Exploring the impact of remoteness on people with head and neck cancer: Utilisation of a state-wide dataset
Abstract
Objective
Living in regional/rural areas can impact outcomes for people with head and neck cancer (HNC). Using a comprehensive state-wide dataset, the impact of remoteness on key service parameters and outcomes for people with HNC was examined.
Methods
Retrospective quantitative analysis of routinely collected data held within the Queensland Oncology Repository.
Design
Quantitative methods (descriptive statistics, multivariable logistic regression and geospatial analysis).
Setting
All people diagnosed with HNC in Queensland, Australia.
Participants
The impact of remoteness was examined in 1991 people (1171 metropolitan, 485 inner-regional, 335 rural) with HNC cancer diagnosed between 2013 and 2015.
Main Outcome Measures
This paper reports key demographics and tumour characteristics (age, gender, socioeconomic status, First Nations status, co-morbidities, primary tumour site and staging), service use/uptake (treatment rates, attendance at multidisciplinary team review and timing to treatment) and post-acute outcomes (readmission rates, causes of readmission and 2-year survival). In addition to this, the distribution of people with HNC across QLD, distances travelled and patterns of readmission were also analysed.
Results
Regression analysis revealed remoteness significantly (p < 0.001) impacted access to MDT review, receiving treatment, and time to treatment commencement, but not readmission or 2-year survival. Reasons for readmission did not differ by remoteness, with dysphagia, nutritional inadequacies, gastrointestinal disorders and fluid imbalance indicated in the majority of readmissions. Rural people were significantly (p < 0.0001) more likely to travel to care and to readmit to a different facility than provided primary treatment.
Conclusions
This study provides new insights into the health care disparities for people with HNC residing in regional/rural areas.
What is already known on this subject
- Head and neck cancer requires complex multidisciplinary care that cannot be accessed locally for most rural people.
- There is currently limited published data on the differences in the presentation, outcomes and service access for rural people with HNC in Australia compared with their metropolitan counterparts. Data from overseas varies considerably and is not easily transferrable to the Australian health care context.
What this paper adds
- This is the first comprehensive state-wide analysis showing statistically significant differences by rurality in Queensland for people with HNC.
- The results highlight the need to directly link the care pathway between the metropolitan treating team with care closer to home and to better understand the care needs and treatment access journeys of people with HNC from rural areas in order to minimise the disparities observed.
- The readmission data suggests the need for greater allied health supports such as dietetics and speech pathology to better manage the post-acute phase of recovery to potentially minimise readmission rates and improve the experience of recovery.
1 INTRODUCTION
Survival rates for head and neck cancer (HNC) have slowly increased over the past several decades with current 5-year survival in Australia reported at 71%.1 However, in order to achieve such improvements, more aggressive and multimodality approaches are now being employed, which has resulted in many people with HNC experiencing greater adverse effects and toxicities, which can persist long term after treatment completion.2, 3 As such, many individuals managed for HNC require ongoing post-acute multidisciplinary team (MDT) management.4, 5 Whilst MDT management has demonstrated improved outcomes in HNC,6 the costly and resource-intensive nature of MDTs7 inevitably results in these services being offered in highly centralised models of care in urban centres. This model can present multiple challenges for people with HNC living in non-metropolitan areas, who must travel to receive their initial care, and then face additional issues accessing ongoing specialised health services upon return home at the completion of treatment.
Within the broad field of cancer care, it is documented that people living outside of metropolitan areas experience health care inequities and have poorer overall cancer survival. Between the years 2010 and 2014, overall 5-year survival for all cancers combined decreased from 63% for metropolitan people to 55% for those from very remote areas.8 This has chiefly been attributed to poorer determinants of health in rural populations,9 delayed referral to specialists,10 delayed time to treatment,11 and lack of access to health services or limited resource availability.9 With specific reference to HNC, between 2010 and 2014, 5-year survival followed a similar trend with 64% for people living in major cities compared with 48% in very remote areas.8 Furthermore, First Nations Australians heavily occupy rural areas, with 19% of those living in very remote areas identifying as First Nations compared with only 1% identifying as non-Indigenous.12 Prior publications have shown that Indigenous people experience even poorer cancer survival outcomes, with 5-year survival reported at 48%8; however, whether survival was different for remote First Nations people was not reported in that report. Currently, most studies specific to HNC which report on differences by First Nations status heavily focus on incidence rates, staging and survival,13, 14 with only one recent study adjusted for remoteness. In that study,15 it was reported that 5-year survival for urban First Nations people was 50.5% (70.4% for all other urban Queenslanders) compared with 32% for remote First Nations peoples (54.8% for all other remote Queenslanders).
Whilst examining cancer variations by remoteness is increasingly a focus of health departments, this type of research in the specific area of HNC is comparatively limited, and has mostly focused only on incidence, treatment rates and mortality as the primary outcome measures.11, 16 Within the Australian context, Tan et al.11 reported differences in time from diagnosis to first treatment and disease recurrence rates in people with HNC from regional and rural areas. Whilst that study confirmed that rurality can impact the care pathways of people managed for HNC, it was limited in sample size, in the geographical regions included, and did not have a balanced metropolitan cohort for comparison. Therefore, further information is needed to comprehensively understand the impact of rurality on HNC care and its outcomes.
Additionally, there has been little focus on the impact of rurality on other markers of quality care such as access to multidisciplinary diagnostic and planning services and hospital readmissions. Such factors are recognised as key indicators known to impact overall health outcomes for people with cancer and other health conditions7, 17-19 and have the potential to be impacted by rurality. For example, in a systematic review of predictors for readmission and 30-day emergency department visits for surgically treated people with HNC, an increasing distance from treatment centre or a rural area of residence were identified as risk factors for readmission and emergency department visits.19 Additionally, rural patients needing post-acute hospital care may be readmitted to local services rather than the service which provided their primary treatment, which creates potential for care fragmentation. This is an important consideration as care fragmentation in people with HNC is associated with substantially higher risk of death.17 Hence further study of the impacts of rurality on such key markers of quality care will further inform the challenges faced by people in rural areas accessing HNC care.
Recognising the existing gaps in knowledge, the current study aimed to undertake a detailed analysis of the impact of rurality on aspects of the care pathway of people with HNC across Queensland, Australia. Using a large state-wide data set of patients diagnosed with HNC, the impact of rurality was examined across multiple markers of quality health care, including MDT access, timing to treatment, readmission rates and conditions leading to post-acute readmission, as well as survival outcomes and travel to access care. This research will provide further insights into how HNC care is impacted by remoteness, and assist health services to undertake targeted and locally relevant service delivery improvement for people with HNC living in rural areas.
2 METHODS
2.1 Setting
Queensland is geographically the second largest state within Australia and spans a total area of over 1.8 million square kilometres. In Queensland, public health care is delivered and managed through 17 separate Hospital and Health Services (HHSs) which collectively service a population nearing 5 million people.20 Of this, approximately 12% of the Queensland population reside in small towns, making it the state with the third largest proportion of residents living within small towns in Australia.21
2.2 Design
Cancer is a reportable disease within Australia and data on all diagnosed cases of cancer is collected and collated into the Queensland Oncology Repository (QOR) managed by Cancer Alliance Queensland (https://cancerallianceqld.health.qld.gov.au). This is a matched and linked dataset (performed deterministically) that integrates data from multiple sources including public and private hospital admissions data, public and private pathology, death data and hospital clinical data systems. Further details on the data sources collated into the QOR is detailed in the report Queensland Head and Neck Cancer Quality Index.16 This study is a retrospective analysis of existing data held within the QOR. Ethical clearance was granted by The Royal Brisbane and Women's Hospital Human Research Ethics Committee HREC/2018/QRBW/44912.
2.3 Participants
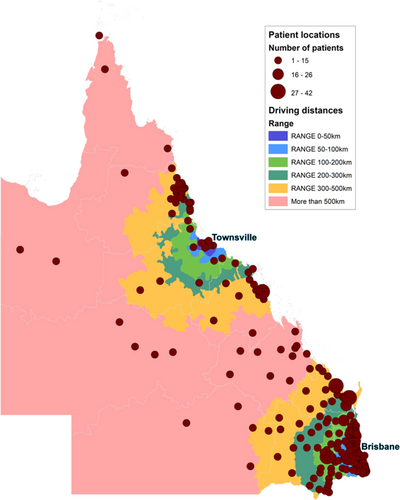
Data was retrieved from the QOR for all people ≥18 years of age, diagnosed with HNC between the years 2013 and 2015 coded with a primary diagnoses of ICD-10-AM22 codes C00.0-C32.0 (including oral cavity, oropharynx, hypopharynx, larynx and other pharynx sites of disease). Cutaneous lesions, tumours of the salivary glands and tumours of the nasopharynx were excluded. People treated exclusively for tumour recurrence during the data collection period were also excluded. The date range of 2013 to 2015 was chosen to reflect current, contemporary care practices in Queensland (the rollout of enhanced Intensity-modulated radiation therapy occurred in 2013) and to allow for 2-year mortality follow-up data to be available (till the end of 2017). During the period of this study, only three locations were equipped to offer definitive HNC tertiary care across Queensland (one hospital located in Townsville) or quaternary care (two hospitals in the capital city, Brisbane) (Figure 1). It is also important to note that the data in the date range of this study was prior to completion of a large health infrastructure project which expanded regional cancer care services within Queensland.23 That funding increased the capacity for regional services to provide HNC services, and therefore, the results reported in this study reflect outcomes prior to that service enhancement.
2.4 QOR data extraction
The QOR data for all patients recorded during the study period was provided in a de-identified format to the research team for further analysis. A modified version of the Australian Statistical Geography Standard (ASGS) Remoteness Structure24 was used to classify the data by remoteness category into 3 groupings based on the individual's postcode of residence – metropolitan, inner regional, and rural (Table S1). This classification system was developed by the Australian Bureau of Statistics (ABS) and was widely used at the time of data extract within QLD Health publications and the QOR publications on other cancer types. The decision was made to use this system to make comparisons with other rural data on other cancer types possible. An exception to this grouping is the metropolitan area of Townsville (originally classified as Rural). Townsville has been classified as Metropolitan because of the availability of high-quality tertiary-level cancer services for both inpatient and outpatient care. Data was then extracted for both the total cohort and then also subgroups of the cohort grouped by remoteness category, in relation to demographics, First Nations status (recorded at least once in any of the linked records), tumour characteristics, treatment type, as well as key service access data including access to MDT review, treatment/no treatment rates, time to first treatment, readmission post-treatment, reasons for readmission, duration of readmission, and also 2-ear survival. Patient postcodes were supplied for the purposes of calculating travel distances and times. Socio-economic status is based on the Socio-Economic Indexes for Areas (SEIFA),25 a census-based measure of social and economic well-being developed by the Australian Bureau of Statistics (ABS) using the Statistical Local Area Levels 2 (SA2). Each SA2 was categorised into the appropriate Socio-Economic Indexes for Areas using the Index of Relative Socioeconomic Advantage and Disadvantage (IRSAD) for Queensland in 2011.20 The ABS use the 2011 SEIFA scores to rank regions into ten groups called deciles. The QOR further aggregates these into three categories: (1) Disadvantaged (decile 1–2, lowest 20%), (2) Middle (decile 3–8, middle 60%), and (3) Affluent (decile 9–10, highest 20%). This data is imported as part of the data linkage procedures for each patient undertaken by the QOR and was supplied in this format to the research team.
Specific rules were applied for some parameters and some limitations within this data set are acknowledged. Treatment facility details were only provided for the first primary treatment and did not delineate if any additional treatment (e.g., post-operative radiotherapy) occurred at any differing location (e.g., if surgery was undertaken at a private metropolitan hospital and post-operative radiotherapy completed through the public referral hospital, only the private metropolitan hospital was noted in the database as the primary treatment site). A MDT review was also considered as being provided if the patient physically attended in person, or if they were not physically present but still had their case presented/discussed. Readmission data was recorded and analysed for post-acute treatment completion admissions only and did not include hospital admissions that occurred prior to or during primary treatment. Data for people who may have presented to emergency departments for management, but who did not proceed to hospital admission, was not available. For analyses pertaining to the reasons for readmissions, a consultant Ear, Nose and Throat surgeon on the study team (MS) reviewed all causes listed on readmission records with a member of the research team (JF) and grouped them into meaningful categories for data interpretation (individual conditions listed within each category are detailed in Table S3). For people who were readmitted more than once, the causes contributing towards each unique admission was recorded as a separate event. Primary or secondary codes for readmission listing the initial cancer diagnosis, tobacco use and type 2 diabetes mellitus without complications were not recorded as a condition on readmission.
Over the past two decades, notable shifts in the morphology of HNC tumours have been observed with an increasing incidence of human papilloma virus (HPV) positive SCC tumours, referred to as p16 positive.26, 27 As such, there is increasing interest in, and inclusion of, this measure in HNC-related research. In the process of data extraction, high levels of missing data were noted for p16 status (59% missing data). Consultation with the QOR team revealed that p16 status had not been consistently entered into the QOR until approximately 2019.16 Hence, available p16 status data was reported only within the demographics and should be interpreted with caution. It was excluded from any statistical analysis. Regarding tumour staging data, data was missing for 646 people (32.4% missing data). Determining the nature of missing data is critical for the correct treatment of missing data in analysis to avoid bias. Hence the pattern of missing data was analysed using matrix plots28 and correlation analysis28, 29 with the main study variables. These steps determined that the missing staging data was “missing at random” and there was no correlation between those with missing data and any variables such as staging or outcomes such as survival. This allowed data from cases with missing data to be removed from the analysis without creating bias, referred to as listwise deletion.30
2.4.1 Statistical analysis of significance
Statistical analysis was led by a contract statistician overseen by study authors (Author 1, Author 3) and conducted using SPSS. Following descriptive analysis, chi-square tests were used to explore differences at the univariate level in each parameter by remoteness. To accommodate multiple comparisons and minimise the effects of Type I error, statistical significance was set at a more conservative level (p < 0.01), with p > 0.01–0.05 discussed as trends. Those identified as significant at the univariate level were then examined further using logistic regression. All parameters identified as p < 0.05 at the univariate level were selected for multivariable analysis using logistic regression.
2.4.2 Travel distances and times
Information about distance travelled to care was also examined in relation to care accessed outside the person's local health service, and the actual distance travelled for care. Distances travelled and times travelled were calculated using Google Maps31 (set to extract times based on the fastest route and average traffic conditions) and then analysed using the geospatial software ArcGis32 by a contract geodesy engineer. Only patient postcodes were supplied by QOR to ensure confidentiality. As such, polygons of individual postcodes were created and distances were calculated from the centre of each postcode polygon. For the heatmap (Figure 1) depicting distances from the two locations offering definitive care at the time (Townsville and Brisbane), driving distances from the centre of the postcode polygon to the tertiary/quaternary hospitals were calculated, regardless of whether they accessed care at another facility (as this map is intended to show the distribution of people with HNC across the state relative to the two definitive sites). For actual driving times undertaken by each person, the distance from the centre of the postcode polygon to the facility that provided actual first primary care or readmission care was calculated and then median values were obtained by remoteness category. Data on whether or not people travelled to their care by means other than a car (e.g., train or plane) is not collected within the QOR, as such travel times were calculated for all individuals assuming travel had occurred by car.
3 RESULTS
3.1 Demographics and tumour characteristics
Examining the total cohort data (Table 1), the number of people diagnosed with primary HNC in QLD within the study period (2013–2015) was 1991, of which 77.7% were men, with 45.3% over 65 years of age, and 94.7% identified as non-First Nations (6 did not state). Squamous cell carcinoma was the most common morphology (93%) with the primary site most often diagnosed being the oropharynx (41.5%), followed by oral cavity (31.2%), larynx (17.9%) and hypopharynx (6.7%). The p16 status was reported for only 41% of the cohort and for those with p16 data available, 61.7% (n = 508/823) were positive. Of those with clinical staging available (67.6%), the majority (73.1%, n = 983/1345) presented with locally advanced disease (Stage 3–4). Most people had no recorded co-morbidities, with one or more co-morbidities recorded for 634 (32%) people. Of those who received treatment, the majority (28.1%) received surgery with adjuvant therapy, followed by 25.4% receiving concurrent chemoradiotherapy and then 24.8% who had surgery alone. Analysis by remoteness category revealed that the majority of people with HNC lived within a metropolitan city (59%), followed by inner regional (24%) and then rural (17%) (Table 1). Subgroup analysis of the group demographics by location revealed only two significant differences between the groups relating to socioeconomic status (p < 0.001) and First Nations status (p < 0.001).
| Sub-group analysis | |||||
|---|---|---|---|---|---|
| Qld total | Metropolitan | Inner regional | Rural | p-value | |
| n (%) | n (%) | n (%) | n (%) | ||
| 1991 (100%) | 1171 (58.1%) | 485 (24.3%) | 335 (16.8%) | ||
| Age group | |||||
| 0–64 | 1088 (54.6%) | 640 (54.6%) | 257 (52.9%) | 191 (57.0%) | 0.523 |
| 65 & older | 903 (45.3%) | 531 (45.4%) | 228 (47.0%) | 144 (42.9%) | |
| Gender | |||||
| Male | 1547 (77.6%) | 890 (76.0%) | 383 (78.9%) | 274 (81.7%) | 0.059 |
| Female | 444 (22.3%) | 281 (23.9%) | 102 (21.0%) | 61 (18.2%) | |
| Primary site | |||||
| Hypopharynx | 156 (7.8%) | 91 (7.7%) | 31 (6.3%) | 34 (10.1%) | 0.052 |
| Larynx | 357 (17.9%) | 189 (16.1%) | 103 (21.2%) | 65 (19.4%) | |
| Oral cavity | 623 (31.2%) | 388 (33.1%) | 131 (27.0%) | 104 (31.0%) | |
| Oropharynx | 827 (41.5%) | 485 (41.4%) | 213 (43.9%) | 129 (38.5%) | |
| Other pharynx | 28 (1.4%) | 18 (5.4%) | 7 (1.4%) | 3 (0.89%) | |
| T-classification (n = 1345) | |||||
| 0–2 | 362 (26.9%) | 236 (28.2%) | 91 (26.8%) | 35 (20.5%) | 0.123 |
| 3–4 | 983 (73.1%%) | 600 (71.8%) | 248 (73.2%) | 135 (79.5%) | |
| Treatment group | |||||
| Surgery only | 493 (24.8%) | 298 (25.4%) | 121 (25.0%) | 74 (22.1%) | 0.03 |
| Surgery + RT/CT | 560 (28.1%) | 329 (28.1%) | 140 (28.9%) | 91 (27.2%) | |
| Concurrent RT/CT | 505 (25.4%) | 313 (26.7%) | 113 (23.3%) | 79 (23.6%) | |
| RT only | 189 (9.5%) | 107 (9.2%) | 40 (8.2%) | 42 (12.5%) | |
| CT only | 38 (1.9%) | 18 (1.5%) | 17 (3.5%) | 3 (0.9%) | |
| No treatmenta | 206 (10.3%) | 106 (9.1%) | 54 (11.1%) | 46 (13.7%) | |
| Socioeconomic status (n = 1988) | |||||
| Disadvantaged | 507 (25.5%) | 168 (14.3%) | 186 (38.4%) | 153 (45.7%) | <0.001* |
| Middle | 1235 (62.1%) | 767 (65.7%) | 289 (59.6%) | 179 (53.4%) | |
| Affluent | 246 (12.4%) | 233 (20.0%) | 10 (2.0%) | 3 (0.9%) | |
| Indigenous status (n = 1985) | |||||
| First nations | 99 (4.9%) | 32 (2.7%) | 18 (3.7%) | 49 (14.6%) | <0.001* |
| Non-indigenous | 1886 (94.7%) | 1137 (97.0%) | 464 (95.6%) | 285 (85.0%) | |
| Co-morbidities | |||||
| 0 | 1357 (68.1%) | 825 (70.4%) | 308 (63.5%) | 224 (66.8%) | 0.063 |
| 1 | 369 (18.5%) | 205 (17.5%) | 104 (21.4%) | 60 (17.9%) | |
| 2 or more | 265 (13.3%) | 141 (12.0%) | 73 (15.0%) | 51 (15.2%) | |
- Abbreviations: CT, chemotherapy; RT, radiotherapy.
- a Not included in significance testing.
- * Significant at 0.01 (bonferroni correction to account for multiple comparisons).
3.2 The impact of remoteness on service use and survival
Unadjusted data for the total cohort, and by remoteness categories, regarding key service access/utilisation is summarised in Table 2. Comparisons by remoteness at the unadjusted level revealed differences in whether a person received an MDT assessment, whether a person had treatment, timing to first treatment and in readmission (Table 2).
| Characteristics | Sub-group analysis | ||||
|---|---|---|---|---|---|
| Qld total | Metropolitan | Inner regional | Rural | p-value | |
| n (%) | n (%) | n (%) | n (%) | ||
| 1991 (100%) | 1171 (58.8%) | 485 (24.3%) | 335 (16.8%) | ||
| MDT review | |||||
| Had MDT | 1616 (81.1%) | 992 (84.7%) | 419 (86.3%) | 205 (61.1%) | <0.001* |
| No MDT | 375 (18.8%) | 179 (15.2%) | 66 (13.6%) | 130 (38.8%) | |
| Received treatment | |||||
| Had treatment | 1785 (89.7%) | 1065 (90.9%) | 431 (88.9%) | 289 (86.3%) | 0.037 |
| No treatment | 206 (10.3%) | 106 (9.1%) | 54 (11.1%) | 46 (13.7%) | |
| Timing to first treatment (n = 1780)a | |||||
| ≤30 days | 825 (46.3%) | 529 (49.8%) | 190 (44.2%) | 106 (36.9%) | <0.001* |
| >30 days | 955 (53.7%) | 534 (50.2%) | 240 (55.8%) | 181 (63.1%) | |
| Readmission post treatmentb | |||||
| Readmitted | 243 (12.2%) | 160 (13.6%) | 45 (9.2%) | 38 (11.3%) | 0.04 |
| Not readmitted | 1748 (87.7%) | 1011 (86.3%) | 440 (90.7%) | 297 (88.6%) | |
| Readmission timing post treatmentc (n = 434) | |||||
| ≤30 days | 255 (58.8%) | 178 (61.4%) | 42 (51.9%) | 35 (55.6%) | 0.26 |
| >30 days | 179 (41.2%) | 112 (38.6%) | 39 (48.1%) | 28 (44.4%) | |
| Length of stayc (n = 434) | |||||
| ≤5 days | 252 (58.1%) | 180 (62.1%) | 41 (50.6%) | 31 (49.2%) | 0.055 |
| >5 days | 182 (41.9%) | 110 (37.9%) | 40 (49.4%) | 32 (50.8%) | |
| 2-year all cause survival | |||||
| Alive | 1501 (75.4%) | 919 (78.5%) | 352 (72.5%) | 230 (68.7%) | <0.001* |
| Deceased | 490 (24.6%) | 252 (21.5%) | 133 (27.5%) | 105 (31.3%) | |
- Abbreviation: MDT, multidisciplinary team.
- a Data not available for 5 people.
- b Readmission by individual patient and not by unique readmissions (i.e. some patients readmitted more than once).
- c Of total unique readmissions.
- * Significant at 0.01 (bonferroni correction to account for multiple comparisons).
3.2.1 Multidisciplinary team review
With respect to MDT access, the likelihood of receiving an MDT assessment was comparable for the metropolitan and regional cohorts, however decreased with increasing remoteness of residence. Specifically, 84.7% of metropolitan and 86.3% of regional people received an MDT review compared with only 61.1% of those from rural areas. At the univariate level this was statistically significant (p < 0.001).
3.2.2 Treatment rates and timing
In total, 10.3% (n = 206) of the total cohort received no treatment (Table 2). Rates of non-treatment increased with rurality with 9.1% of metropolitan people not receiving treatment compared with 11.1% for inner regional and 13.7% for rural people which was trending towards significance at the unadjusted level (p = 0.037). Less than half (41.6%) of the total cohort commenced treatment within 30 days of diagnosis, with a further 37% commencing within 60 days. The likelihood of treatment commencement within 30 days decreased by remoteness (49.8% of metropolitan, 44.2% of regional and 36.9% of rural people) and was statistically significant at the unadjusted, univariate level (p < 0.001).
3.2.3 Post-acute readmission
In total, 243 people were readmitted to hospital within 90 days of treatment completion, representing 12.2% of the total cohort (Table 2). Of these, 97 people (39.9% of all readmissions) were readmitted on more than one occasion, resulting in 434 unique post-treatment readmissions available for analysis. Breakdown of readmission by remoteness at the univariate level was trending towards statistical significance (p = 0.04) with 13.6% (n = 160) of the metropolitan cohort, 9.2% (n = 45) of the inner regional, and 11.3% (n = 38) of the rural cohorts being re-admitted. Most people (58.75%) were readmitted within the first 30 days of treatment completion. Length of stay ranged from 1 to 97 days, with a median of 4 days. Readmission timing and length of stay by remoteness were not statistically significant.
Across the total cohort, people treated with concurrent chemo/radiotherapy represented most readmissions at 37.6%, followed by surgery with adjuvant chemo/radiotherapy 34.7%, radiotherapy alone 14.5% and surgery alone 9.9% (Table S2). Readmissions were recorded across 61 different settings (see Figure S1). In the metropolitan cohort, people were more likely to be readmitted to the same facility that provided their initial primary care (42.8%) compared with inner regional (40%) and rural people (15.7%). Further characteristics of people who were readmitted are shown in Table S2. Within the readmitted cohort, the only demographic that was significant at the univariate level by remoteness was age (p = 0.01), with 43.7% of metropolitan people readmitted being over age 65 compared with 64.4% for inner regional and 63.1% for rural.
In addition to rates of readmission, reasons for readmission were also examined from the QOR dataset. In total, there were 479 conditions listed as contributing towards post-acute hospital readmission that were grouped into meaningful categories for interpretation (Table S3) and are presented by remoteness in Table 5. The top five admitting categories were found to be the same for metropolitan and the regional/rural cohorts. For both, dysphagia, fluid imbalance, nutritional inadequacy, gastrointestinal disorders and electrolyte imbalance were the top five conditions (Table 3).
| Admitting condition category | Number of times reported n= | Percentage of cohort admissions % | |
|---|---|---|---|
| Regional & Rural cohort (n = 144 unique admissions) | Dysphagia | 35 | 24.3% |
| Fluid imbalance | 30 | 20.8% | |
| Nutritional inadequacy | 24 | 16.6% | |
| Gastrointestinal disorders | 24 | 16.6% | |
| Electrolyte imbalance | 21 | 14.6% | |
| Oral mucositis & pain | 20 | 13.9% | |
| Respiratory infection & failure | 12 | 8.3% | |
| Gastrostomy management | 12 | 8.3% | |
| Kidney disease & failure | 12 | 8.3% | |
| Disorders of blood pressure | 11 | 7.6% | |
| Haematological disorders | 10 | 6.9% | |
| Palliative care | 10 | 6.9% | |
| Infection and sepsisa | 9 | 6.3% | |
| Metropolitan cohort (n = 290 unique admissions) | Dysphagia | 67 | 23.1% |
| Nutritional inadequacy | 67 | 23.1% | |
| Gastrointestinal disorders | 67 | 23.1% | |
| Fluid imbalance | 65 | 22.4% | |
| Electrolyte imbalance | 61 | 21.0% | |
| Oral mucositis & pain | 41 | 14.1% | |
| Kidney disease & failure | 37 | 12.8% | |
| Haematological disorders | 34 | 11.7% | |
| Stomatitis | 26 | 8.9% | |
| Disorders of blood pressure | 25 | 8.6% | |
| Infection and sepsisa | 25 | 8.6% | |
| Respiratory infection & failure | 20 | 6.9% | |
| Dermatitis and skin burns | 15 | 5.2% |
- Note: Only conditions reported in 5% or more of admissions are reported in table.
- a Not elsewhere specified in another category.
3.2.4 Two-year survival
A total of 675 (33.9%) people were deceased at the time of data extraction in 2018, with a 2-year all-cause survival rate of 75% for the total cohort. When examining survival by remoteness, there was a 78.5% survival rate within the metropolitan cohort, compared with 72.5% for regional and 68.7% for rural. This was significant at the unadjusted univariate level (p < 0.001).
3.3 Variables associated with remoteness: Multivariable analysis
Multivariable analysis was completed using multinomial logistic regression to control for all factors that may impact the outcomes observed by remoteness in the unadjusted analyses. The first stage involved univariate analysis using chi-square tests to assess the association between the categorical dependent variables (MDT review, had treatment, first treatment within 30 days, readmission rate and 2-year survival) against the independent variables (gender, age, remoteness, socioeconomic status, First Nations status, co-morbidities, tumour staging and treatment type). These results are presented in Table 4, with remoteness being associated with: MDT review (p < 0.001), treatment (p < 0.001), timing to first treatment (p < 0.001), and 2-year survival (p < 0.001) at the univariate level. Readmission was also included in the regression analysis as it was trending towards significance (p = 0.04).
| Independent variables | Categorical dependent variables (p-values) | ||||
|---|---|---|---|---|---|
| MDT review | Had treatment | Treatment within 30 days | Readmission | Survival | |
| Gender | 0.380 | 0.279 | 0.165 | 0.894 | 0.641 |
| Age | 0.993 | 0.000* | 0.473 | 0.079 | 0.000* |
| Remoteness | 0.000* | 0.021 | 0.000* | 0.040 | 0.000* |
| Socioeconomic status | 0.023 | 0.068 | 0.005* | 0.006* | 0.160 |
| Indigenous status | 0.002* | 0.022 | 0.331 | 0.054 | 0.016 |
| Co-morbidity | – | 0.003* | – | 0.000* | 0.000* |
| T-classification | – | 0.000* | – | 0.002* | 0.000* |
| Treatment type | – | – | – | 0.000* | 0.000* |
- * Significant at 0.01 (bonferroni correction to account for multiple comparisons).
Each of the significant categorical variables was then examined in a series of logistic regression models. The regression models that were found to be significantly associated with remoteness were (a) Had MDT review, (b) Had treatment, and (c) Treatment timing >30 days (Table 5). The odds ratio for receiving an MDT review between the rural cohort compared with metropolitan was significant (OR = 0.292, 95% CI [0.221, 0.400]), however, not between inner regional and metropolitan (OR = 1.219, 95% CI [0.884, 1.680]). No other variables besides remoteness were associated with not receiving an MDT review. Regarding having treatment, regression analysis revealed a significant difference between the metropolitan cohort and both the inner regional (OR = 0.550, 95% CI [0.340, 0.891]) and rural people (OR = 0.449, 95% CI [0.253, 0.798]) (Table 5). Additional influencing factors associated with non-treatment were age over 65 years and T-classification of 3–4. For the model examining treatment within 30 days, the odds ratio between the rural people compared with metropolitan was significant (OR = 1.527, 95% CI [1.156, 2.018]) however not between the inner regional compared with metropolitan (OR = 1.168, 95% CI [0.924, 1.477]). A socioeconomic status of affluent was the only other variable associated with receiving treatment within 30 days (OR = 0.696, 95% CI [0.493, 0.981]).
| Model | Variables | B | SE | Wald | df | p | Odds ratio | 95% CI for odds ratio | |
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| (a) Had MDT review | Remoteness | 84.231 | 2 | <0.001 | |||||
| Metro [reference] | |||||||||
| Inner regional | 0.198 | 0.164 | 1.459 | 1 | 0.227 | 1.219 | 0.884 | 1.680 | |
| Rural | −1.212 | 0.151 | 64.779 | 1 | <0.001 | 0.297 | 0.221 | 0.400 | |
| Socioeconomic | 1.626 | 2 | 0.444 | ||||||
| Low [reference] | |||||||||
| Middle | −0.088 | 0.143 | 0.381 | 1 | 0.537 | 0.916 | 0.693 | 1.211 | |
| Affluent | 0.160 | 0.238 | 0.450 | 1 | 0.502 | 1.174 | 0.735 | 1.873 | |
| Indigenous Status | – | – | – | ||||||
| Non-indigenous [reference] | |||||||||
| First nations | −0.238 | 0.242 | 0.963 | 1 | 0.326 | 0.789 | 0.491 | 1.267 | |
| (b) Had treatment | Remoteness | 10.084 | 2 | 0.006 | |||||
| Metro [reference] | |||||||||
| Inner regional | −0.597 | 0.246 | 5.902 | 1 | 0.015 | 0.550 | 0.340 | 0.891 | |
| Rural | −0.800 | 0.293 | 7.441 | 1 | 0.006 | 0.449 | 0.253 | 0.798 | |
| Age | – | – | – | ||||||
| 0–65 [reference] | |||||||||
| >65 years | −1.662 | 0.240 | 47.811 | 1 | <0.001 | 0.190 | 0.118 | 0.304 | |
| Indigenous status | – | – | – | ||||||
| Non-indigenous [reference] | |||||||||
| First nations | −0.473 | 0.477 | 0.983 | 1 | 0.321 | 0.623 | 0.245 | 1.587 | |
| Co-morbidity | 0.216 | 2 | 0.898 | ||||||
| 0 [reference] | |||||||||
| 1 | 0.103 | 0.281 | 0.135 | 1 | 0.713 | 1.109 | 0.639 | 1.925 | |
| 2+ | 0.110 | 0.302 | 0.132 | 1 | 0.717 | 1.116 | 0.617 | 2.017 | |
| T-classification | – | – | – | ||||||
| Stage 1–2 [reference] | |||||||||
| Stage 3–4 | −1.423 | 0.346 | 16.931 | 1 | <0.001 | 0.241 | 0.122 | 0.475 | |
| (c) Timing to first treatment >30 days | Remoteness | 9.047 | 2 | 0.01 | |||||
| Metro [reference] | |||||||||
| Inner regional | 0.155 | 0.120 | 1.681 | 1 | 0.195 | 1.168 | 0.924 | 1.477 | |
| Rural | 0.423 | 0.142 | 8.882 | 1 | 0.003 | 1.527 | 1.156 | 2.018 | |
| Socioeconomic | 4.371 | 2 | 0.112 | ||||||
| Low [reference] | |||||||||
| Middle | −0.101 | 0.117 | 0.739 | 1 | 0.390 | 0.904 | 0.719 | 1.138 | |
| Affluent | −0.363 | 0.175 | 4.282 | 1 | 0.039 | 0.696 | 0.493 | 0.981 | |
| (d) Readmission | Remoteness | 3.007 | 2 | 0.222 | |||||
| Metro [reference] | |||||||||
| Inner regional | −0.351 | 0.232 | 2.287 | 1 | 0.130 | 0.704 | 0.446 | 1.110 | |
| Rural | 0.120 | 0.280 | 0.183 | 1 | 0.669 | 1.127 | 0.651 | 1.952 | |
| Socioeconomic | 12.507 | 2 | 0.002 | ||||||
| Low [reference] | |||||||||
| Middle | 0.469 | 0.239 | 3.845 | 1 | 0.05 | 1.598 | 1.000 | 2.552 | |
| Affluent | 1.086 | 0.310 | 12.262 | 1 | <001 | 2.964 | 1.613 | 5.444 | |
| Co-morbidity | 36.546 | 2 | <0.001 | ||||||
| 0 [reference] | |||||||||
| 1 Co-morbidity | 0.789 | 0.216 | 13.291 | 1 | <0.001 | 2.201 | 1.440 | 3.365 | |
| 2+ Co-morbidity | 1.269 | 0.223 | 32.327 | 1 | <0.001 | 3.557 | 2.297 | 5.510 | |
| T-classification | – | – | – | ||||||
| Stage 1–2 [reference] | |||||||||
| Stage 3–4 | 0.130 | 0.257 | 0.256 | 1 | 0.613 | 1.139 | 0.689 | 1.883 | |
| Treatment group | 31.703 | 5 | <0.001 | ||||||
| No treatment [reference] | |||||||||
| CT only | 2.229 | 1.254 | 3.161 | 1 | 0.075 | 9.290 | 0.796 | 108.455 | |
| RT only | 2.951 | 1.042 | 8.016 | 1 | 0.005 | 19.128 | 2.480 | 147.546 | |
| Surgery only | 1.565 | 1.058 | 2.190 | 1 | 0.139 | 4.784 | 0.602 | 38.029 | |
| XRT/CT | 3.208 | 1.017 | 9.956 | 1 | 0.002 | 24.740 | 3.372 | 181.535 | |
| Surgery + XRT/CT | 2.988 | 1.018 | 8.625 | 1 | 0.003 | 19.853 | 2.702 | 145.863 | |
| (e) 2-years survival | Remoteness | 3.519 | 2 | 0.172 | |||||
| Metro [reference] | |||||||||
| Inner regional | −0.261 | 0.180 | 2.092 | 1 | 0.148 | 0.770 | 0.541 | 1.097 | |
| Rural | −0.353 | 0.231 | 2.332 | 1 | 0.127 | 0.702 | 0.446 | 1.105 | |
| Age | – | – | – | ||||||
| 0–65 years [reference] | |||||||||
| >65 years | −0.926 | 0.164 | 31.900 | 1 | <0.001 | 0.396 | 0.287 | 0.546 | |
| Indigenous status | – | – | – | ||||||
| Non-indigenous [reference] | |||||||||
| First nations | −0.183 | 0.375 | 0.239 | 1 | 0.625 | 0.833 | 0.399 | 1.736 | |
| Co-morbidity | 53.490 | 2 | <0.001 | ||||||
| 0 [reference] | |||||||||
| 1 | −0.940 | 0.190 | 24.368 | 1 | <0.001 | 0.391 | 0.269 | 0.567 | |
| 2+ | −1.362 | 0.204 | 44.720 | 1 | <0.001 | 0.256 | 0.172 | 0.382 | |
| T-classification | – | – | – | ||||||
| Stage 1–2 [reference] | |||||||||
| Stage 3–4 | −1.302 | 0.228 | 32.620 | 1 | <0.001 | 0.27 | 0.174 | 0.425 | |
| Treatment group | 110.580 | 5 | <0.001 | ||||||
| No treatment [reference] | |||||||||
| CT only | 2.247 | 0.545 | 17.026 | 1 | <0.001 | 9.462 | 3.254 | 27.517 | |
| RT only | 1.500 | 0.329 | 20.757 | 1 | <0.001 | 4.483 | 2.351 | 8.549 | |
| Surgery only | 2.350 | 0.315 | 55.640 | 1 | <0.001 | 10.489 | 5.656 | 19.450 | |
| XRT/CT | 2.736 | 0.287 | 90.653 | 1 | <0.001 | 15.423 | 8.782 | 27.087 | |
| Surgery + XRT/CT | 2.566 | 0.282 | 82.833 | 1 | <0.001 | 13.013 | 7.489 | 22.613 | |
- Note: bold = significant at 0.01, “–” denotes value not calculated for binary variables.
The other models examining readmission and survival were not found to be associated with remoteness. Rather, readmission was found to be significantly influenced by affluent socioeconomic status, having more co-morbidities, T-classification 3–4, and treatment type (most notably receiving XRT). For 2-year survival, the factors most influencing the likelihood of being deceased at 2-years were age (over 65 years), presence of one or more co-morbidities, T-classification 3–4 disease and treatment type (Table 5).
3.4 Travel to access care
Regarding travel to services, the data revealed that there were 70 facilities across QLD that were accessed by this cohort for some aspect of their primary care. Of these, the three tertiary/quaternary facilities provided 65% of primary care for all people with HNC across the state. For people needing radiotherapy, there were 12 smaller services that were accessed to provide this care (including three private facilities that provided care for 3% of people with HNC); however, the majority was provided by the tertiary/quaternary facilities in Brisbane and Townsville which provided radiotherapy care for 81.2% of people.
Exploring the issue of travel to access care, subgroup analysis revealed that the inner regional and rural cohorts were significantly (p < 0.001) more likely to receive their primary care treatment outside of their health service of residence (52% metro, 66.8% inner regional, 68.9% rural accessed care outside their residential health service), which included travelling for surgery (p < 0.001, 26.5% metro, 57.1% inner regional, 58.2% rural travelled for surgery). Examining actual geographical distance travelled to services providing primary care revealed that across the total cohort, 66.5% lived within 100 km one-way driving distance, 10.1% lived 100–200 kms one-way driving distance and 23.4% lived further than 200 kms one-way driving distance of the three locations that were equipped to offer definitive HNC tertiary or quaternary care (one hospital site located in Townsville and two hospital sites across capital city, Brisbane) between 2013 and 2015 (see Figure 1). Examining actual travel times (by car) from home postcode to site of the first treatment, the median travel was 46 min for the metropolitan cohort, compared to 2 h and 33 min for people from inner regional areas, and then 13 h and 21 min for those in rural areas. For those who were readmitted, median travel times to the facility of readmission was 53 min for the metropolitan cohort, compared to 2 h and 30 min for those from inner regional areas, while rural people had a median of 11 h and 21 min driving time.
4 DISCUSSION
Administrative and clinical data has historically been collected for billing and funding purposes, however, it is increasingly being used to better understand differences in the management and outcomes of specific cancer types. Within the field of HNC in Queensland, such data has only recently been examined within a Queensland Health report16 which documented broad demographic, incidence and mortality rates; however, variances by remoteness was not a central feature of that report and no statistical analysis for significance was completed. Hence, the current study is the first conducted in Queensland which has comprehensively examined the impact of remoteness on people with HNC.
Although this research included a cohort of individuals residing within a specific region of Australia, general demographic characteristics such as gender ratios, age at diagnosis, predominant morphology, primary tumour site and treatment modality were in line with other existing published data.16, 33 No co-morbidities were recorded for the majority of people (68%), which is in keeping with existing literature.16, 34 For those who received treatment, no significant differences were observed in treatment type by rurality. Whilst examining other variables that may affect treatment type in this cohort was outside of the scope of this study, prior work has shown that treatment variations in HNC are commonly associated with more advanced disease,35 disease recurrence36 and in people of older age.37 No treatment was recorded for 10.3% of all people, which was also expected, and at the lower end of other studies such as those from the USA which have reported rates of HNC non-treatment as high as 23.9%.38 Staging was also found to be incomplete in a proportion of this dataset (32.4% missing data), however this is a common issue reported in other publications of HNC data sets.16, 39 As such this cohort presents with comparable demographics and data availability to other reported cohorts. However, the local issues relating to available services and distance to care are unique to the current Queensland setting.
Regression analysis revealed that receiving an MDT review, receiving treatment, and timing to treatment commencement were significantly influenced by remoteness, with rural people less likely to have an MDT review, less likely to have treatment, and less likely to begin treatment within 30 days of diagnosis. Each of these identified differences placed rural people at a treatment disadvantage. It is recognised best practice that people with HNC should be promptly referred to MDT clinics for assessment and management.4 Whilst the total cohort rates of MDT access (81%) were similar to overseas reports for general cancer care,40 only 61.1% of people with HNC from rural areas were seen by an MDT which was significantly less than those from metropolitan areas. With communication technologies supporting health care services to overcome the issue of geography as a barrier to care (e.g., telehealth), the reasons why people from rural areas had less MDT access during the time period studied by this research warrants further investigation. Although the rural cohort in this study was not found to have poorer survival outcomes at 2 years, access to MDT for rural people needs to be improved, as previous research has identified that delayed referral to HNC specialists has been found to be associated with significantly higher risk of death at 3-year follow-up.10 Positively, the results showed that access to services, timing to treatment and outcomes were not statistically significantly different for people identifying as First Nations compared with non-Indigenous, although some results were trending towards poorer outcomes. Differences may therefore be more influenced by lower SES status and affluence levels and would be important areas for future work. This is in contrast to other work which has shown 5-year survival is significantly lower for First Nations Australians diagnosed with HNC,8, 15 however those results were not adjusted for other variables. The data presented in this study only extended to 2-year survival and examination of extended survival beyond this would be a valuable area for future study.
Rates of non-treatment were also impacted by remoteness, with lower proportions of rural people receiving treatment. Non-treatment was also associated with an older age and higher disease staging. In regards to receiving treatment within 30 days, previous studies have shown differences exist by remoteness in timing from diagnosis to first treatment by rurality.11, 16 This pattern was also observed in the current study, with greater delays to care reported among people from rural areas. The Optimal Care Pathway for People with Head and Neck Cancer states that treatment should commence within 4 weeks of diagnosis,4 however in this study 63.1% of rural people commenced treatment beyond 30 days. The underlying reasons why rural people have a greater delay in treatment commencement could not be ascertained in this study and would be an important area for future study.
Readmission post-treatment in this study occurred in 12.2% of people with HNC. Rates of readmission reported in the HNC literature vary significantly due to the heterogeneity in this cohort, with some reported as low as 3%41 and others as high as 16%.42, 43 However, much of the published literature focuses on surgical cohorts, with comparatively less readmission data available on those people treated with non-surgical modalities. In the current study, people managed with radiotherapy (with or without surgery and/or chemotherapy) represented the majority of readmissions. With increasing rates of non-surgical treatments being offered, this warrants further attention and inclusion in the scientific literature. This includes the need for greater consideration being given to the best ways to support these people after treatment in their recovery to reduce the high rates of post-acute hospital readmission.
Whilst, ideally, people in rural areas should be able to access care as close to home as possible to minimise the travel burden, the current data highlighted that 68.9% of rural and 66.8% of inner regional patients had to travel outside their residential health service to access primary care. This is a direct result of MDT services for primary HNC care being located in larger metropolitan centres. While it is recognised that hospitals within larger dedicated cancer services are related to improved survival outcomes,6, 18 the burden of travelling for care away from family and local supports must be acknowledged.
Equally, the current data highlighted issues regarding readmissions with only 15.7% of rural people in this cohort being re-admitted to the same facility that provided their primary care. This raises concerns around potential fragmentation of care along the HNC pathway. Care fragmentation is a known risk factor for poorer overall outcomes and death in surgically treated HNC cases from the United States17, 44 and a scenario that disproportionately affects non-metropolitan populations.44 Although poorer survival was not found in this study, prior work in QLD, Australia, has indicated that care fragmentation can significantly impact the quality of care delivery and the experience of recovery in rural people with HNC.45 Further examination of how care fragmentation impacts on recovery and ways to minimise these impacts is warranted given the high degree of readmission to alternative health facilities identified in the current study. Accessing health services closer to home is desirable for people from rural areas, it is important for health services to ensure there is access to quality care and that clinical information pertinent to their cancer recovery is accessible across facilities. Given the geographical dispersity of the Queensland population, further analysis of care pathways and service access in rural areas is needed to understand how rurality impacts the way in which people with HNC engage in services and their experience of accessing care away from the metropolitan cancer centres. Better understanding of the care and recovery pathways for rural people with HNC may provide future solutions to improving care and access to services across Queensland. For example, the potential benefits of more widespread digital health systems, allowing better communication of health information between facilities and coordinating care between metropolitan and rural services may help address some of the challenges rural people with HNC face.
With studies reporting high proportions of unplanned hospital readmissions are potentially avoidable,46, 47 it is critical to understand the causes contributing to such presentations in order to reduce the frequency and ultimately the associated costs. Studies that have reported on conditions leading to emergency department presentation or hospital readmission in HNC cohorts commonly cite an infectious aetiology as the main condition.41, 43 In the current study, the top admitting condition categories reported were Dysphagia, Nutritional inadequacy, Gastrointestinal disorders, Electrolyte imbalance and Fluid imbalance. Many of the conditions listed within these top three categories may reflect poor oral intake (possibly from lack of appetite, nausea, odynophagia and/or dysphagia), which are known to frequently impact people with HNC.2, 3 Many people with HNC are malnourished before therapy commencement and hospital readmission rates have been shown to correlate with body weight loss percentage.48
Collaborative management of people with HNC through coordinated and joint speech pathology and dietetics services has shown a reduction in readmissions for people with HNC.49 Importantly, these top contributing conditions did not vary greatly by remoteness and highlight the need for all people with HNC to have good access to post-acute support services to manage these difficulties. It does, however, need to be acknowledged that access to allied health services is known to be disproportionally poorer for rural people. As such, further research is needed into how the post-acute phase of recovery is impacted by remoteness and how access barriers to health services impacts the experience of recovery for rural people.
When examining travel along the treatment journey for people with HNC from regional and rural areas, this study confirmed that non-metropolitan people were significantly more likely to travel to receive care and to receive treatment outside of their health service of residence. Considering only a handful of facilities in the state of Queensland at the time this data was collected were equipped to offer definitive HNC services and were found to provide over 80% of primary treatment for this cohort, this result is not surprising. However, it is important to acknowledge as travelling to receive care is known to contribute to many stressors, including financial, emotional, employment and psychological stress for people with HNC and their families.50 Further, the travel times undertaken by the regional and rural groups to access both primary treatment and readmission care for this cohort were considerable. Whilst travelling to access cancer care is a necessity for most people in rural areas, health services need to be mindful of how travelling impacts a person's well-being, ability to have family/social supports present and, ultimately, how this impacts their ability to engage with health services.
4.1 Limitations
4.1.1 Missing and incomplete data
Using administrative data to understand clinical presentations does have limitations51 and some data was not available. Data studies conducted elsewhere in HNC have similarly reported data unavailability,39 however have still presented clinically significant findings. Matrix plots and correlation analysis revealed missing data cases could be reliably excluded from multivariable analysis without creating bias. As such, results presented in ‘Variables associated with remoteness’ are not impacted by the missing data. It is acknowledged that although staging and p16 data was incomplete, it is routinely assessed in the diagnostic process. Through this research, the clinical significance of including these in the repository has been realised and future evaluations using these variables will be possible. Additionally, only the total number of co-morbidities coded on hospital admission data was supplied to the research team. As such, no analysis of the co-morbidities beyond total numbers could be undertaken and would be an area for future study.
4.1.2 Readmission data limitations
The current readmission data does not include emergency department attendance without a true inpatient readmission. As such, the data presented likely under reports post-acute service needs as studies in HNC emergency department care do not correlate to inpatient readmission (8.43% vs. 3.2%).41 Given the retrospective nature of this study, assumptions had to be made regarding the grouping/categorisation of causes for readmission. While this data was analysed by a senior Ear, Nose and Throat specialist, they are only assumptions and may not be fully accurate, for example, iron deficiency can result from poor nutritional intake (due to dietary choices or dysphagia), poor gut absorption or from blood loss. Therefore, some caution is recommended when interpreting the groupings of conditions.
4.1.3 Date ranges included
The date range of 2013–2015 was chosen as it allowed for accurate 2-year survival data. Whilst a wider date range including more recent years would be desirable, the survival data for later years was inconsistent at the time of data extract.
4.1.4 Site of treatment
It was not deemed within the scope of this analysis to explore differences in outcomes between treatment centres. All three primary treatment centres (regional and metro) are classified as Level 5/6 Cancer Units and are benchmarked against standards to provide equitable and quality services across QLS. Furthermore, individual differences in patient journeys are commonplace and therefore the issue of comparing the true impact of “site of treatment” on outcomes is complex and would require dedicated focus to this question.
4.1.5 Changes in service structures
It is acknowledged that there may be more active use of telehealth to rural populations brought on by greater investment in rural cancer care in recent years as well as increased telehealth activity bought on by the COVID-19 pandemic. This may have changed how rural people engage with specialist services (e.g., timing to treatment and post-acute care to reduce unnecessary hospital readmission) and provides an opportunity for future investigation.
5 CONCLUSION
This study illustrates differences experienced by people with HNC by remoteness within Queensland, Australia. In this data set, it was identified that people from rural areas were more likely to not receive an MDT review, to not receive treatment and to have a delay in treatment commencement compared with people residing in metropolitan areas. They were also more likely to need to travel to access care, more likely to need to seek treatment in a health service outside of their area of residence and more likely to be readmitted post-acutely to a different facility than where they received primary care. For those individuals who were readmitted post primary care, the most common condition categories contributing towards admission related to Dysphagia, Nutritional inadequacy, Gastrointestinal Disorders, Electrolyte Imbalance and Fluid Imbalance. Overall, the current outcomes highlight the need to directly link the care pathway between the treating team with care closer to home and to better understand the care needs and treatment access journeys of people with HNC from regional/rural areas in order to minimise the disparities in outcomes. The readmission data suggests the need for greater allied health supports such as dietetics and speech pathology to better manage the post-acute phase of recovery to potentially minimise readmission rates and improve the experience of recovery.
AUTHOR CONTRIBUTIONS
Jasmine Foley: Conceptualization; investigation; writing – original draft; methodology; validation; visualization; formal analysis; project administration; data curation. Laurelie R. Wishart: Conceptualization; investigation; writing – original draft; methodology; validation; data curation; formal analysis; supervision. Elizabeth C. Ward: Conceptualization; investigation; writing – original draft; methodology; validation; visualization; formal analysis; data curation; supervision. Clare L. Burns: Conceptualization; methodology; validation; supervision. Rebecca Packer: Conceptualization; methodology; validation; supervision. Shoni Philpot: Investigation; data curation; methodology. Lizbeth M. Kenny: Validation; investigation. Maurice Stevens: Validation; investigation.
FUNDING INFORMATION
This research was supported by an Australian Government Research Training Program (RTP) Scholarship awarded to Jasmine Foley as part of a University of Queensland doctoral program.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
ETHICAL APPROVAL
Ethical approval was sought and granted by The Royal Brisbane and Women's Hospital and Health Service Human Research Ethics Committee (HREC/2018/QRBH/44912) and The University of Queensland Human Research Ethics Committee prior to commencement of the study.
CONSENT TO PARTICIPATE
Individual consent was not able to be obtained from the patients from which the data is derived, therefore access to data was granted following approval by the Department of Health under the guidelines stipulated within the Public Health Act 2005 (Ref QCOS/032227/RD007656).




