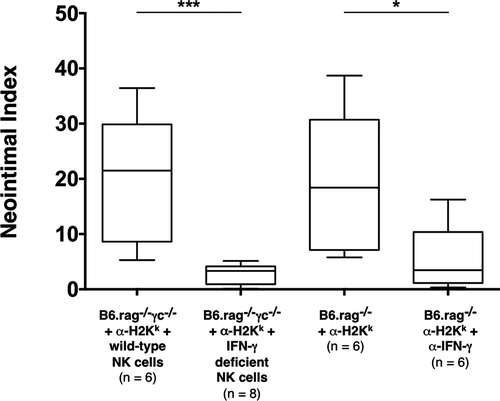
NK cell activation leads to the production of a number of inflammatory mediators, particularly INF-γ 29. Thus, we hypothesized that NK cells may mediate CAV through the secretion of this cytokine. As described previously, administration of DSA and transfer of wild-type NK cells to B6.rag−/−γc−/− recipients resulted in pronounced CAV in all of the allografts recovered. However, administration of DSA into B6.rag−/−γc−/− recipients that instead received adoptively transferred B6 NK cells deficient in IFN-γ production resulted in complete amelioration of these lesions (NI 3.3% ± 2.9% in IFN-γ–deficient NK cell recipients vs. 20.4% ± 20.1% in wild-type NK cell recipients; p = 0.0007). To determine if pharmacologic blockade of this cytokine would result in the same effect, C3H allografts were transplanted into B6.rag−/− recipients that received DSA. In addition, recipient animals were also administered anti-IFN-γ mAb. CAV severity was significantly lower in this group as compared to previously described control animals (NI 3.5% ± 4.2% in anti-IFN-γ mAb recipients vs. 18.4% ± 18.1% in control recipients; p = 0.0411). Quantitatively, there was no significant difference between the CAV lesions in the group that received pharmacologic cytokine blockade and the previously described group that utilized IFN-γ–deficient NK cells (NI 3.5% ± 4.2% in anti-IFN-γ mAb recipients vs. 3.3% ± 2.9% in IFN-γ–deficient NK cell recipients; p = 0.491) (Table 2 and Figure 3). Taken together, these data show that lack of NK cell–derived IFN-γ production or systemic neutralization of this cytokine significantly attenuated CAV.
| Group | Donor | Recipient | Treatment | Frequency of CAV |
|---|---|---|---|---|
| 1a | C3H | B6.rag−/− | a-H2k | 5/6 |
| 2 | α-H2k + α-IFN-γ | 1/6 | ||
| 3b | C3H | B6.rag−/−γc−/− | No Treatment | 0/5 |
| 4c | α-H2k + B6 wild-type NK cells | 6/6 | ||
| 5 | α-H2k + B6.IFN-γ−/− NK cells | 0/8 | ||
| 6 | α-H2k + B6.pfp−/− NK cells | 4/6 | ||
| 7 | C3H.lpr | B6.rag−/−γc−/− | α-H2k + B6 wild-type NK cells | 6/8 |
| 8 | α-H2k + B6.pfp−/− NK cells | 0/6 |
Perforin and Fas play alternate but obligatory roles in NK cell–mediated CAV
Given the additional role of NK cells as mediators of contact-dependent target cell lysis, we also examined the contribution of these pathways on CAV development. NK cells depend primarily on the perforin system to mediate target cell lysis, but they can also express FasL upon activation and can utilize this pathway as well to mediate cell death 30-33. Therefore, we hypothesized that either perforin or Fas/FasL interaction could be a key mechanism by which NK cells facilitate injury within our model. To test whether perforin deficiency would have an effect on CAV, C3H allografts were transplanted into B6.rag−/−γc−/− recipients that received DSA. Recipients also received adoptively transferred B6 NK cells deficient in perforin production. Lesions triggered by perforin-deficient NK cells appeared to be somewhat less severe than controls, but the quantitative difference on statistical analysis was not significant (NI 9.1% ± 8.6% in perforin-deficient NK cell recipients vs. 21.5% ± 20.1% in control recipients; p = 0.240) (Table 2 and Figure 4).
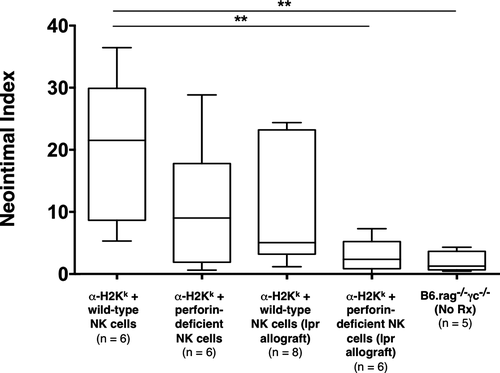
Alternatively, to test if Fas/FasL interaction was required for this form of chronic rejection, Fas-deficient C3H.lpr allografts were transplanted into B6.rag−/−γc−/− recipients that received DSA, as described previously, and adoptively transferred wild-type B6 NK cells. Due to lack of expression of Fas on cardiac allograft (target) cells, the NK cells would be unable to utilize this pathway to mediate cell lysis. On histological examination, the vascular lesions again appeared to be minimally reduced (with wider variability) but were not statistically significant from controls (NI 5.1% ± 8.8% in recipients bearing C3H.Ipr cardiac allografts vs. 21.5% ± 20.1% in control recipients; p = 0.081) (Table 2 and Figure 4).
Although deficiency in either pathway alone did not appreciably alter the development of CAV lesions, we then sought to test the hypothesis that perforin and Fas/FasL represent alternative but essential mechanisms of NK cell–mediated chronic allograft rejection, as found in prior studies of cytotoxic T cell–mediated acute rejection 20, 21. Therefore, we combined the transfer of perforin-deficient NK cells with the transplantation of Fas-deficient C3H.lpr allografts to achieve simultaneous disruption of perforin and Fas/FasL pathways in B6.rag−/−γc−/− recipients receiving DSA. Interestingly, none of the animals within this group (0/6) showed significant CAV lesions, demonstrating that concurrent disruption of NK cell–derived perforin and allograft Fas expression resulted in a marked reduction of CAV in comparison to controls (NI 2.4% ± 2.6% in perforin and Fas-deficient recipients vs. 21.5% ± 20.1% in control recipients; p = 0.0043) (Table 2 and Figure 4). Notably, we also confirmed that perforin-deficient NK cells retain the capacity to produce IFN-γ (Figure S1).
Adoptively transferred NK cells persist within recipients and are detectable posttransplantation
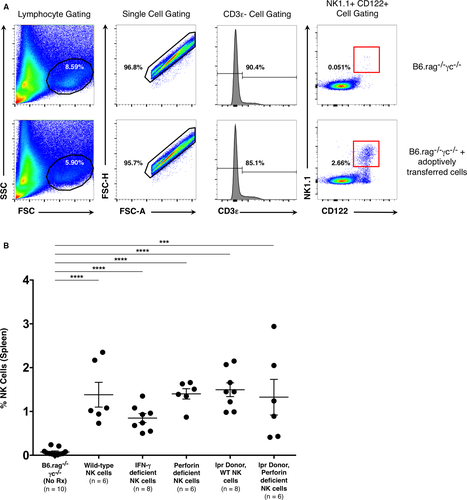
A key issue underlying the interpretation of these experiments is whether the NK cell phenotypes utilized impacted engraftment and survival of these cells in adoptive transfer recipients. Prior studies have shown that wild-type NK cells undergo homeostatic expansion when adoptively transferred into a lymphopenic environment; importantly, these cells are long lived and retain their functionality 34, 35. To ensure that adoptively transferred NK cells survived in the B6.rag−/−γc−/− recipients described previously, whole spleens were recovered from recipient animals at the time of allograft recovery. Analysis by flow cytometry confirmed the presence of NK cells as characterized by a CD3ε−, CD122+, NK1.1+ phenotype. This analysis also established that the adoptively transferred cells from all experimental groups survived and persisted in the recipients, with no significant difference between the treatment groups (p = 0.226) (Figure 5). Recipient spleens were also analyzed for the presence of either CD4+ (CD45.2+, CD3ε+, CD4+) or CD8+ (CD45.2+, CD3ε+, CD8+) T cells. Neither T cell population could be detected (data not shown), further confirming that T cells were unlikely to contribute to the aforementioned responses.
Discussion
Despite extensive characterization of acute allograft rejection in both vascularized and nonvascularized transplant models, there is surprisingly little evidence elucidating the precise mechanism(s) that underlie the immune response in chronic allograft rejection. In addition, the exact role of DSA and their contribution to this process also remain unclear. Prior results indicate that NK cells can play a key role in antibody-mediated chronic rejection by interacting with DSA through an Fc receptor–dependent manner to trigger CAV independently of other cellular or complement components 14. NK cells are potent mediators of innate immunity, and NK cell responses in solid organ transplantation have been studied in both animal models and clinical studies 36-41. However, much of the current understanding of NK cell biology is derived from basic in vitro cellular analysis and studies based on viral infectious diseases and tumor immunity. In the setting of transplantation, the role of these cells is much less clear, as NK cells have been shown to have both pathologic and regulatory effects depending on the timing of cellular interactions and the nature of the allograft itself 42.
In the present study, we show that CAV inflicted by NK cells requires NK cell expression of IFN-γ. IFN-γ is considered the “prototypical” proinflammatory cytokine and has been shown to have both pathogenic and regulatory roles in the T cell–mediated alloimmune response. On one hand, IFN-γ production correlates strongly with acute rejection; for example, it is required for efficient CD8+ T cell–mediated islet cell rejection 19 and for the rejection of MHC class II skin allografts 43, 44. The presence of donor IFN-γ receptors is also required for CD4+ T cell–mediated acute cardiac rejection 22. Paradoxically, however, the presence of IFN-γ has also been associated with allograft prolongation. In murine skin and cardiac models of costimulation blockade, IFN-γ production facilitates long-term allograft survival, presumably through limiting expansion of allograft-reactive activated T cells 45. NK cells are known to be key producers of a number of cytokines, notably IFN-γ. In the context of virally infected cells, this cytokine has direct cytotoxic effects and perpetuates a continued inflammatory response because it is a potent activator of both NK cells and macrophages 46, 47. IFN-γ also induces IL-12 secretion, which further increases NK cell–mediated toxicity and promotes additional IFN-γ production 29, 48, 49. The present results indicate that NK cell IFN-γ production does not merely correlate with CAV but is actually a rate-limiting effector pathway in this response.
NK cells are also “early responders” to sites of inflammation and infection, and a key differentiator of NK cells from conventional cytotoxic T cells is the ability to kill target cells rapidly without the requirement for prior immunization 50. Similar to cytotoxic T cells, NK cells directly induce target cell apoptosis, mainly through secretion of perforin but also through Fas/FasL interactions 30-33. As the sensitivity to cytolytic pathways is likely dictated by the target cell 20, 51, both pathways were interrogated as potential contributors to triggering CAV. Our data show that individual disruption of either NK cell–derived perforin or allograft Fas expression did not significantly alter the development of CAV. However, the simultaneous disruption of NK cell perforin production and allograft Fas expression largely attenuated lesion development. This suggests that CAV is mediated by alternative, but obligate utilization of these cytolytic processes. Taken in combination, our data indicate that both IFN-γ and the alternative pathways of perforin and Fas/FasL are rate limiting in this model of chronic rejection.
Based on current findings, we would propose the possibility of a “two-hit” model of NK cell–mediated CAV in vivo. As the first step, IFN-γ clearly plays an important role in the NK response, and it is possible that this cytokine first “conditions” the target cells to be more efficiently killed by a given cytolytic pathway. One possible mechanism is through upregulation of NK cell–activating ligands or downregulation of NK cell inhibitory ligands. The current prevailing view of NK cells is that they distinguish between healthy and damaged cells by integrating signals from stimulatory and inhibitory receptors expressed on target cells. The resultant sum of these signals then results in either NK cell activation or quiescence 52. Both the secretion of cytokines and the activation of cytolytic machinery are dependent on this interplay of surface and intracellular receptors 53. Classically, the main inhibitory ligand recognized on target cells is MHC class I, leading to the “missing self” concept of target cell recognition (reviewed in reference 54). However, the lack of MHC class I molecules alone is not sufficient for activation of NK cells 55, suggesting that regulation of other ligands must be altered to induce an NK cell response. Another possibility is that IFN-γ is involved by making target cells more sensitive to contact-dependent cytotoxic killing. For example, expression of platelet-activating factor receptor (PAFR) on tumor target cell membranes is required to render the membrane susceptible to NK cell perforin-mediated damage, and IFN-γ has been shown to induce PAFR expression on target cells 56. Therefore, it is possible that target cell susceptibility to NK cell–mediated lysis is not an inherent property of these cells but requires the expression of appropriate cell-surface ligands to occur.
Interestingly, taken as a whole, these results are quite similar to those of previous studies of CD8+ T cell–mediated acute islet allograft rejection and CD4+ T cell–mediated acute cardiac allograft rejection. Diamond and Gill (19) showed that IFN-γ was essential for efficient CD8+ T cell–mediated rejection of islet allografts. A follow-up study by Sleater et al (21) demonstrated that individual disruption of either T cell–derived perforin or allograft Fas expression alone did not significantly delay CD8+ T cell–mediated rejection but that simultaneous interruption of T cell–derived perforin and allograft Fas expression completely diminished CD8+ T cell–mediated rejection. In CD4+ T cell–mediated acute cardiac allograft rejection, expression of the IFN-γ receptors on donor cells was required 22. Furthermore, Grazia et al (20) showed that the individual disruption of either CD4+ T cell–derived perforin or donor Fas expression had no significant impact on the tempo of rejection but that simultaneous removal of both donor Fas expression and CD4+ T cell–derived perforin abrogated the response. Although our model system utilizes the additional requirement of DSA, the parallels between cytotoxic T cells and NK cells are striking. Taken together, we would postulate that these varied contact-dependent cellular pathways of acute and chronic immune-mediated injury might utilize analogous effector mechanisms to govern their response.
Limitations of these studies should be mentioned. Although NK cells require IFN-γ within this process, the exact downstream effect of this cytokine is currently unknown. The timing of this process is unclear as well; the majority of allograft injury could be initiated early during the peritransplant period or may be a sustained response over time. Lastly, this model was specifically developed to query the mechanisms involved in NK cell–mediated chronic allograft dysfunction, and thus contribution from the adaptive immune system (T cells) was intentionally eliminated. NK cells can either inhibit or enhance T cell responses (reviewed in reference 57) and, in a physiologic transplantation setting, the overall importance of the NK cell response in this setting remains unclear.
In conclusion, results show that NK cell–mediated CAV requires both IFN-γ production as well as contact-dependent cytotoxic activity. These results also highlight the redundancy of the immune system and raise the possibility that both acute and chronic contact-dependent allograft rejection may have similar requirements for inflicting tissue injury, whether it is through an adaptive (CD4+ or CD8+ T cell) or an innate (NK cell) mediated immune response.
Acknowledgments
This work was supported in part by National Institutes of Health (NIH)/National Center for Advancing Translational Science Colorado CTSA Clinical and Translational Sciences Award (CTSA) Grant UL1 TR001082 and in part by a Fellowship Grant from the International Society for Heart and Lung Transplantation (ISHLT). The contents are the authors’ sole responsibility and do not necessarily represent official NIH or ISHLT views. The authors would like to thank Dan Koyanyagi for his expertise in the preparation of all histology samples.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




