The Barrier to HIV Transmission Provided by Genital Tract Lactobacillus Colonization
Abstract
While resistance to HIV transmission is due to multiple mechanisms such as the epithelium, a lower genital tract microbiota dominated by Lactobacillus appears to play an important role. This article reviews selected recent research on genital tract microbiota in women including how microbiota impacts HIV resistance and factors affecting Lactobacillus colonization.
Introduction
Approximately 85% of HIV infections in women occur due to heterosexual transmission [CDC. Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report 2012; 17(No. 4). Published December 2012]. Therefore, understanding factors that affect resistance to and/or risk of HIV infection in women is of critical importance.
The immune system appears to have limited effectiveness in preventing HIV transmission to or from women. For example, there is essentially no protective adaptive immune response to HIV in most people at risk. Further, HIV vaccines so far are of limited effectiveness.1 Innate immunity may contribute to resistance through expression of antimicrobial peptides.2, 3 However, increased levels of some antimicrobial peptides have been found in seroconverters4 suggesting a limited role in resistance, and innate immunity/inflammation may also increase susceptibility in the genital tract by recruiting target cells to the genital tract, modulating their susceptibility to infection, and degrading the epithelial barrier.5, 6
The epithelial barrier of the cervix and vagina has long been considered a component of innate resistance to infection with HIV. A recent study by Carias et al.,7 however, indicates while most HIV virions do not penetrate the epithelium, a small proportion of virus can penetrate the epithelial layers of the cervix and vagina to a depth where it can encounter target cells. The microbiota of the lower female genital tract dominated by Lactobacillus can also be considered an innate barrier. This article reviews selected recent research on genital tract microbiota in women including how microbiota impacts HIV resistance.
Evidence for a role of the Lactobacillus in resistance to HIV transmission
Epidemiologic studies show that the genital microbiota in women affects three different modes of HIV transmission. A number of studies have found that bacterial vaginosis (BV), a condition where levels of Lactobacillus are relatively decreased in the lower genital tract, increases the risk of a woman acquiring HIV during heterosexual contact.8 A recent study by Cohen et al.9 found BV was also associated with an increased risk of female-to-male transmission.
An alteration of microbiota to bacterial vaginosis has in several studies also been associated with increased mother-to-child transmission.10 While BV might be expected to increase the risk of infection of the fetus during birth while passing through the vagina, some evidence points to BV increasing in-utero infection of the fetus.10 While the mechanism(s) by which BV could increase in utero infection have not been established, this could be due to effects such as amniotic inflammation, increased leukocyte infiltration, or increased pre-term birth.
In many, but not all studies, BV is associated with higher levels of HIV in the genital tract, also called HIV shedding.11-13 This could help explain why vaginosis is linked to increased risk of female-to-male transmission, although in the Cohen study, increased genital virus may have only partially explained the increased transmission.9
The mechanism of resistance to HIV due to Lactobacillus
Studies by us and others14-16 that have used molecular methods to define the genital microbiota show that when Lactobacillus becomes the dominant type of bacterium in the genital tract, it tends to make up >80–90% of all the microbiota, and conversely, when it is less than this amount, it generally is in the range of 0–20% of the microbiota (Fig. 1). This suggests that when Lactobacillus becomes dominant in the genital tract, it suppresses growth of the other genital bacteria. The mechanisms that suppress the other genital bacteria may in many cases also act on HIV that is introduced during sexual activity or suppress the amount of virus shedding in HIV-infected women. There are several proposed ways that Lactobacillus protects against infection by HIV (Table 1).
| Direct anti-HIV effects |
| Low pH |
| Lactic acid |
| H2O2 |
| Bacteriocins |
| Indirect anti-HIV effects |
| Prevents bacterial vaginosis and STDs |
| BV and STDs increase HIV target cell number and activation |
| BV and STDs break down the protective mucous barrier |
| STDs can cause ulceration of the epithelium |
- BV, bacterial vaginosis; STD, sexually transmitted diseases.
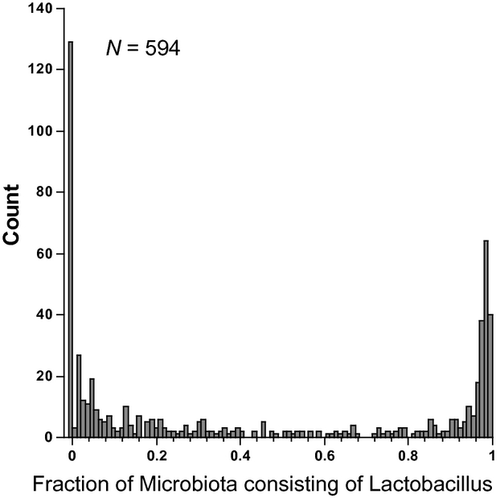
The pH of the genital tract of women with a microbiota dominated by Lactobacillus has long been recognized to be very acidic, and this is thought to be one of the major mechanisms for resistance to BV, sexually transmitted diseases (STDs), and HIV. A pH cutoff of 4.5 is used in the Amsel test with higher than 4.5 suggestive of BV. Lactic acid produced by bacterial fermentation is mainly responsible for the low pH found in women with a dominant Lactobacillus microbiota.17
In women that have a genital microbiota dominated by Lactobacillus, differences in the species of Lactobacillus appear to affect the vaginal pH.15, 18 The median pH of 105 women with a microbiota consisting of mostly Lactobacillus crispatus was 4.0, while 135 women with mostly Lactobacillus iners was 4.4. Smaller numbers of women had microbiota dominated by Lactobacillus gasseri (N = 25) and Lactobacillus jensenii (N = 21), and the median pH in these groups was 5.0 and 4.7.18 In another study, 100% women with a microbiota consisting of mostly L. crispatus had a vaginal pH under 4.5, while in contrast, only 74% of women with dominant L. iners had a pH under 4.5.15
The exact pH that is present in the vagina may be different than what is measured with pH paper when genital fluids are exposed to the air. Thus, a recent study measured the pH of vaginal fluid under conditions more closely approximating the CO2 levels found that vaginal fluid from women with a microbiota dominated by Lactobacillus had an average pH of 3.5 which is substantially lower than reported in previous studies.19
Lactic acid secretion by Lactobacillus contributes to the low pH of the genital environment, but may also have some antimicrobial properties of its own.20 A solution at pH 4.0 inactivated about 90% of HIV in 30 min, but a pH 4.0 solution containing 0.3% lactic acid (a concentration achievable in vivo) resulted in a 100× greater reduction in infectious virus.20
Production of H2O2 by Lactobacillus has been associated with protection. For example, development of BV has been recognized for some time to be associated with a lack of vaginal H2O2-producing lactobacilli in several longitudinal studies,21, 22 while prolonged colonization with Lactobacillus is associated with the presence of H2O2-producing lactobacilli.23 In vitro, H2O2 can kill HIV.24 However, the concentration of H2O2 generated in vivo appears to be very low compared with the amount required for killing of STDs and BV-associated bacteria, possibly because H2O2 production is restricted in an anaerobic environment.25 Also, genital fluid and semen were found to inhibit the antimicrobial activity of H2O2.25
Bacteriocins are small proteinaceous antimicrobial compounds that kill closely related microorganisms.26 A number of bacteriocins have been found in Lactobacillus isolates including vaginal strains.26 For example, a recent paper sequenced a vaginal isolate from a healthy Nigerian woman and found that the genome contained a 7-gene cluster apparently involved in biosynthesis of a bacteriocin they designated as pentocin KCA1 penA. However, as these authors note, ‘A number of bacteriocins have been reported in vaginal bacteria, but the extent to which they influence the microbiota composition remains to be determined’.27
Domination of the microbiota by Lactobacillus may not only provides direct resistance to HIV, but likely provides indirect effects as well. For example, Lactobacillus protects against STDs such as Herpes, Chlamydia, and Neisseria gonorrhea.28, 29 Those STDs are associated with an increased risk of HIV infection possibly by affecting the epithelial barrier or causing inflammation. Similarly, Lactobacillus colonization reduces the risk of developing BV and BV may increase the risk of HIV by causing inflammation, degrading the protective mucous layer and other effects.30, 31
Factors that affect colonization by Lactobacillus
As colonization of the lower genital tract of women by Lactobacillus appears to be important for preventing HIV transmission, it is critical to understand factors that affect its colonization. Treatment of women with antibiotics can temporarily suppress BV organisms, but this does not substantially aid in long-term Lactobacillus colonization.32 Several of the biologic factors that can disrupt or prevent colonization by Lactobacillus are known. For example, a study by Cherpes et al.22 followed 773 women over a year and found that occurrence of BV was associated with a number of factors including smoking, vaginal intercourse, receptive anal sex before vaginal intercourse, sex with an uncircumcised male partner, and lack of vaginal H2O2-producing lactobacilli. Those authors posited that a commonality of many of these risk factors for BV development could be that they ‘…adversely affect survivability or maintenance of H2O2-producing lactobacilli’. For example, alkaline semen can raise the pH of the vagina potentially allowing bacteria such as Gardnerella vaginalis, which makes much less acidity and likely raises the pH of vagina, to become established in this niche and outcompete Lactobacillus.17 Douching, which disrupts the pH of the vagina, has also been linked to developing BV.33 Vaginal lubricant use and anal sex before vaginal sex also increase the risk of developing BV.22, 34, 35 There is also evidence that certain types of bacteria, found in subsets of women, may affect re-establishment of Lactobacillus after treatment for BV.36, 37
It has for a long time been recognized that glycogen is deposited in the vaginal epithelium and that its appearance during development coincides with colonization by Lactobacillus.38 This has led to the concept that glycogen is a nutrient crucial for Lactobacillus colonization. Measurement of vaginal glycogen has been performed by several methods and, in most cases, has been reported in relatively subjective or semiquantitative ways. For example, many of the earlier studies examined vaginal biopsy sections on slides after staining with various stains such as iodine or periodic acid schiff staining.38-40 The thickness of the glycogen layer and the visually subjective intensity of staining were then reported. Also, staining of shed epithelial cells on smears with subjective scoring (+, ++, etc.) has been used to quantify glycogen.41 Potentially important from the standpoint of Lactobacillus colonization, however, is the concentration of glycogen in the lumen of the vagina. This level is likely critically important for bacterial growth and is not given by most of those methods. The factors that affect the amount of glycogen available for utilization by Lactobacillus are numerous and include many unknowns including the rate at which epithelial cells are shed and degraded, the numbers of cells shed that contain glycogen, the amount of glycogen in the cells, and the rate of glycogen clearance (Table 2).
| Total epithelium thickness |
| Epithelial glycogen thickness |
| Amount of glycogen in Epithelial cells |
| Rate of shedding of cells from epithelium |
| Rate of release of glycogen by shed or non-shed cells |
| Rate of glycogen breakdown/clearance |
Our laboratory recently has begun measuring the amount of free glycogen in genital fluids collected by lavage as this measure would potentially be less subjective and more closely associated with colonization by Lactobacillus. Our initial studies showed that humans have much higher levels of genital glycogen than macaques42 providing a possible explanation for the lack of colonization by Lactobacillus in this important animal model of genital HIV infection. More recent studies by our group (manuscript submitted) show that in 185 lavage samples collected from women in the Women's Interagency HIV Study (WIHS), free glycogen was strongly and significantly associated with a low vaginal pH and the fraction of the microbiota consisting of Lactobacillus as determined by pyrosequencing.
Although there is much evidence that glycogen is involved in supporting colonization by Lactobacillus, there are still several mysteries for understanding how this bacterium is able to maintain dominance in the genital tract. For example, if glycogen can be utilized by bacteria other than Lactobacillus, does glycogen also contribute to growth of anaerobes or other pathogens? Under conditions of high acidity due to a microbiota dominated by Lactobacillus, growth of the other bacteria is inhibited. However, when the pH increases, do the anaerobes deplete the glycogen and prevent the re-establishment of a dominant Lactobacillus? Another unresolved issue is that many genital isolates of Lactobacillus do not appear to be able to use glycogen as an energy source in vitro. Thus, a number of studies including those by Stewart-Tull,43 and more recently, Martin et al.44 showed that most genital Lactobacillus isolates could not use glycogen. Wylie and Henderson45 found that of 42 genital tract Lactobacillus isolates, only one could use glycogen obtained from oysters. To determine whether the source of glycogen could affect Lactobacillus growth, glycogen was also isolated from human vaginal tissue. Only three isolates of 42 could grow on human glycogen. Similar results have been observed in our laboratory where genital isolates of L. gasseri (Fig. 2), L. jensenii (not shown) and Lactobacillus johnsonii (not shown) are not capable growing when glycogen is the only carbohydrate source in the medium.
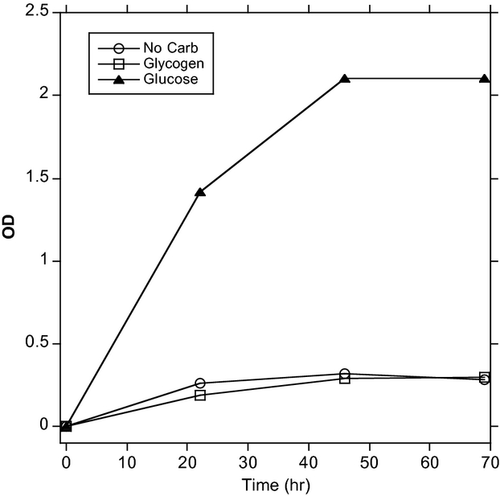
While many Lactobacillus genital isolates cannot utilize glycogen, they can use smaller carbohydrates such as glucose (Fig. 2) or maltose. Interestingly, we recently found that most of the women that we have so far tested have an activity in their lower genital tract secretions that breaks down glycogen into the smaller carbohydrates (manuscript in preparation). We are currently investigating whether this glycogen-olytic activity can in combination with glycogen help support growth of Lactobacillus either in vitro or in vivo.
Conclusion
The microbiota of the female genital tract appears to play an important role in the HIV epidemic. Further studies to determine the factors that affect the colonization of the female genital tract by Lactobacillus are of critical need.
Funding
This work was supported by NIH Grants P01 AI082971 and P30 AI 082151.




