Immune cell profiling of vaginal blood from patients with early pregnancy bleeding
Sebastian Gidlöf and Martin A. Ivarsson contributed equally to this study.
Abstract
Problem
Vaginal bleeding during early pregnancy is estimated to occur in 20% of all pregnancies and it is often difficult to predict who will ultimately miscarry. The role of immune cells in early pregnancy loss is poorly understood.
Method of Study
In this prospective cohort study, 28 pregnant women presenting with first-trimester vaginal bleeding donated vaginal blood, peripheral venous blood, and saliva during their initial emergency room visit, and at a follow-up. The composition, frequency, and phenotype of immune cells in the vaginal blood were determined using flow cytometry. The proteome of serum and saliva was analyzed with OLINK proximity extension assay and correlated to vaginal immune cell phenotype and outcome of pregnancy. The course and outcome of pregnancies were followed and recorded.
Results
Vaginal blood contained all main immune cell lineages including B cells, NK cells, T cells, and monocytes/macrophages. Notably, vaginal blood immune cells expressed tissue residency markers including CD49a. Women who subsequently miscarried had a higher frequency of vaginal blood CD49a+ NK cells compared to those who did not miscarry, and this correlated with serum levels of granzyme A and H, as well as CSF1, CAIX, and TWEAK. Women in the miscarriage group also had a higher frequency of peripheral blood T cells expressing CD49a.
Conclusions
Our study provides novel insight into human reproductive immunology in relation to miscarriage. Tissue-resident NK cells in vaginal blood alone or in combination with serological biomarkers hold potential as prognostic factors in the prediction of pregnancy outcome in women with early pregnancy bleedings.
1 INTRODUCTION
Vaginal bleeding during early pregnancy occurs in approximately 20% of all pregnancies.1, 2 Globally, this symptom imposes a significant burden to women, their families, and healthcare systems. Even though chromosomal aberrations are the underlying cause of most sporadic miscarriages, the etiology of the individual case is often not identified.3, 4 In the clinical setting, it is difficult to predict the outcome of vaginal bleeding with a viable embryo/fetus in utero, and repeated follow-ups are often necessary. Attempts have been made to determine the risk of pregnancy loss based on clinical, biochemical, and sonographic features, but strong prognostic factors predicting who will ultimately miscarry or have a favorable pregnancy outcome are lacking.5 The uncertain prognosis is a cause of great anxiety among the vast majority of these patients.
During normal pregnancy, the maternal immune system adapts both systemically and locally to allow the semi-allogeneic embryo and placenta to grow inside the uterus. Tolerance is established and maintained throughout the pregnancy through placental and maternal anti-inflammatory cytokines and hormones, skewing the local immune cell lineages to immune-tolerogenic phenotypes.6 In early pregnancy, estrogen induces an expansion of suppressive regulatory T cells (Treg) both locally in the uterus and in the circulation.7 Tregs inhibit the proliferation and activation of CD4+ and CD8+ T cells and suppress the cytotoxic function of NK cells as well as inhibiting maturation and activation of dendritic cells and macrophages. Failure in these adaptations may result in rejection of the embryo causing miscarriage and it is postulated that underlying immunological aberrations may cause up to 50% of all miscarriages.8 Special decidual natural killer cells (dNK), that are phenotypically and functionally distinct from circulating peripheral blood NK cells, comprise up to 70% of the decidual leukocytes.9 Decidual NK cells and macrophages (dMac) can both be seen interacting with the invading trophoblasts, and studies indicate this interaction is key to normal placentation.10, 11 Indeed, malplacentation can lead to spontaneous abortion.12, 13 Both dNK and dMacs produce chemokines and angiogenic factors that regulate spiral artery remodeling and function in vitro.14, 15 In contrast to conventional NK cells, dNK cells have mostly regulatory and anti-inflammatory functions.16
Whether bleeding in the fetal-maternal interface is a consequence of changed immune cell composition and function is not known. Several serum biomarkers have been evaluated for the prediction of miscarriage following first-trimester vaginal bleeding. Pillai et al. found cancer antigen 125 (CA125/MUC16) to be the most promising marker for pregnancies gestational age 6−12 weeks, with a high negative predictive value in finding the pregnancies that were likely to continue.17 Further, low levels of placental growth factor (PlGF) and soluble vascular endothelial growth factor (VEGF) receptor 1 (sFlt-1) in maternal serum have been associated with an increased risk for miscarriage.18 Saliva is a non-invasive source of putative biomarkers,19 and low levels of PlGF in maternal saliva have been linked to preeclampsia risk.20 Whether saliva levels of PlGF or any other biomarker can be linked to miscarriage remains to be investigated.
Here we have, for the first time, applied high-parameter flow cytometry and proteomics on biological samples from patients presenting with first-trimester vaginal bleeding. Our primary aim was to identify immune cell populations in the vaginal blood using flow cytometry with venous blood as a reference. The second aim was to correlate pregnancy outcomes: miscarriage or continued pregnancy, to information on immune subset composition, and to serum and saliva proteome data. Our results represent important steps forward in the understanding of the pathogenesis of miscarriage.
2 MATERIAL AND METHODS
2.1 Subjects
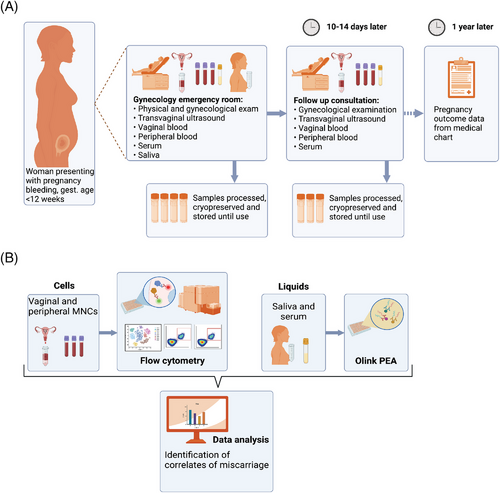
Women presenting with early pregnancy bleeding at the gynecological emergency unit at Karolinska University Hospital from January 2020 to December 2022 were recruited to this prospective cohort study. The study participants received both written and oral information about the study before they were asked to participate. The inclusion criteria were recent or ongoing vaginal bleeding, viable intrauterine pregnancy <12 weeks of gestation or pregnancy of unknown location confirmed by ultrasound examination, age 18−40 years, BMI of 19−35 kg/m2, and sufficient knowledge of spoken and written Swedish to give written informed consent. The exclusion criteria were non-viable pregnancy, ectopic pregnancy, severe bleeding requiring immediate surgery or clinically unstable vital parameters, signs of genital infection, and chronic illness. A total of 29 women consented to the study. One woman was later diagnosed with extrauterine pregnancy and excluded from the study. None of the included women had signs of molar pregnancy nor had any symptoms of ongoing local or general infection. Clinical information regarding gynecological history and conception was collected. The amount of vaginal bleeding was assessed by the study participant according to a four-grade scale bleeding pictogram (Figure S1).
Data from a total of 28 women were used in final analysis. The median age was 31 years (range 22−40) and median BMI 23.7 (range 16.5−34.7) kg/m2. One of the women included in the study was later found to have a BMI of 16.5 kg/m2. She was not excluded from the analysis. Another woman was diagnosed with anti-phospholipid syndrome later in pregnancy and was also kept in the analysis. Results from these two women did not differ significantly from the rest of the study population.
2.2 Sampling of blood, serum, and saliva
The study participants underwent a clinical examination and a transvaginal ultrasound in the gynecological emergency unit, and blood from the vagina was collected using a speculum. The vaginal blood was transferred from the speculum to a 50 mL Falcon tube prefilled with cold 10 mL cell RPMI 1640 media (Hyclone) supplemented with 10% Fetal Calf Serum (FCS) (ThermoFisher) (referred to as R10), Heparin (40 U/mL, LEO Pharma), Penicillin/Streptomycin (100 μg/mL, ThermoFisher), Gentamicin (50 μg/mL, ThermoFisher), and Amphotericin B/Fungizone (2.5 μg/mL, ThermoFisher) as previously described for collection of menstrual blood.21 Venous blood samples (Hemogard EDTA K2, BD Vacutainer), serum (Hemogard SST II, BD Vacutainer), and saliva (50 mL Falcon tube) were collected within 30 min of vaginal blood sampling. For saliva sample collection, the study participants were asked to rinse their mouths with water to remove food residues and then wait at least 10 min to allow saliva to pool in the mouth before giving the sample. Vaginal blood and saliva samples were kept at 4°C until processing. Venous blood and serum samples were kept at room temperature.
All women with threatened miscarriage were offered a follow-up clinic visit after 10−14 days where transvaginal ultrasonography was performed to evaluate the pregnancy. Venous blood and serum samples were taken at this second-time point, and in the case of still ongoing vaginal bleeding, a second vaginal blood sample was collected. Out of the 28 women who participated in the study, 17 came to the follow-up consultation. Among them, eight were able to provide a second vaginal blood sample. Of these eight participants, four had miscarried while the remaining four had a continuing pregnancy. The course and outcome of pregnancy including perinatal outcome was collected from the patient's electronic medical records. The study and experimental design are displayed in Figure 1A,B.
3 METHOD
3.1 Cell isolation
Early in the study we observed that nearly all the vaginal blood samples contained pieces of tissue. There was no visible difference in the amount of tissue present in vaginal blood samples when comparing the miscarriage group with the ongoing pregnancy group. To ensure all cells were extracted from these, while also ensuring samples were treated equally, all vaginal samples were subjected to enzymatic digestion before isolation of cells. A combination of DNAse and type II collagenase was used to break down tissue, as previously described.22 Briefly, tissue/vaginal blood was agitated with a magnetic stirrer at 37°C in RPMI containing 0.2 mg/mL DNAse and collagenase. This was followed by straining using 70 μm cell strainer (Miltenyi Biotec). Mononucleated cells from vaginal and peripheral blood were isolated using standard density gravity centrifugation (Ficoll/Lymphoprep) and were frozen and stored in liquid nitrogen. The number of mononucleated vaginal cells obtained and subsequently frozen ranged from 0.2−6 million mononucleated cells.
3.2 Saliva isolation
Saliva was kept at 4°C from the collection and through isolation. Samples were centrifuged at 4.000 rpm for 20 min in a centrifuge set to 4°C and saliva supernatants aliquoted into two or four 1.5 mL Eppendorf tubes depending on volume. Protease inhibitor (cOmplete™, Roche) was added to each tube before the sample was frozen and stored at −80°C.
3.3 Flow cytometry
For antibodies used, see Table S1. We confirmed by flow cytometry that vaginal blood cells could be frozen and thawed without effect on the markers included in the panel (data not shown). Frozen cells were thawed in warm R10, washed twice, and resuspended in FACS buffer (PBS supplemented with 2% FCS and 2 mM EDTA). All vaginal cells from a vial (up to 6 million mononucleated cells) were stained as previously described.23 1−2 million PBMC were stained in parallel. The samples were fixed with BD FIX/PERM and analyzed on a BD A5 Symphony flow cytometer. Analysis of cytometry data was done using FlowJoTM v10 (BD LifeSciences), and R-scripts. UMAP visualization was done using umap-learn python script.24
3.4 OLINK
Serum and saliva samples were thawed and diluted in PBS, randomized to a 96-well plate and shipped on dry ice to OLINK Proteomics (Uppsala, Sweden) for proteome analysis using Olink Target 96 Immuno-Oncology panel (Supplementary Data). The concentration of each protein was expressed in Olink's arbitrary unit normalized protein expression (NPX) in log2 scale.
3.5 Statistics
Analysis of flow cytometry results was conducted with FlowJoTM v10 (BD LifeSciences) and downstream analysis completed with GraphPad Prism v9.0. Flow cytometry and proteome data was tested for normal distribution using the D'Agostino-Pearson normality test. Flow cytometry data failed the normality test and downstream comparisons were performed applying the Mann-Whitney U test. Serum proteome data was normally distributed, and regression analysis was performed with Pearson correlation coefficients using R version 4.2.2. (R core team). Volcano plots were generated by the EnhancedVolcano 1.12.0 R–package.25 Benjamini-Hochberg correction of multiple testing was used to reduce the false discovery rate. Pearson correlations were done with R–scripts ggplot2 and ggpubr. Hierarchical clustering was done with pheatmap. Saliva proteome data included very few samples and nonparametric Spearman correlation was used. In figures * = p < .05, ** = p < .01. Figure 1 and Figure S1 were created with BioRender.com.
4 RESULTS
In total, data from 28 women diagnosed with early pregnancy bleeding were analyzed. The baseline clinical characteristics and information on the course and outcome of pregnancies is presented in Table 1. Fourteen of these women were subsequently diagnosed with a miscarriage and 14 continued their pregnancy beyond fetal viability. At the time of enrollment in the study, there were no significant differences observed in age, gestational age according to the last menstrual period, BMI, blood pressure, body temperature, or blood levels of CRP and hemoglobin between the two groups. Furthermore, no significant distinctions were found in terms of previous gynecological history or the number of miscarriages (Table 1).
| Miscarriage group | Ongoing pregnancy group | ||
|---|---|---|---|
| Group size | n | 14 | 14 |
| General characteristics | Age, median (range) | 30 (25–40) | 31 (22–39) |
| BMI, median (range) | 25,4 (21,3–34,7) | 23,1 (16,5–29,7) | |
| Systolic blood pressure mmHg, median (range) | 120 (100–136) | 119 (96–138) | |
| Diastolic blood pressure, mmHg, median (range | 77 (64–86) | 74 (60–87) | |
| Pulse bpm, median (range) | 84 (74–105) | 79 (65–111) | |
| Temperature°C, median (range) | 37,0 (36,8–37,7) | 37,1 (36,3–37,4) | |
| CRP mg/L, median (range) | <5 (<5) | <5 (<5–12) | |
| Hemoglobin g/L, median (range) | 127 (117–142) | 123 (101–148) | |
| u–HCG positive, n (%) | 14 (100) | 14 (100) | |
| Gynecological history | Menstrual cycle length days, median (range) | 29 (26–34) | 29 (23–30) |
| Menstrual duration days, median (range) | 5 (2–6) | 5 (4–10) | |
| Previous pregnancies n (%) | |||
| 0 | 4 (29) | 3 (21) | |
| 1 | 2 (14) | 3 (21) | |
| 2 | 2 (14) | 2 (14) | |
| ≥3 | 6 (43) | 6 (43) | |
| Previous miscarriages n (%) | |||
| 0 | 8 (57) | 8 (57) | |
| 1 | 2 (14) | 2 (14) | |
| 2 | 3 (21) | 1 (7) | |
| ≥3 | 1 (7) | 3 (21) | |
| Current medication | n (%) | ||
| Low molecular heparin | 0 | 1 (7) | |
| ASA | 0 | 2 (14) | |
| Prednisolone | 0 | 2 (14) | |
| Vaginal progesterone | 2 (14) | 2 (14) | |
| Asthma medication | 3 (21) | 0 | |
| Iron supplements | 2 (14) | 2 (14) | |
| Levothyroxine | 2 (14) | 1 (7) | |
| No medication | 9 (64) | 10 (71) | |
| Conception | Spontaneous, n (%) | 12 (86) | 11 (79) |
| IVF, n (%) | 2 (14) | 3 (21) | |
| Current pregnancy characteristics | Gestational age days (LMP), median (range) | 54 (38–70) | 55 (40–84) |
| Gestational age days (CRL), median (range) | 44 (37–56) | 58 (40–82) | |
| Intrauterine pregnancy, n (%) | 10 (71) | 13 (93) | |
| Pregnancy of unknown location, n (%) | 4 (29) | 1 (7) | |
| Yolk sac visible, n (%) | 7 (54) | 12 (86) | |
| Subchorionic hemorrhage, n (%) | 2 (14) | 6 (43) | |
| Bleeding pictogram | n (%) | ||
| Spotting | 2 (14) | 4 (29) | |
| Light | 6 (43) | 7 (50) | |
| Moderate | 3 (21) | 3 (21) | |
| Heavy | 3 (21) | 0 | |
| Vaginal bleeding no. of days, median (range) | 2 (1–8) | 1 (1–10) | |
| Course of pregnancy | Delivery at term, n (%) | n/a | 11 (76) |
| Uneventful pregnancy, n (%) | n/a | 7 (50) | |
| Premature delivery (<37 weeks of gestation), n (%) | n/a | 2 (14) | |
| Stillborn, n (%) | n/a | 0 | |
| IUGR, n (%) | n/a | 2 (14) | |
| Preeclampsia, n (%) | n/a | 2 (14) |
4.1 Vaginal blood from pregnant women with first trimester bleedings contains all main lymphocyte subsets including tissue resident cells
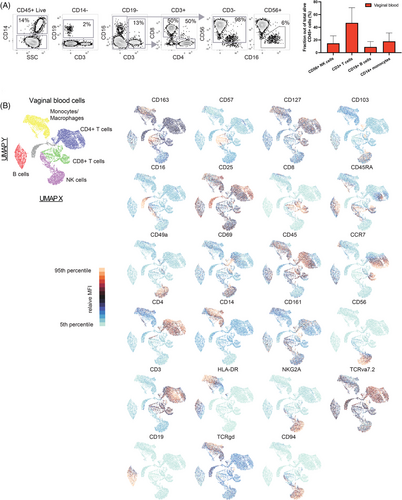
To map the overall composition of immune cells we used a 24-colour flow cytometry panel. We determined that lymphocytes are alive and detectable in vaginal blood from women presenting with early pregnancy bleeding. The vaginal blood contained CD14+ cells (range 2.8%−55% of live CD45+ cells), CD19+ B cells (1.1%−37%), CD3+ T cells (8.4%−80%), and CD56+NK cells (0%−45%) (Figure 2A). A Uniform Manifold Approximation and Projection for Dimension Reduction (UMAP) projection was made to illustrate the composition (Figure 2B). This shows that immune cell composition can be analyzed in vaginal blood from women with early pregnancy bleeding and contains all major immune cell subsets.
4.2 Vaginal blood immune cells exhibit distinct immune cell composition compared to matched peripheral blood
After mapping the immune cell composition of vaginal blood, we next compared it to matched peripheral venous blood samples. A combined UMAP using data from both vaginal and peripheral blood cells indicated similarities as well as differences (Figure 3A and Figure S2A). Pairwise comparisons of vaginal blood mononucleated cells and peripheral blood mononucleated cells indicated no significant differences in the composition of B cells, T cells, and conventional NK cells (Figure 3B-D). However, a higher frequency of CD14+HLA-DR+ monocytes was found in vaginal blood compared to peripheral blood. Conversely, subsets of CD14+CD163+ and CD14+CD16+ monocytes were found in higher frequencies in peripheral blood (Figure 3E,F).
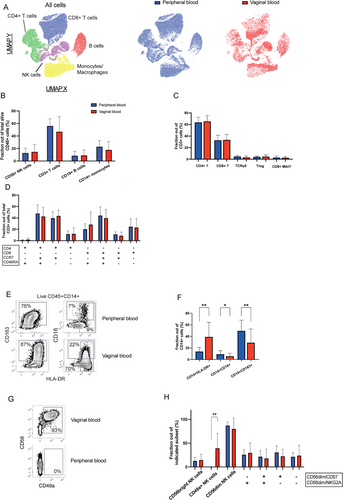
As vaginal blood may contain cells originating from the uterus, we included tissue-residency markers including CD49a in our cytometry panel. A high fraction of uterine NK cells express CD49a,16, 26 and interestingly, the vaginal blood from many patients contained this subset (Figure 3G,H). This subtype of NK cells is the dominating immune cells in the decidua during the first trimester of pregnancy. Its presence in the vaginal blood, therefore, indicates that cells have dislodged from the fetal-maternal interface. A more detailed analysis of the cells strengthened this notion since the same cells also expressed dNK cell markers CD103 and CD69 (Figure S2B). Markers of conventional NK cells, CD16 and CD57, were absent on these cells but were expressed by conventional NK cells in vaginal and peripheral samples (Figure S2C). Differentiation status of the conventional NK cells, as indicated by expression of NKG2A and CD5727 found in vaginal blood showed no difference in composition compared to peripheral blood (Figure 3H).
Taken together, the results showed that vaginal and peripheral blood shared a similar pattern in the composition of most immune cells. However, there were distinct differences with the enrichment of tissue-resident NK cells and CD14+HLA-DR+ myeloid cells.
4.3 Women who miscarry exhibit distinct vaginal blood NK cell and peripheral blood T cell profiles
Having determined that vaginal blood samples contained uterine immune cells we wanted to investigate if pregnancy outcome was related to the immune cell composition. We compared patients who had miscarried after their emergency room visit (n = 14), with those who continued their pregnancy until term (n = 14). Women who miscarried showed a distinct immune cell profile as indicated by a UMAP separating miscarriage from ongoing pregnancy patients (Figure 4A). Notably, a clear abundance of CD49a+ NK cells in vaginal blood was visible in the miscarriage group, indicating possibly a breach of the uterine integrity allowing decidual cells to escape the uterus (Figures 3A and 4B). Notably, the same group of women showed an increased frequency of CD49a+ T cells in peripheral blood (Figure 4C). A deeper analysis revealed that CD4+ vaginal blood effector memory T cells (CCR7-CD45RA-) were significantly increased in the miscarriage group (Figure 4D). Neither of these three measurements correlated with each other (data not shown). Further, we found no difference in the frequency of CD14+HLA-DR+, CD14+CD163+, or CD14+CD16+ monocytes in vaginal blood between the two groups.
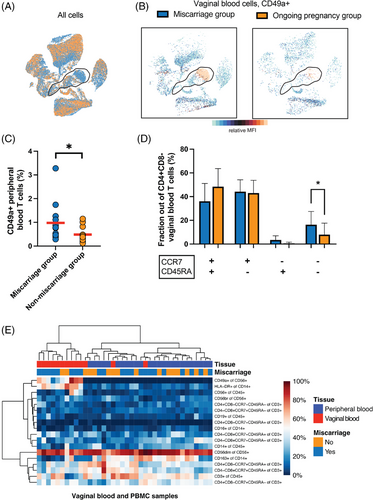
To reveal the importance of each immune cell subset measurement with respect to correlation with miscarriage we performed a hierarchical clustering using the cytometry data (Figure 4E), which indicated that CD49a+ dNK cell frequency showed the strongest link to the miscarriage group.
For assessment of longitudinal dynamics, the frequency of immune cells was compared between the two time-points of sampling. Markedly, in both the miscarriage- and the ongoing pregnancy group we found no difference in the frequency of peripheral blood major immune cell subsets or of the subgroups studied (data not shown).
Altogether, this shows that the vaginal blood from women who subsequently miscarry contain a higher frequency of CD49a+ dNK cells and CD4+ effector memory T cells compared to women who continue pregnancy until term.
4.4 Serum proteome analysis reveals correlates of vaginal immune cell phenotype and miscarriage
Next, we set out to assess the dynamics of maternal serum proteome in relation to pregnancy outcome and to our cytometry data. We chose to use a panel including immune-oncology related factors to ensure coverage of maximum number of immune-related molecules as well as the markers introduced above such as PlGF, CA125/MUC16, and VEGF. Having identified an elevated frequency of CD49a+ NK cells in the vaginal blood of miscarriage patients, we correlated serum protein levels to frequency of CD49a+ dNK cells in vaginal blood of this group. Colony stimulating factor (CSF1) (r = −0.74; p = .014), granzyme A (r = −0.64; p = .045), granzyme H (r = −0.71; p = .021), TNF-related weak inducer of apoptosis (TWEAK) (r = −0.74; p = .014), and carbonic anhydrase IX (CAIX) (r = −0.73; p = .038) were all significantly negatively correlated (Figure 5A). When correlating the frequency of peripheral blood CD49a+ T cells in the miscarriage group with serum protein levels, soluble CD28 was significantly negatively correlated (r = −0.66; p = .036) (Figure 5B). Additional correlations with CD49a+ vaginal NK cells and peripheral blood T cells, respectively, are available as Supplementary Data (all non-significant).
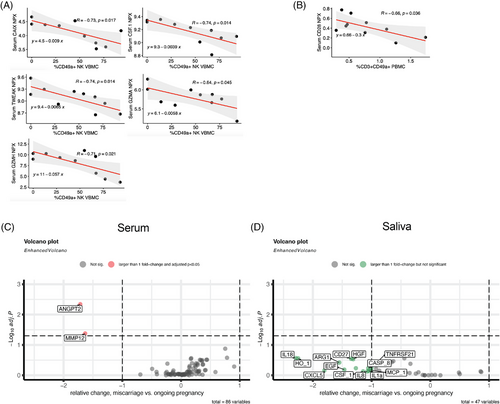
Next, we aimed to detect any associations between the levels of our measured serum proteins in the miscarriage group in comparison to the ongoing pregnancy group. We found two proteins, angiopoietin 2 (ANGPT2) and matrix metalloproteinase 12 (MMP12), with a fold change > 1 and adjusted p < 0.05 in the miscarriage group (Figure 5C).
A similar analysis with OLINK saliva data revealed no statistically significant correlation with vaginal blood CD49a+ dNK cells in the miscarriage group. When comparing saliva protein abundancy between the two groups, we found no statistically significant associations after correction for multiple comparisons (Figure 5D).
In summary, we found covariations between abundance of CD49a+ NK cells in vaginal blood and several maternal serum proteins. Fold change analysis of serum protein ratios identified a significant fold change for ANGPT2 and MMP12 in the miscarriage group.
5 DISCUSSION
Early pregnancy bleeding is common. It often causes psychological distress in women and generates a significant burden to health care systems. Reliable prognostic factors for pregnancy outcome of a viable fetus accompanied by vaginal bleeding in the first trimester would be useful in the clinical setting and could potentially reduce the number of follow-up visits and treatment delay for women with threatened miscarriage. The local uterine immune cells are pivotal in preparation for pregnancy and in maintaining a beneficial milieu for the conceptus, and immune aberrations have been associated with a higher risk of miscarriage.28 To our knowledge, the immune cells in vaginal blood from threatened miscarriages have not been previously broadly characterized. Here, we performed a high-dimensional flow cytometry characterization of immune cells in vaginal blood from early pregnancy bleedings. The key finding of this study was that vaginal blood from women who later had a miscarriage contained a higher frequency of tissue-resident CD49a+ NK cells suggestive of decidua origin.
In the first trimester decidua, dNK cells make up the largest proportion of all immune cells and are predominantly CD49a+.9, 26 These tissue resident CD49a+ NK cells produce fetal growth promoting factor29 and their frequency in menstrual blood and decidua have been shown to be decreased in women with recurrent miscarriage compared to healthy controls.30 In peripheral blood, however, normal ranges of NK cells vary substantially among healthy individuals31 and peripheral blood NK cells expressing tissue residency markers are rare. We have previously shown that dNK cells can be replenished from the circulation and acquire a tissue-resident phenotype,22 but if peripheral blood NK cell numbers or phenotype reflects uterine events is not clear. In this current study, tissue-resident dNK cells found in vaginal blood is in line with this previous knowledge. The proportion of CD49a+ dNK cells was larger in vaginal blood from women who subsequently miscarried supporting the interpretation that these cells leaked from the fetal-maternal interface when the tissue was disrupted.
In our cohort, the proportion of tissue resident T cells in peripheral blood was larger in the miscarriage group compared to the ongoing pregnancy group. However, no such difference was observed in the frequency of tissue resident T cells in vaginal blood between the two groups. The tissue residency marker CD49a is an integrin which binds to extracellular matrix components and facilitates for the cell to stay in the tissue. NK cells and T cells can acquire a tissue resident phenotype once they home in different tissues of the human body as well as in the uterus.22, 32 An increased fraction of tissue resident T cells in peripheral blood could be explained by uterine T cells leaking into the circulation when the maternal-fetal interface is broken. When correlating frequency of peripheral blood CD49a+ T cells with frequency of vaginal CD49a+ NK cells in the miscarriage group, we found no correlation. These findings could be explained to some extent by the relatively small sample size of the study, however, the interpretation is challenging and future larger studies may shed light on this.
Macrophages are another large group of immune cells residing in the first trimester decidua and throughout pregnancy.33 Monocytes in tissues differentiate to macrophages in response to surrounding cytokine signals and can polarize to both a pro-inflammatory type 1 (M1 cells) and an anti-inflammatory, immune-tolerogenic type 2 (M2 cells). Gene expression studies have shown that macrophages in the decidua are dominantly of M2 CD14+HLA-DR+CD209+ phenotype.34 This is in line with our finding that CD14+HLA-DR+ myeloid cells were found in the vaginal blood. Future studies should include additional macrophage markers to reveal more about their phenotype in vaginal blood.
Immune cells are present in all parts of the female genital tract and blood collected from the vagina could potentially be contaminated with immune cells from the vagina, ecto- and endocervix. The lower female genital tract has a high load of microbes and are exposed to the external environment, rendering the local immune cells important defenders against pathogens. There are, however, big differences in immune cell composition in the decidua compared to the lower female genital tract where NK cells comprise only 2.7% of all CD45+ cells, whereas T cells are the most abundant.35 Within the CD4+ T cells in the cervix, the vast majority exhibit an effector memory phenotype.35 In our cohort, we noticed that the women who subsequently miscarried reported more heavy vaginal bleeding and a contamination of cervical effector memory CD4+ T cells might explain why we found this cell subset at increased frequencies. In the ectocervix, CD14+ monocytes are the most abundant APCs, with most of them being CD11c- macrophages. In contrast to the ectocervix, the endocervical population density of macrophages is much lower. Vaginal blood from women with threatened miscarriage is likely to contain blood from both the maternal circulation and the maternal-fetal interface, as well as pregnancy tissue and lower female genital tract cells. However, flow cytometry analysis has the power to distinguish cell populations specific to the decidua. It is possible that the vaginal blood samples have been contaminated with ectocervical mononuclear cells. However, it is not sufficient to explain all the differences in frequencies of vaginal blood NK cells and monocytes compared to peripheral blood.
The soluble proteins were analyzed in serum from the maternal circulation and not vaginal blood, although a vaginal blood protein analysis would have further described the present environment of the pregnant uterus. This alternative was not within the scope of this study and is likely to be difficult due to the small amount of vaginal blood samples available for collection. One limitation of this study was that the amount of vaginal blood collected was not measured which might have implications for the total number of measurable cells. One way to overcome this could be to weigh vaginal blood tubes pre- and post-sample collection in future studies.
The cohort of women included in the study exhibited no significant variations in clinical parameters or previous gynecological history. Specifically, there was no statistically significant difference in the number of prior pregnancy losses between the miscarriage group and the ongoing pregnancy group. However, it is important to acknowledge that the relatively small sample size might account for the absence of significant differences in the history of miscarriage. To evaluate how these or other clinically distinguishable parameters correlate with flow cytometry findings would be worth exploring in larger-scale studies. Correlations between serum protein levels and miscarriage do not consolidate causality and we cannot rule out other possible explanations for our findings. To strengthen the prognostic significance of the serum proteins correlating with CD49a+ dNK cells in our study, larger studies should be performed.
This is the first study designed to broadly investigate the content of vaginal blood in women presenting with early pregnancy bleeding. Women were enrolled in the study in the clinical gynecological emergency setting and without any pre-medication that could potentially alter conditions in the decidua. We have thoroughly characterized the immune cell phenotype and frequency and the proteome in serum and saliva in this cohort.
6 CONCLUSION
This study describes the immune cell composition and phenotype in vaginal blood from early pregnancy bleedings. We conclude that vaginal blood collected from women with early pregnancy bleeding contains all major immune cell subsets. Tissue resident dNK cells were more abundant in vaginal blood from women who subsequently miscarried. This finding has the potential implication of becoming a clinically relevant tool in prediction, diagnosis, and treatment of first trimester miscarriage.
ACKNOWLEDGMENTS
We thank research midwives Ann-Christine Wideberg and Maria Fursäter for technical assistance. This project was supported by the Swedish Research Council, the Center for Innovative Medicine at Karolinska University Hospital and Karolinska Institutet, the Novo Nordisk Foundation, Karolinska Institutet, and by the Swedish Society for Medical Research.
CONFLICT OF INTEREST STATEMENT
The authors report no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.The data are not publicly available due to privacy or ethical restrictions.