Decay rate and persistence time of Cape buffalo (Syncerus caffer caffer) dung in the forest of Western Ethiopia
1 INTRODUCTION
Dung counts were first used in the 1940s (Bennett, English, & McCain, 1940), and are mostly employed in woodland and forested habitats, where direct counts are difficult to apply (Acevedo et al., 2008). They have been used to estimate the population of elephants (Loxodonta africana) and other forest-dwelling mammals (Plumptre & Harris, 1995) for >50 years (Neff, 1968). Dung counts have been rarely employed for Cape buffalo (Syncerus caffer caffer) because they are most often found in open savannah and savannah woodland habitats where direct methods such as counts are often used to estimate population size. However, the difficulty of direct methods in population estimates of Cape buffalo in forested ecosystem has led to the application of indirect survey methods as described by Laing, Buckland, Bums, Lambie, and Amphlett (2003).
Population estimates through dung counts require precise determination of defecation rate, dung count in the area and mean persistence time of dung (Barnes & Jensen, 1987). However, very little information is available on the decay rate and persistence time of Cape buffalo dung which hampers the current ability to apply dung-based methods to estimate population size. Information about dung count as a means of density estimate for Cape buffalo was limited because they have not yet been recorded in dense forests lacking open and grassland habitats. Hence, site-specific dung persistence time for the target species should be determined to ensure accurate population estimates of species (Laing et al., 2003). This study tested the hypotheses that persistence time of buffalo dung would be faster during the wet season than the dry season. Similarly, faster decay and disappearance of elk, Cervus canadensis, pellet were reported from moist than drier habitats of boreal forest in north-western Canada (Jung & Kukka, 2016).
In forested ecosystems, the population size of Cape buffalo had not been estimated because thick forest makes the use of direct methods, such as counts, too logistically difficult. Estimation of dung persistence time is a crucial method to estimate population from dung counts. Therefore, the objective of this study was to determine site-specific mean dung persistence time of Cape buffalo in forested landscape.
2 MATERIALS AND METHODS
2.1 The study area
- Dense forest: Consists of primary forests with tall trees forming relatively closed upper canopy. It has shade-tolerant shrubs such as Dracaena afromontana and dense herb layer like Hypoestes forskaolii. Shade-tolerant grasses such as Setaria poiretiana and Panicum hochstetteri and man seedlings of dominant tree species were available.
- Open forest: Characterised by large mature trees, but with clear evidences of past human disturbances such as garden plants, graves and traditional worship grounds. Open forest has an incomplete canopy cover, open from the ground, shrubs and bushes distributed irregularly. The ground is predominantly covered by P. hochstetteri during both seasons.
- Riparian forest: The riverine parts are comprised of impenetrable dense thickets, interwoven lianas with species such as Rubus steudneri, Rubus apetalus and Rosa abyssinica. However, most of the riparian banks were relatively open and comprises P. hochstetteri, Cyperus distans, S. poiretiana and Cyperus fischerianus.
- Plantation forest: This habitat comprises Eucalyptus, Cupressus lusitanica, Grevillea robusta and Pinus patula species. It is open from the ground with understory covers such as S. poiretiana, C. distans, Cynodon dactylon and herbs such as H. forskaolii and Achyranthes aspera.
JWPF receives a unimodal annual rainfall. The mean annual rainfall in the area from 1992 to 2014 was 1805 mm, with the highest mean monthly rainfall record 324 mm in July and the lowest of 9 mm in December. The mean monthly maximum temperature was 28°C recorded in February and March, but the mean minimum was 12°C recorded in July and August.
3 METHODS
Mean dung persistence time of Cape buffalo dung was determined in JWPF in order to estimate dung density (Laing et al., 2003). In the study area, finding dung was very difficult due to the dense understory cover and low density of Cape buffalo. Hence, a total of 50 fresh dung piles (<48 hr) were obtained and marked in different habitats (Barnes & Jensen, 1987; Buckland et al., 2001). The age of dung was estimated based on dung decay stages described by Barnes and Jensen (1987). Among these, 27 and 23 dung piles were marked during the wet and dry seasons, respectively. The distribution of dung piles in different habitats includes the following: open forest = 14 dung piles (wet = 8; cry = 6), dense forest = 12 dung piles (6 dung piles during both seasons), plantation forest = 11 dung piles (wet = 7; dry = 4) and riparian forest = 13 dung piles (wet = 6; dry = 7). Each marked dung was assigned to different dung decay stages as described by Barnes and Jensen (1987) (Table 1).
| Stages of decay | Characteristics of dung pile stages |
|---|---|
| 1 | Intact dung, fresh (<48 hr), moist and thin layer with odour |
| 2 | Intact dung, fresh, dry layer and no odour |
| 3 | Some of the dung is disintegrated, but more than 50% of it is distinguishable as dung |
| 4 | Only <50% of the dung is distinguishable, but most of it is disintegrated |
| 5 | Almost all dung piles disintegrated forming an amorphous shape or flat mass |
| 6 | Dung piles decayed to the stage it would be impossible to observe at 2 m distance. It can be seen only at closer distant directly underfoot |
We did not use dung piles found at areas of high use by animals or people (e.g., water points, roads and buffalo trails) because of excessive trampling. Moreover, dung piles which assumed to be independent in the same habitat were marked to keep the heterogeneity of microhabitats within each habitat. Dung monitoring was carried out during the dry (January—February 2016) and wet (May—June 2016) seasons. During dung monitoring experiment, habitat types where the dung had fallen were recorded and rechecked at weekly (every Sunday) interval as described by Barnes and Jensen (1987). Dung piles were monitored during the wet and dry seasons to obtain the mean dung persistence time of Cape buffalo in the area. Accordingly, the maximum dung persistence time during the wet and dry seasons was 38 and 55 days, respectively. More than 75% of dung piles were decayed at these maximum days. Hence, further follow-up of the remaining dung piles was stopped as reported by Barnes and Jensen (1987) assuming that the dung decay time of the remaining dung piles has insignificant impact on the mean dung persistence time. Dung piles were followed until most of the dung piles have decayed (Plumptre & Harris, 1995) or totally disappeared by any mechanism (Marques et al., 2001). Genstat version 18 was used to estimate the mean dung decay (disappearance) time. The dung disappearance time between habitats and seasons was tested by two-way analysis of variance (ANOVA) and dependent sample t test, respectively.
4 RESULTS
4.1 Dung persistence time
Dung persistence time showed a slight difference, but was insignificant across habitats during the wet and dry seasons in JWPF. During the wet season, the mean dung persistence time was shorter in dense forest (29 days, 95% CI: 26–32) and riparian forest (32 days, 95% CI: 30–34). However, it was relatively delayed in open (38 days, 95% CI: 36–40) and plantation forests (38 days, 95% CI: 34–42). The mean (±SE) dung persistence time was similar in different habitats during the dry season. The mean (±SE) number of days in which dung decayed was estimated to be 32.7 ± 3.6 and 51.8 ± 2.7 days for the wet and dry seasons, respectively. Dung persistence time ranged from 25 days to 38 days during the wet season and from 46 days to 55 days during the dry season. The difference in the persistence time of dung in different habitats during the wet (F3, 19 = 2.68, p = 0.076 and dry (F3, 16 = 0.369, p = 0.776) seasons was not significant. However, there was a significant interaction between the effects of habitat and season on persistence time of dung piles (F3, 39 = 2.94, p = 0.004).
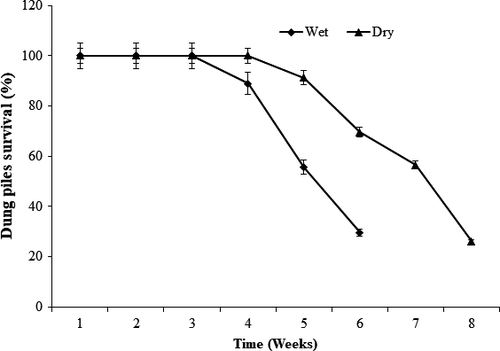
However, the difference in the mean persistence time of dung between the two seasons was significant (t = 19.88, df = 41, p < 0.05). The mean number of days in which dung decayed was 42 ± 3.8 days in JWPF. Dung disappearance was delayed during the first three and 4 weeks during the wet and dry seasons, respectively. Since then, the process was rapid during the wet season compared to the dry season (Figure 2).
5 DISCUSSION
The complex interactions of abiotic and biotic factors (Marques et al., 2001) result in seasonal, intersite and intrasite variability in dung decay rates (Theuerkauf et al., 2009). Moist environments may accelerate the disappearance of pellets compared to drier environments (Massei, Bacon, & Genov, 1998). Similarly, we found that Cape buffalo dung decayed faster during the wet season than the dry season in JWPF. As described by Lehmkuhl, Hansen, and Sloan (1994), moisture promotes invertebrate diversity, activity and organic decomposition of dung. Mulama (1997) also reported similar findings for elephants in Ghana and Kenya, respectively. Seasonal variations in the dung decay rate in our study area could be attributed to variation in rainfall and abundance of decomposers between seasons. Dung beetles, flies and high rainfall were the main causes for the faster dung decay rate during the wet season, in this study, as similar to Anderson and Coe (1974). A similar incident was reported by Mulama (1997) in studying the dung decay rate of elephants in Aberdare National Park, Kenya. During the dry season, dung decomposers were reduced, and termites act as key decomposers (Anderson & Coe, 1974). Furthermore, the production of loose or watery dung during the wet and condensed or thick dung during the dry seasons could have contributed to the seasonal variation in the dung decay rate in JWPF. In our study, olive baboon (Papio anubis) also played a significant role in the dung decay rate as they destruct dung, probably, to search for worms and insects.
Decay rate of pellet in ungulates may vary significantly among different habitats (Massei et al., 1998). For example, Jung and Kukka (2016), elk pellets decay slowly in the habitats that had the greatest moisture. To the contrary, dung of Cape buffalo was decayed faster in dense and riparian forests than in open and plantation forests, both during the wet and dry seasons in JWPF. Similarly, Massei et al. (1998) reported that moisture promotes pellet disappearance rates of ungulates. In our study, dung decay was delayed in open and plantation forests due to the fact that the exposure of dung to wind on nondecomposable leaf debris of Eucalyptus and Pinus patula removes water from it and decreases organic decomposition. To the contrary, Tsaparis, Katsanevakis, Ntolka, and Legakis (2009) reported that physical decay of pellet was higher in open habitats than forested areas in the arid Mediterranean due to alternate exposure to different climatic conditions compared to the sheltered forested habitats. In our study, dung decayed slowly in open and plantation forests which could be ascribed to its openness and shelter unlike open habitats.
In our study, dung persistence time in different habitats was relatively similar during the wet and dry seasons. However, dung persistence time was longer during the dry season than the wet season in all habitats. The mean dung persistence time (42 days) in JWPF was comparable to that found by Bekhuis, DeJong, and Prins (2008), who reported 45 days for forest buffalo (Syncerus caffer nanus) from Campo Ma'an National Park, Cameroon. The mean dung persistence time of elephants was greater than buffalo at least by two- to three-fold (Mulama, 1997; Vanleeuwe & Probert, 2014). This has revealed a marked difference between the dung decay rates of different species. Moreover, effects of dung decomposing factors vary spatially and temporally (Sutton, Whitemore, & Chadwick, 1983). Variation in dung decay rate and persistence time was associated with various ultimate and proximate factors (Jachmann, 2001) though they were not quantified and tested in this study. Therefore, further detail study is required to identify the ultimate and proximate factors that affect the decay rate and persistence time of Cape buffalo dung in JWPF.
ACKNOWLEDGEMENTS
We thank Addis Ababa and Wollega Universities for providing financial support. We are also grateful to Oromia Forest and Wildlife Enterprise for allowing this research to be conducted in Jorgo-Wato Protected Forest and our field assistants for their support during data collection.




