Autophagy: Basic Principles and Relevance to Transplant Immunity
Abstract
Autophagy developed into a rapidly expanding field detailing its molecular mechanism and relevance in health and disease. Autophagy is an evolutionarily conserved process that summarizes a pathway in which intracellular material is degraded within the lysosome and where the macromolecular constituents are recycled. This “self-eating” process was originally described in a cell under starvation but now numerous studies established autophagy as a cellular response to stress. As a consequence, the autophagy machinery interfaces with most cellular stress–response pathways, including those involved in controlling immune response and inflammation. Autophagy also influences adaptive immunity through its effect on antigen presentation, naïve T cell repertoire selection and homeostasis and TH cell polarization. Data are emerging that dysregulated autophagy has an impact on human pathologies including infectious diseases, cancers, aging and neurodegenerative conditions. This review focuses on recent findings elucidating the ability of autophagy to be of significance in the transplant setting.
Abbreviations
-
- ATG
-
- autophagy-related gene
-
- cFLIP
-
- cellular caspase-8-like inhibitory protein
-
- DAMPs
-
- damage-associated molecular patterns
-
- ER
-
- endoplasmic reticulum
-
- HMGB1
-
- high mobility group box protein 1
-
- IFN
-
- interferon
-
- IR
-
- ischemia and reperfusion
-
- LC
-
- light chain
-
- mTOR
-
- mechanistic target of rapamycin
-
- mTORC
-
- mTOR complex
-
- NLR
-
- NOD-like receptor
-
- NLRP
-
- NOD-leucine-rich repeat pyrin domain containing protein
-
- NOD
-
- nucleotide-binding oligomerization domain-containing protein
-
- PAMPs
-
- pathogen-associated molecular patterns
-
- PI3K
-
- phosphatidylinositol 3-kinase
-
- PRR
-
- pattern recognition receptors
-
- RIPK
-
- receptor interacting protein kinase family
-
- ROS
-
- reactive oxygen species
-
- TH
-
- T helper
-
- TLR
-
- Toll-like receptor
-
- Treg
-
- regulatory T cell
Introduction
The term autophagy was first described 45 years ago and refers to a group of diverse processes. There are at least three different types, including macroautophagy, chaperone-mediated autophagy and microautophagy. Macroautophagy, usually referred to as autophagy, is the subject of this review. Autophagy is different from other cytoplasmatic digestive methods and represents a catabolic process able to capture and eliminate large diverse substrates (e.g. intra- and extracellular proteins, damaged organelles and invading microorganisms) 1. These targets can be sequestered within a double-membrane vesicle (the autophagosome) and delivered to the lysosome for degradation (Figure 1). Clearing the cell of unwanted constituents and recycling cytoplasmic material are the two central physiological functions of autophagy with the goal to maintain normal cell physiology, macromolecular synthesis and energy homeostasis even during stressful conditions. Consequently, dysfunction of the autophagy machinery leads to accumulation of abnormal proteins or damaged organelles and is linked to human diseases such as diabetes, autoimmunity, cancer, neurodegenerative and infections 2. Increasingly, autophagy-related research has focused on its role in inflammation and immune responses but only recently was the connection of autophagy with transplant immunity explored (Table 1) 3-5.
| Interaction | Function |
|---|---|
| Innate immunity | |
| Cytokines | Autophagy inhibition increases IL-1α, IL-1β and IL-18 secretion in response to LPS stimulation 21 |
| Intracellular organisms | Xenophagy is a selective form of autophagy in which intracellular pathogens can be eliminated. This pathway can be initiated by SLRs or by NOD2-ATG16L1 interaction 1 |
| Inflammasome | Basal autophagy suppresses inflammasome activation by clearing the cytoplasm of debris and defective organelles that can function as endogenous inflammasome agonists 25; autophagy-based unconventional secretory pathway for extracellular delivery of IL-1β depends on inflammasome 46 |
| Toll-like receptors | TLRs and autophagy influence each other. Autophagy stimulated by TLRs enhances antigen presentation by dendritic cells 47 |
| Adaptive immunity | |
| Dendritic cells | Autophagy increases MHC class II presentation of cytoplasmatic antigens (self or viral) 47; autophagy transports endogenous protein from cytoplasm into the lumen of antigen processing compartments 48 |
| T cells | Autophagy affects T cell survival after TCR activation and destabilizes the immunological synapse; autophagy affects central tolerance by thymic naïve T cell repertoire selection 27 and their survival and function by removing mitochondria and the endoplasmatic reticulum 29, 31; Beclin-1 facilitates T effector cell death during tolerance induction 3 |
| B cells and plasma cells | Autophagy is needed for the development and survival of B1 cells 37; in absence of autophagy plasma cells secrete excessive amount of immunoglobulins 39 |
- LPS, lipopolysaccharide; SLRs, sequestosome 1-like receptors; NOD, nucleotide-binding oligomerization domain-containing protein; ATG, autophagy-related gene.
In this review, we describe briefly the molecular regulation of autophagy and discuss the current state of knowledge of its role in cell survival, innate and adaptive immunity, alloimmunity, autoimmunity, ischemia and reperfusion (IR) injury.
Molecular Regulation
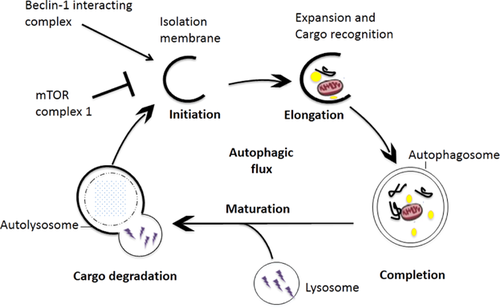
Autophagosomes are believed to emerge at least in part from the endoplasmic reticulum (ER) membranes. The molecular basis of autophagosome formation has been extensively studied in yeast cells and most of the autophagy-related gene (ATG) are conserved in mammals 6. The autophagosome formation involves three morphological stages: initiation (formation of phagophores), elongation and completion of the autophagosome and maturation (fusion of autophagosomes with lysosomes to form autolysosomes) (Figure 1) 1.
The processes of autophagosomal formation is tightly regulated. Serine/threonine protein kinase ULK1 (ATG1 in yeast) and ULK2 are being controlled by the nutritional regulator mechanistic target of rapamycin (mTOR). Upon activation, ULK1 forms a complex with the Beclin-1 (ATG6 in yeast), class III phosphatidylinositol-3-phosphate kinase (VPS34) and ATG14-like protein (ATG14L). This upstream complex stimulates the formation of the isolation membrane of the autophagosome. Next, ubiquitin-like conjugation systems, ATG5–ATG12, conjugate with ATG16L1. To facilitate elongation and closure, this newly formed complex activates the microtubule-associated protein 1 light chain 3 (LC3)–PE conjugation system by addition of a phosphatidylethanolamine group to carboxyl terminus of mammalian paralogues of ATG8, which is formed by LC3A, LC3B, LC3C, γ-aminobutyric acid receptor-associated proteins 1.
Targeted deletion of ATG5, ATG6 (Beclin-1) and ATG7 in the mouse leads to perinatal lethality. Therefore, conditional KOs and tissue-specific deletions are required to study this process in vivo 6. It is important to note that while the role of autophagy is often tested using deletion of ATG proteins there is growing array of nonautophagic functions for ATG proteins. As a consequence, some of the assumed autophagy-related functions can be in fact autophagy-independent properties 7.
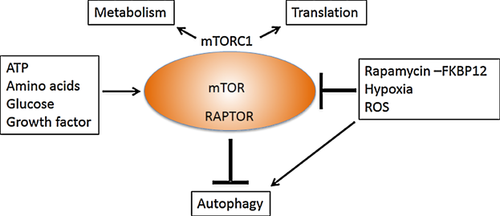
Autophagy occurs at a basal level and can be induced in response to environmental signals including nutrients, cytokines and microbial pathogens (Table 2). Starvation is a potent inducer of autophagy by inhibition of mTOR. mTOR functions as a central sensor of cellular energy and belongs to a macromolecular complex, mTORC1 1.
| Induction | Suppression |
|---|---|
| PAMPs: LPS, PGN | BCL-2 |
| DAMPs: HMGB1, ROS, ATP, DNA complexes | STAT3 |
| PRR: NOD-like receptors, Toll-like receptors | Cytokines: IL-4, IL-13, SDF1, IGF1 |
| Cytokines: IFNγ, TNFα, TGFβ |
- PAMPs, pathogen-associated molecular pattern, LPS, lipopolysaccharide; PGN, peptidoglycan; DAMPs, damage-associated molecular pattern; ATP, adenosine triphosphate; PRR, pattern recognition receptors; NOD, nucleotide-binding oligomerization domain-containing protein; HMGB1, high mobility group box protein 1; ROS, reactive oxygen species; BCL, B cell lymphoma; STAT, signal transducer and activator of transcription; SDF, stromal cell-derived factor; IGF1, insulin-like growth factor 1; IFN, interferon; TNF, tumor necrosis factor.
Measuring Autophagy
Monitoring autophagy is challenging given that this process is dynamic and the number of autophagosomes observed at any specific time point can reflect either increased formation due to increased autophagic activity, or reduced rate of their conversion to autolysosome 6. While electron microscopy is the best way to visualize autophagosomes, it is not very useful to quantitatively assess autophagy activity or flux. LC3 is the only known mammalian protein that specifically associates with the autophagosome membrane. The conversion of the cytosolic truncated form of LC3 (LC3-I) to its autophagosomal membrane-associated form (LC3-II), assessed by immunoblotting, indicates the formation of autophagosomes. It is important to note that these methods are steady-state analysis and rely on the induction of autophagy, not the completion. This is in contrast to autophagic flux monitoring, which analyzes the complete process of autophagy. Autophagy flux is best assessed using inhibitors of lysosomal proteolysis (e.g. chloroquine, bafilomycin or leupeptin) allowing examining the lysosomal turnover of intra-autophagosomal LC3-II 6. Autophagic flux can be expressed as the difference in LC3-II signal with and without lysosomal protein inhibitors. Automated fluorescence and luminescence-based strategies can measure the turnover of specific substrates, including LC3B, p62/SQSTM1 (sequestrome 1) and polyglutamine-protein aggregates. GFP-LC3 in transfected cells or transgenic mice can be used to monitor autophagic flux in vitro or in vivo 8. In addition, novel assays based on flow cytometry allow autophagic flux measurements in mixed populations of primary cells 9. However, to date no conventional autophagic flux assay is suitable and validated for clinical samples.
Autophagy and Cell Survival
The role of autophagy in promoting cell death is controversial. Some of the controversy is caused by the definition of cell death induced by autophagy. Criteria proposed to define cell death by autophagy include that it is independent of apoptosis, in the presence of autophagic flux, and the inhibition of autophagy prevents cell death 10. At least in mammals neither excessive nor impaired autophagy has been convincingly demonstrated to be a direct, caspase-independent cause of cell death without signs of apoptosis. This led to the term “autophagy-associated” cell death rather than autophagy-induced cell death.
Crosstalk with cell death pathways
Important regulators (p53, phosphatidylinositol 3-kinase [PI3K]/Akt axis, Bcl-2, ER stress) control and trigger both apoptosis and autophagy, signifying the complex interrelation of these pathways 10. Autophagy and apoptosis often occur in the same cell but autophagy usually manifests well before apoptosis. If apoptosis is blocked (e.g. by removing pro-apoptotic proteins BAX, BAK) autophagy induction is intensified. In addition, cells can be more susceptible to apoptosis when autophagy is inhibited 10. The sequential activation of both processes is explained by the fact that similar molecular switches are involved in the regulation of intrinsic stress that determine whether a cell undergoes apoptosis or autophagy (e.g. NFκB, HMGB1, high mobility group box 1 protein [HMGB1], p53). Of interest is the role of p53 because siRNA to inhibit p53 is currently used in a clinical trial to prevent ischemic injury after kidney transplantation (ClinicalTrials.gov ID NCT00802347). The protein p53 can suppress autophagy in its basal state and when activated (e.g. by hypoxia) induces autophagy, which might be clinically beneficial early after kidney transplantation 4.
Autophagy is pro-survival
The fact that genetic deletion of critical autophagy genes accelerates cell death supports the notion that autophagy is an adaptive response and acts as a protective, pro-survival mechanism. The degradation of proteins and organelles enables the cell to use alternative sources of substrates when nutrients are limited. It is proposed that exhausting these reserves may lead to autophagy-associated cell death 11.
Autophagy can be pro-survival by reducing the susceptibility to apoptosis but few mechanistic data explain the mechanisms. One possible method is that autophagy inhibits TRAIL-induced apoptosis by selectively degrading active caspase-8 12. Autophagy also decreases p62/SQSTM1, a protein that if accumulated can stimulate the production of reactive oxygen species (ROS) and cell death 13. Finally, a more general mechanism through which autophagy reduces the tendency of cell to undergo cell death is the removal of degrading mitochondria (mitophagy), which can increase the threshold for apoptosis 10 (Figure 2).
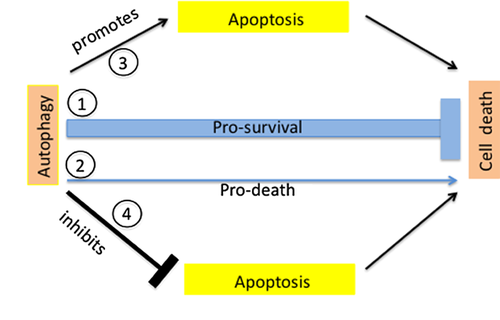
Autophagy is pro-death
First evidence that demonstrated true autophagic cell death was obtained in model organisms such as Drosophila where autophagy was necessary for cell death of salivary gland cells 14. The early steps of autophagosome formation generate a platform for caspase-dependent apoptosis activation (ATG3 and ATG5) 15. Autophagy may also stimulate apoptosis by depleting endogenous apoptotic inhibitors 10 (Figure 2).
In addition to stimulating cell death by apoptosis, autophagy controls the sensitivity to necroptosis. Members of the receptor interacting protein kinase family (RIPK1,3) are key members in the regulation of necroptosis. In human renal cancer cell lines, mTOR inhibition-induced autophagy stimulation eliminated RIPKs, whereas autophagy inhibition induced RIPK-dependent necroptosis 16. In primary T cells cellular caspase 8-like inhibitory protein (c-FLIP) represents a potential regulator cell death. c-FLIP deficient T cells exhibited enhanced autophagic flux in the naïve state and underwent receptor-interacting protein-1-dependent necroptosis after TCR stimulation 17.
Beth Levine's group recently described a new type of cell death, coined “autosis,” which was triggered by selective induction of autophagy. Autophagy-inducing peptide (Tat-Beclin-1; Table 3) caused cell death that could not be rescued by inhibitors of apoptosis and necroptosis. This new form of autophagy-triggered cell death was distinct from apoptosis or necrosis and was also observed during rat cerebral hypoxic–ischemic injury 18.
| Compound | Effect on autophagy | Mechanism of action and target |
|---|---|---|
| Hydroxychloroquine | Inhibitor | Lysosomal acidification |
| 3-MA, Wortmannin | Inhibitor | Class III PI3K |
| Anti-TNFα | Inhibitor | Pro-autophagic cytokine block |
| P140 phosphopeptide | Inhibitor | Down-regulation of autophagic flux at the autolysosome stage |
| Tat-Beclin-1 | Inducer | Interacts with a negative regulator of autophagy |
| Temsirolimus, sirolimus | Inducer | mTOR (incl. other actions) |
| Cyclosporine | Inducer | Mitochondrial permeability |
| Tamoxifen | Inducer | Beclin-1 |
| Vitamin D | Inducer | mTOR inhibition |
| Bortezomib | Inducer | mTORC1 inhibition |
| Carbamazepine, valproate | Inducer | Inositol levels |
- 3-MA, 3-methyladenine; PI3K, phosphatidylinositol 3-kinase; TNF, tumor necrosis factor; mTOR, mechanistic target of rapamycin; mTORC, mTOR complex.
Additionally, autophagy-related functions in cell death pathways can be a consequence of autophagy-independent properties. ATG12 conjugation of ATG3 is important in mitochondrial homeostasis and cell death that does not affect starvation-induced autophagy 19.
Take together, the importance of autophagy-associated cell death, in particular of immune cells, remains poorly understood. Due to conflicting findings, the results in this area of investigation should be interpreted carefully and within the cellular context. When and how autophagy can be pro-survival and pro-death depends on the extent of injury, the microenvironment and the activation of stress regulators allowing cells to survive toxic changes.
Autophagy in Immunity
The immunological roles of autophagy have become well-established physiological functions in nearly all aspects of immunity and are summarized in detail elsewhere 1, 20 (Table 1).
Innate immunity
The importance of innate immunity in transplantation is well-described 4. Pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) activate innate immunity via pattern recognition receptors (PRR). PRR include Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain-containing protein (NOD)-like receptor (NLRs) recognition molecules. Activation of these innate receptors can regulate autophagy and act in the recruitment of autophagy proteins to phagosomal membranes 20 (Table 2).
Autophagy has anti-inflammatory and pro-inflammatory effects in innate immunity. In addition, several innate mechanisms like T helper (TH) 1 and TH2 type cytokines can regulate autophagy (Table 2). A balance between these activating or amplifying pathways and inhibitory signaling determines the net outcome in terms of induction or inhibition of autophagy.
Toll-like receptors
Cell-derived DAMPs can signal through specific TLRs expressed on immune or nonimmune cells 4. TLRs were the first class of innate receptors to be connected to autophagy 21. TLR4 activation, both Toll/IL-1R domain-containing adaptor inducing interferon (IFN) (TRIF) and MyD88-dependent, trigger autophagy through a direct interaction with Beclin-1. ATG5, Beclin-1 and PI3K are required for the formation of autophagosomes by TLR4 stimulation. One molecular mechanism linking TLR signaling and autophagy induction is the association of Beclin-1 and MyD88-containing protein complexes, affecting Bcl-2/Beclin-1 interactions 20.
HMGB1 is an intranuclear protein implicated in danger signaling. Under stress condition such as starvation, the oxidized form of HMGB1 translocates from the nucleus into cytoplasm and promotes autophagy by binding to Beclin-1 and displacing Bcl-2. In addition to HMGB1, other DAMPs are known to induce autophagy (Table 2) 22.
NOD-like receptors
NOD 2 and the related sensor NOD1, are cytosolic receptors and binding to bacterial peptidoglycan induces autophagy. Murine NOD1 and NOD2 interact with ATG16L1 and modulate autophagy in the context of Crohn's disease 23. Recent data found ATG16L1 functions at an upstream step of the NOD signaling and NOD-regulatory capacity of ATG16L1 was independent of its role in canonical autophagy 7. NOD2 stimulation with muramyldipeptide induced autophagy in dendritic cells and, as a result, up-regulated MHC class II expression and activated CD4 cells 24.
Inflammasome
In response to cellular danger (PAMPs and DAMPS), the inflammasomes are activated making them optimal sentinels for cellular stress and injury. The NOD-leucine-rich repeat pyrin domain containing protein (NLRP) named NLRP3 assembles and oligomerizes into a common structure, which collectively activated the caspase-1 cascade, thereby leading to the production of pro-inflammatory cytokines especially IL-1β and IL-18.
Autophagy has a negative role in inflammasome activation. Under sterile conditions and in unstimulated cells basal autophagy clears the cytoplasm of debris and defective organelles, thereby preventing spurious activation of the inflammasome. In contrast, autophagy blockade leads to an accumulation of depolarized mitochondria (impaired mitophagy) that leak endogenous inflammasome agonists, such as mitochondrial DNA and ROS, which activate the NLRP3 inflammasome 25, 26.
Autophagy and antigen presentation
Autophagy was implicated in cross-presentation, an alternative pathway of MHC class I presentation. Via this pathway, CD8 T cells respond to exogenous antigens and phagocytozed material 1, 20. During MHC class II presentation, autophagosomes fuse with MHC class II loading compartments, thereby delivering cytosolic proteins to MHC class II molecules 27 (Table 1). Recent data reported the formation of autophagosome-like structures originating from MHC class II loading compartments in dendritic cells, suggesting an unconventional antigen presenting cell-specific type of autophagy 28.
Autophagy and T cells
T cells are particularly dependent on autophagy for their development, survival and proliferation. Several autophagy proteins are critical for these functions and include Beclin-1, ATG3, ATG5 and ATG7. After exiting the thymus, T cells depend on autophagy and mitochondrial content reduction for their maturation 29 (Figure 3). After activation via TCR and costimulation, cytokine culturing and prolonged serum starvation autophagy are induced in T cells 30. Autophagy is a pro-survival process in activated T cells and there is evidence that this process can promote secretion of cytokines such as IL-2 and IFNγ and influence TH cell polarization 30.
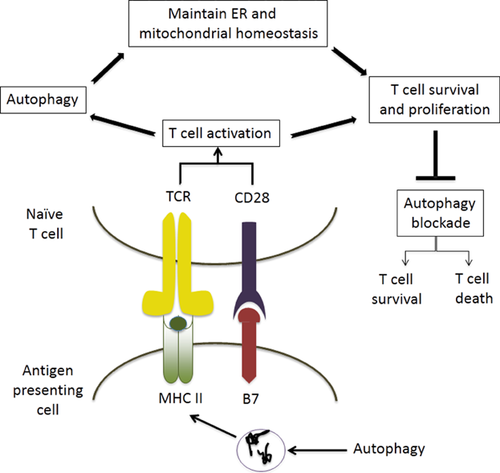
Beclin-1+/− T cells show normal numbers in the periphery and normal proliferation after activation 3. In contrast, complete blockade of autophagy (ATG3, ATG5 or ATG7 deficiency or beclin1Fl/FlCD4Cre) leads to reduced T cells in the periphery of naïve mice and impaired proliferation after stimulation 29-31.
However, depending on cellular contexts and stimuli, autophagy has not only pro-survival but also pro-death roles in T cells (Figure 3). Autophagy was shown to control cell death induced by IFNγ signaling in TH cells and autophagy-deficient TH2 cell line are more resistant to growth-factor withdrawal-induced cell death 32, 33. Other data supporting the pro-death role involve IFNγ inducible, immunity-related GTPase−/− (IRGM 1). When stimulated with IFNγ IRGM−/− T cells die in an autophagic-dependent manner 33. Consistent with this, becn1+/− effector T cells showed reduced cell death after transfer into allogeneic recipients 3.
In vitro experiments revealed that regulatory T cells (Tregs) from becn1+/− mice exhibited comparable capacity to suppress proliferation of WT T cells. The co-transfer of WT or becn1+/− Tregs equally suppressed proliferation of scurfy CD4+ T cells when compared to the massive expansion observed in Rag2−/− mice given scurfy CD4 T+ cells alone 3. However, it is still unclear how autophagy in one cell type affects other cells and immune networks. Additionally, the metabolic regulator mTOR senses nutrient availability, influences cell functionality, and mTORC1 directs Treg function in vivo 34. Whether these effects involve autophagy-dependent mechanisms is not known.
The homeostatic functions of autophagy are believed to be critical as an anti-aging process and modulation of autophagy can influence lifespan 2. In the immune system, senescence was associated with an altered responsiveness and immune function. Aged T cells were found to be more resistant to tolerance induction by enhancing CD8 T cell alloimmunity 35. In humans primary senescent CD8 T cells (from donors older than 60 years of age) have decreased basal and starvation-induced autophagy compared to cells obtained from younger donors (less than 28 years of age) 9. Whether and if so how age-induced impairment in autophagy contributes to alloimmunity is not known. In addition, while advanced age after transplantation is clearly linked with increased infectious complications and altered memory cell repertoire, it is unknown whether these phenomenon can be explained with dysregulation of autophagy.
Autophagy in B cells and plasma cells
Autophagy plays a complex role in B cells. In contrast to mature T cells, mature peripheral B cells do not require autophagy for survival 36. Autophagy is important for B cell survival during development and ATG5 deletion results in a dramatic reduction in B-1 B cells 37. On the other hand, autophagy is an alternative death pathway, as antigen receptor stimulation in the absence of costimulation induces autophagy-associated cell death 38. Thus, B cell receptor ligation-induced autophagy may be critical in negative selection of self-reactive B cells, thereby preventing autoimmunity.
Recent data established that autophagy controls immunoglobulin secretion in plasma cells by containing ER maintenance. Somewhat surprisingly, the deficiency of autophagy (ATG5−/−) leads to excessive immunoglobulin production 39. It will be interesting to test the role of autophagy in plasma cells during alloantibody production.
Autophagy in autoimmunity
The cortex of the thymus, responsible for central tolerance induction in the T cell compartment, is one of the tissues with the highest constitutive, starvation-independent autophagy content. When thymi were transplanted under the renal capsule of athymic nude mice, recipients of ATG5−/− thymi showed infiltration of autoreactive CD4+ T cells into multiple organs including autoimmune colitis. These data suggest that autophagy-enhanced MHC class II presentation is important in the selection of naïve T cells repertoire and impaired autophagy allows for the escape of autoreactive T cells and the loss of self-tolerance 27. In contrast to these data, cell-specific deletion of ATG7 in the thymic epithelium did not cause autoimmunity, implying that autophagy in thymic epithelial cells was dispensable for negative selection of autoreactive T cells 40. Since the ATG12-ATG5-ATG16 multimeric complex is required for autophagosome synthesis and is not formed in the absence of ATG5 or ATG7, the deficiency of these two critical proteins cause the same phenotype. Future studies need to test whether and under which circumstances autophagy plays a role in maintaining self-tolerance.
Autophagy in alloimmunity
Stable transplantation tolerance can be induced in murine systems using multiple therapeutic manipulations. While multiple studies indicate that regulatory processes are necessary for tolerance induction, regulation must be accompanied by depletion of effector cells to reduce the pathogenic T cell burden. If T cell death was blocked, allografts were rejected despite tolerogenic regimens. Apoptosis of alloreactive T cells was believed to be the primary mechanism of cell death under these circumstances 41.
We tested the role of an essential autophagy protein, Beclin-1, on heart transplant survival in mice 3. Long-term allograft survival induced by donor-specific transfusion plus anti-CD154 required homozygous lymphocyte expression of Beclin-1. Following adoptive transfer into allogeneic recipients, autophagy-deficient, Beclin-1 heterozygous effector T cells exhibited enhanced proliferation with diminished cell death and increased production of IFNγ. Whereas the induction and function of Tregs in Beclin-1 heterozygous mice was normal, effector T cells from these mice were resistant to Treg-mediated suppression 3. These data identify a requisite role for Beclin-1 in facilitating effector T cell death during tolerance induction.
The mTOR inhibitors rapamycin and everolimus are already used as part of immunosuppressive regimens with their main effector mechanism being to inhibit T cell proliferation. Importantly, inhibition of mTOR induces autophagy and the use of rapamycin, in combination with costimulatory blockade, provides strong synergy and favors peripheral tolerance 41.
Hypoxic and ischemic injury
Recent finding identified autophagy as a process that can be regulated by hypoxia in cells and tissues (Figure 4). Hypoxia-induced autophagy requires Beclin-1 and involves ROS and HIF-1α stabilization. Accumulating evidence from animal models of warm IR injury and humans shows that IR injury with or without transplantation was modulated by autophagy. In tubular cells, in response to hypoxia, autophagy occurred before apoptosis and there was a time-dependent increase in autophagic flux in tubular cells after reperfusion 42.
Data clearly showed that autophagy was induced after IR injury and several recent studies clearly revealed the reno-protective effect of autophagy during IR injury. IR injury in autophagy-deficient (ATG5 or ATG7) tubular cells was more severe compared to WT. In addition, ATG-deficient kidneys showed rapid accumulation of p62, a key autophagy substrate, more ROS markers and increased tubular apoptosis 43, 44. The observation that autophagy preceded apoptosis suggests it is an early response to cell stress and not a result of apoptosis.
Using autophagy reporter mice (CAG-RFP-EGFP-LC3) autophagic activity peaked after 1 day and returned to baseline after 3 days. Interestingly, most tubules with activated mTOR showed no signs of autophagy, whereas inhibition of mTORC1 induced autophagy and limited regenerative cell proliferation 8. These data suggest a role of the mTORC in renal repair and support reports that rapamycin delays renal recovery after ischemic insult 4.
Autophagy is known to decline with age and the kidney is a target organ of aging. It is well known that older kidneys have a higher risk for posttransplant ischemic injury 4. Aging kidneys fail to induce autophagy in tubular cells in response to hypoxia. Interestingly, calorie restriction triggered starvation-induced autophagy and was able to restore the hypoxia-induced autophagy in older kidneys. The anti-aging protein NAD-dependent deacetylase SIRT1 was mechanistically crucial in restoration of autophagy in aged kidneys after hypoxia 45.
It is still not well understood how and under which circumstances autophagy is protective and what signaling pathways lead to tubular autophagy. To discover the most effective molecular targets in these complex signaling networks, it is critical to identify nexuses where and how innate sensing receptors (e.g. TLR), and cell survival or death pathways intersect.
Immunosuppressive drugs and autophagy-related processes
The list of currently used drugs that have the ability to regulate autophagy-related processes is lengthy and includes several immunosuppressive drugs (Table 3). Rapamycin and second-generation mTOR inhibitors (e.g. temsirolimus, everolimus, deferolimus) are inducers of autophagy by virtue of their ability to inhibit mTORC1 kinase. However, mTOR inhibition also affects other vital function including cell growth and proliferation (Figure 4).
ER stress induced by cyclosporine is a cellular regulator of autophagy. Cyclosporine was shown to induce autophagy in vivo in injured tubular cells and autophagy inhibition increased tubular cyclosporine toxicity, suggesting that autophagy is a protective mechanism 5.
Other immunosuppressive drugs that have potential effects on autophagy include bortezomib and rituximab. Their exact mechanisms of action and the functional importance especially in immune cells remain to be examined.
Clinical Applications
There is a clear link between genetic mutations in critical autophagy genes and pathophysiological processes in humans that include cancer, cardiovascular, autoimmune (e.g. systemic lupus erythematosus), metabolic and neurodegenerative disorders 2. This strong association with common diseases make autophagy an attractive target for developing new treatments. Several clinical trials primarily in the field of oncology are currently ongoing. Unfortunately, clinical trials that aim to target autophagy are limited by the incomplete understanding how and when this pathway contributes to the process, and the lack of specific biomarkers of autophagic activity in vivo 2. The development of specific autophagy-inducing agents such as the cell-permeable Tat-Beclin-1 peptide, are critically important to advance the field with clinical trials avoiding the pleiotropic effects of some of the currently tested drugs 18. In transplantation mTOR inhibitors are already used as part of immunosuppressive regimens with their main effector mechanism being to inhibit T cell proliferation. In clinical transplantation it is to date not clear whether the intended effects or side effects of mTOR inhibitors are mediated through their ability to induce autophagy.
Perspectives and Conclusions
Autophagy influences many aspects of innate and adaptive immunity with potential relevance in organ transplantation. It seems many if not all innate response pathways have become integrated with autophagy and evolutionarily this has extended to adaptive immunity. Autophagy dysregulation may involve altered activation of autophagic proteins or altered autophagic activity (flux) and, as a result, can cause or drive chronic inflammation and autoimmune disorders.
Although tremendous advances have been made in our understanding of autophagy on a cellular level, many unanswered questions remain before this pathway can be exploited to therapeutically in transplantation.
Acknowledgments
This study was supported by American Society of Nephrology (to BS). We thank Fernando Mocian, MD, PhD, for insightful discussions.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




