Yellow fever – An old foe with new developments
Abstract
Introduction
Yellow fever is caused by an RNA flavivirus. Immunisation in conjunction with vector control is at the forefront of yellow fever control and elimination.
Objective
This narrative review describes the impact and importance of yellow fever vaccinations for northern Australian health practitioners.
Design
Selected key policies, studies and medical guidelines are reviewed and presented.
Finding
Large yellow fever outbreaks, associated with vector spread, have occurred in the last decade in Africa and South America, increasing the risk of international spread of the virus. Mobile populations, like travellers or migrant workers, continue to be at risk of yellow fever. Quality assurance, including yellow fever centre accreditation and initiatives to decrease fraudulent yellow fever vaccination documentation, has evolved in the past few years. Fractional dosing of yellow fever vaccines has been shown to provide protection for 1 year in outbreak scenarios, but further studies are needed.
Discussion
Although Australia is yellow fever-free, the disease could be introduced by viraemic persons as a competent Aedes mosquito vector is present in northern Australia. In addition to surveillance and vector control, health education and yellow fever vaccination remain the best lines of defence. In the event of an outbreak, a response via fractional dosing could prove to be effective in controlling the virus.
Conclusion
Health care providers in northern Australia should be aware of the risks of yellow fever and its introduction to northern Australia and be able to discuss vaccination status with their clients when needed.
What is already known on the subject
- Yellow fever is a very rare disease in non-endemic countries that has no specific treatment.
- Symptoms are generalised and therefore insufficient for determining a diagnosis. Diagnosis relies on clinical suspicion and laboratory confirmation in specialised laboratories and public health units.
- Yellow fever vaccination should be considered in cases of travel to, from, or through a yellow fever risk area. This holds true, even if the risk exposure is low or not mandated by health regulations.
- Due to the presence of the mosquito vector (Aedes species) of the yellow fever virus, northern Australia is at risk of yellow fever introduction.
What this paper adds
- All health personnel and vaccination centres in Australia providing yellow fever vaccination must be accredited on a regular basis.
- International efforts to reduce the risk of counterfeit vaccination documents are underway. Correct documentation regarding the vaccination status or the letter of exemption is warranted, and exceptions are limited.
- Fractional dosing of the yellow fever vaccine can be an effective tool to control large yellow fever outbreaks. Thus, this method could be used in the case of an outbreak in Australia, but further studies are needed.
1 INTRODUCTION
Yellow fever is a mosquito-borne, hemorrhagic disease affecting both human and non-human primates. The yellow fever virus belongs to the genus Flavivirus. It is believed to have originated in Africa and spread to South America through the transatlantic slave trade.1 Currently, yellow fever is endemic in large regions of South America and Africa.2 The WHO and other health agencies like Smartraveller (www.smartraveller.gov.au) provide regularly updated country requirements and information on yellow fever outbreaks.3, 4 Risk populations for yellow fever, besides those in endemic areas, are mobile populations, such as travellers, migrants (workers) and displaced peoples. This increased risk can be attributed to a lack of disease awareness, inadequate mosquito avoidance practices, non-vaccination and a lack of natural immunity.5, 6
In endemic areas, yellow fever virus transmission is maintained by cycling between mosquito vectors and infected non-human primates.1 An infected mosquito can start a savanna cycle by biting and infecting a human (Figure 1). Travelling, infected humans can give rise to the urban cycle and serve as a link between the sylvatic and urban cycles.6 Epidemics can occur in both cycles, but without a non-human primate host present, urban epidemics can be eliminated by vaccination. While northern Australia does not have non-human primates, it is possible to introduce yellow fever into northern Australia by a viraemic traveller, establishing an urban cycle.
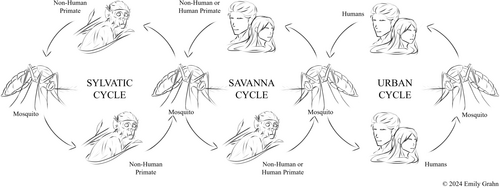
Australia's high volume of travellers, lack of awareness, presence of a competent vector and lack of immunity make this area a potential target for a future outbreak.
1.1 Risk of infection
Despite the advances in vector control and vaccine availability, there are recurring yellow fever outbreaks. It becomes difficult to complete a risk assessment on the yellow fever virus due to seasonal variations in virus circulation, in addition to local endemic communities having acquired immunity either through vaccination or natural infection.8 Furthermore, most yellow fever cases are asymptomatic or only show mild symptoms, especially in endemic settings, with around 15%–20% of cases developing severe symptoms.8 This can lead to the false assumption that “there is no yellow fever where I am going.”
The risk of illness due to yellow fever has been estimated to be 1 in 20 000 for South America and 1 in 2000 for West-Africa for a two-week trip.7 Due to a substantial case fatality rate of around 30%–60% associated with severe yellow fever,7 yellow fever vaccination becomes warranted in almost all cases when visiting an endemic or potential risk areas.
1.2 Diagnosis and treatment
- Isolation of the yellow fever virus; or
- Detection of the yellow fever virus by nucleic acid testing; or
- Seroconversion or a four-fold or greater rise in yellow fever virus-specific serum IgM or IgG levels between acute and convalescent serum samples in the absence of vaccination in the preceding 3 weeks; or
- Detection of yellow fever virus antigen in tissues by immunohistochemistry.
Confirmation of laboratory results by a second arbovirus reference laboratory is required in the absence of travel history to areas with known endemic or epidemic activity. Epidemiological evidence is defined as a history of travel to a yellow fever-endemic country in the week preceding the onset of illness.
Some infected persons may present as asymptomatic, while others will develop unspecific symptoms such as fever, chills, headache, body aches/fatigue/weakness and vomiting.10 These symptoms are common in returning international travellers seeking health care and can be caused by several tropical and non-tropical infections.11 The differential diagnostic approach is beyond the scope of this article; however, it must include the ruling out of malaria in cases where patients have spent time in a risk area for malaria. This must be done regardless of the malaria chemoprophylaxis status and mosquito avoidance behaviour.
Most yellow fever patients improve within a week. A minority of patients may progress to the second, more severe, stage of the disease.8 To date, there is no specific treatment for yellow fever, though, yellow fever patients can be provided complex supportive treatment.8 High case fatality rates can be reduced in intensive-care settings like Australia, Europe and North America.
1.3 Australian yellow fever vaccination centres
Yellow fever vaccinations have been subject to health regulation in many countries. To prevent the international spread of disease, Australia is a signatory to the International Health Regulations 2005 (IHR) adopted by the WHO.12 This means that travellers to, from and through countries are often required, or recommended, to hold a valid international Certification of Vaccination and Prophylaxis to document their yellow fever vaccination status. Under the Biosecurity Act of 2015, the Commonwealth Department of Health has statutory responsibility for the control of yellow fever and the subsequent vaccination centres.13 As of November 26th, 2018, individual health care providers are accredited to administer the yellow fever vaccine after successful completion of the online Yellow Fever Vaccination Course (https://mycollege.acrrm.org.au/search/find-online-learning/details?id=22729).14, 15 To maintain Yellow Fever Vaccination Centre status, all medical practitioners (and nurse practitioners approved by the relevant state/territory health authority) administering yellow fever vaccines need to renew accreditation every three years.15, 16
1.4 Yellow fever vaccination
With the introduction of yellow fever vaccines in the 1930s, regular booster vaccination was recommended.17 In a 2013 WHO position paper on yellow fever vaccines, the WHO announced that a single dose of the vaccine is sufficient to confer a life-long protective immunity against the virus,18 making the formal amendment in 2016.19 This should be clearly stated in international certifications of vaccination or prophylaxis as “life of person vaccinated.” In cases where yellow fever vaccination is required by a health authority, the yellow fever vaccine should be administered at least 10 days prior to entry into the country.15 If the yellow fever vaccination is only recommended, the vaccine can be given until departure as the immune response starts to build up quickly enough during the incubation phase of typically three to 6 days to prevent severe disease.16
The yellow fever vaccine is a live, attenuated virus cultured in pathogen free embryonic hen eggs and is typically well tolerated.20 In several trials, up to 25% of all vaccine recipients reported mild systemic side events that interfered with normal daily activity.21 These side effects include flu-like symptoms, headache, fever and chills, myalgia within three to 7 days and mild local reactions like erythema or pain at the inoculation site.18
There are two rare, severe side effects associated with the yellow fever vaccination. These are the yellow-fever-vaccine-associated neurotropic disease (YEL-AND) and the yellow-fever-vaccine-associated viscerotropic disease (YEL-AVD). A study found that reporting rates for YEL-AND are 8 of 100 000 000 doses distributed and 4 of 100 000 000 doses distributed for YEL-AVD worldwide.22 There are certain confounding factors that complicate the above analysis and results. Primary, post-marketing surveillance differs considerably between low- and high-income countries, thus potentially skewing reporting numbers. Additionally, data on co-morbidities that can contribute to the likelihood of adverse effects, such as thymus disorder or human immunodeficiency virus infection, might be missing or unknown. Finally, age data would be useful as the risk for adverse vaccine effects increases with age, notably in clients older than 60 years of age.22
Despite the possibility of adverse effects from the vaccine, the risk of morbidity or mortality due to vaccination in this setting is lower than the risk of death due to a yellow fever infection. As mentioned, the risk of illness during a two-week trip to South America is 1 in 20 000.7 Case fatality rates for symptomatic infections fluctuate and depend on the severity of the disease, underlying medical conditions and supportive treatment options available in low-income countries. Therefore, for this hypothetical, we will use a higher-end fatality rate of 50%. The risk of illness from yellow fever for a one-day trip would be 1 in 280 000. With the hypothetical case fatality rate, the risk of death due to yellow fever illness for a single-day trip to South America is 1 in 560 000. Literature suggests the risk of YEL-AVD diagnosis is approximately 1 in 300 000 vaccinations.22 The risk of dying from YEL-AVD is then 1 in 600 000 at an assumed fatality rate of 50%. This illustrates that even in low endemicity settings, the vaccine has less risk of fatality than yellow fever, even for single-day trips.
There are contraindications and precautions for the yellow fever vaccination. The Australian Immunisation Handbook currently lists anaphylaxis to vaccine components, infants younger than 6 months, severe immunocompromised people and people with thymus disorder (Table 1) as absolute contraindications.23
| Contraindications |
|---|
| Anaphylaxis |
| Anaphylaxis after a previous dose of any yellow fever vaccine |
| Anaphylaxis after any component of a yellow fever vaccine |
| Anaphylaxis to eggs |
| Thymus Disorders |
| Myasthenia gravis |
| Thyoma |
| Thymectomy |
| DiGeorge syndrome |
| Thymic damage from chemoradiotherapy or graft-vs.-host diseases |
| Infants under 9 months of agea |
| People who are immunocompromisedb |
| Precautions |
|---|
| Adults aged ≥60 years |
| Women who are pregnant or breastfeeding |
| People with possible IFNAR1 deficiency |
| People with HIV |
- a Countries experiencing a mass outbreak of yellow fever may choose to immunise infants from as young as 6 months of age.
- b Yellow fever vaccination can be considered case by case. This needs to consider factors such as risk of exposure and level of immunocompromised.
- cAdopted from the Australia Immunisation Handbook.23
These clients should be advised not to travel to regions at risk for yellow fever. If travel is unavoidable, clients should be counselled regarding mosquito avoidance and provided with a letter of exemption. Adults older than 60 years, people with mild immunosuppression, women who are pregnant or breastfeeding and infants older than 6 months can be vaccinated after a benefit–risk assessment.23 Usually, the risk during the trip outweighs the risk of vaccination (see example above); however, a careful assessment is needed and should be documented in the patient's file.
There are a limited number of WHO prequalified yellow fever vaccines. Updated lists can be found on the United Nations prequalified vaccine site (https://extranet.who.int/pqweb/vaccines/prequalified-vaccines).24 Among these vaccines, there are a few that are more commonly distributed and some that are not available in certain countries. This fluctuation and differing demand can lead to shortages and stockpiles. For example, YF-Vax®, produced by Sanofi, is licensed in the United States, while Stamaril® is not. Despite being a part of the same company, Sanofi, Stamaril® was not available for distribution in the US prior to the YF-Vax® manufacturing shortage in 2016.25 Even so, while Stamaril® is now available, it is still not FDA-approved and is listed as an investigatory drug (EAP) rather than a fully licensed alternative.26 In Australia, the main yellow fever vaccine is Stamaril®.23 While there has yet to be an event such as that seen in the US, a national stock of one vaccine could lead to similar issues.
1.5 Letters of exemption and vaccine certificates
A letter of exemption can be given to the client in case there is a medical contraindication towards yellow fever vaccination. Several points are important to consider when providing a letter of exemption (Table 2).
| Important considerations | |
|---|---|
| 1. | Use the medical contraindications to vaccination section of the International Certificate of Vaccination or Prophylaxis or use a letterhead stationery from your approved Yellow Fever Vaccination Centre in Australia |
| 2. | Clearly state that the yellow fever vaccine is contraindicated on medical grounds |
| 3. | Display the official stamp provided by your health authority |
| 4. | Signed by hand by the clinician or authorised health worker |
| 5. | Avoid changes or amendments, as these could invalidate the exemption |
| 6. | Although not required, state an end date (e.g., last day of the trip) as the medical condition might change in the future |
| 7. | A hard and soft copy (e.g., pdf file in a personal email account) of the letter of exemption should be kept in a safe place |
In the case of pre-existing patient conditions such as HIV infection/AIDS, since these infections may lead to questioning at international borders, the term “on medical grounds” is preferred. Clients need to be made aware that they could be denied entry into the country or be quarantined for 6 days to rule out a current yellow fever infection, even with a valid letter of exemption. Travellers with a letter of exemption might be forced to take the yellow fever vaccination at airports or police/military checkpoints based on local health regulations or new risk–benefit analysis,27 e.g., mandatory vaccination as a public health approach or asked to pay a fine or “special fee.” To maintain the widespread acceptance of letters of exemption and to reduce the risk of fraudulent letters or exemptions, they should only be provided for the valid reasons mentioned above.
An increasing problem is the issue of counterfeit vaccine certificates. This poses significant concerns regarding proper disease tracking, especially in terms of introducing infected persons into non-endemic areas with competent vectors.28 To combat the issue of counterfeit certificates, Nigeria, for example, has introduced an e-registry for vaccination cards.29 A traveller can register for their ecard by paying 2000 NGN (Nigerian Naira) through a government-run website,30 and then bring that receipt, along with a valid passport, to any Port Health Services Office in Nigeria.29 As of 1st July 2019, the previous yellow fever vaccination certificates have become invalid.29
1.6 The Australian setting
Travellers arriving in Australia from a suspected or confirmed yellow fever area within 6 days without a documented yellow fever vaccination are usually permitted to enter Australia, but are counselled by a biosecurity officer from the Department of Agriculture and Water Resources.31 Upon entry into Australia without a yellow fever vaccination, the advice is not to travel to northern Australia for 6 days as the vector is present, and this aspect should be discussed before the client leaves Australia. This does not warrant a medical exemption; rather, a letter of medical certificate could be given. However, it is also possible that the traveller may be quarantined on arrival. For example, the Iguazu waterfalls is at risk of yellow fever, though it is located within Argentina, a majority yellow fever-free country. Though the traveller may not have visited the area, and hence have no risk of yellow fever infection and therefore no need for yellow fever vaccination, border and biosecurity officers in an Australian airport may not be able to confirm this information, and thus deny entry or enforce quarantine.32
1.7 Outbreak control with fractional vaccine dosing
In the case of a viraemic person who gives rise to a localised yellow fever outbreak in northern Australia, the virus could be contained by vector mosquito control and ring vaccination.33 Yellow fever vaccine lots vary in their potency, though they are typically well above the minimum of effectiveness recommended by the WHO.34 Fractional dosing takes the vaccine and creates a usually 5-fold lower sub-dose, injecting that into the patient, inducing, a similar immunological and virological pattern to a full dose.35 Therefore, this approach provides an effective public health intervention, especially for countries with limited resources or vaccine allocations. Usage of the vaccine during an outbreak would be limited by the stocks available; therefore, by utilising fractional doses of vaccines to confer temporary protection, authorities can reach a wider range of people with the reduced amount of vaccines. During a large-scale 2016 outbreak occurring in Angola, the Democratic Republic of Congo (DRC) and Uganda, 18 million fractional doses were sent out.
To study the effectiveness of fractional dosing, a fractional dosing campaign was done in Kinshasa, the capital city of the DRC, during this yellow fever outbreak.36 This study included non-pregnant adults and children older than 2 years and utilised a fractional vaccine dosing approach, targeting 7.6 million persons during a 10-day period in August 2016. Embedded within the campaign was a study to assess the body's immune response to fractional dosing in a large-scale public health intervention.36 Participants received one-fifth of the standard dose (0.1 mL) of the Stamaril® vaccine. Neutralising antibody titres in the blood were assessed just prior to vaccination, in other words, measuring how many protective antibodies against the disease were found at baseline. Results found that out of 493 participants who were seronegative for the protective antibodies at baseline, 482 (98%; 95% confidence interval [CI], 96 to 99) underwent seroconversion after receiving the dose. This illustrates the efficacy of a lower dose in conferring a protective immune response. They also assessed antibody protection 1 year after the initial vaccination, finding that out of the 684 participants, 666 (97%; 95% CI, 96 to 98) showed continued seropositivity for yellow fever protective antibodies.36
Though this provides evidence towards the usefulness of fractional dosing in an outbreak scenario, this study did not include a control group, and to complicate the judgement, there was a waning of the outbreak during the study period. These factors could alter conclusions on vaccine effectiveness, and further studies regarding other yellow fever vaccines are needed to investigate the fractional dosing approach in pregnant women, children younger than 2 years and human immunodeficiency virus patients. It is also not clear whether the fractional dosing approach leads to protection for the life of the person vaccinated.
Due to this concern, in addition to historical data, the World Health Association now proposes fractional dosing as a dose-sparing option for outbreak response.37
2 CONCLUSION
Similar to the recent introduction of Japanese encephalitis into southern parts of Australia formerly not at risk,38 Australia is at risk for a potential introduction of yellow fever by viremic travellers into its north. Northern Australia possesses a virally competent vector, making this area at a higher risk for transmission should the virus be introduced. For these reasons, health care providers in northern Australia need to be aware of the risk of yellow fever introduction and discuss vaccination status with their clients when appropriate. Should an outbreak happen in this area, fractional dosing accompanied by vector control measures could prove effective.
AUTHOR CONTRIBUTIONS
Emily Grahn: Validation; investigation; writing – review and editing; visualization. Jacqueline Picard: Conceptualization; methodology; investigation; writing – original draft; writing – review and editing. Lars Henning: Conceptualization; investigation; methodology; project administration; supervision; validation; writing – original draft; writing – review and editing.
ACKNOWLEDGEMENTS
Open access publishing facilitated by James Cook University, as part of the Wiley - James Cook University agreement via the Council of Australian University Librarians.
CONFLICT OF INTEREST STATEMENT
Nothing to disclose, no conflicts of interest or outside funding.




