Liana cutting for restoring tropical forests: a rare palaeotropical trial
Abstract
enLiana growth following forest disturbance is threatening the tropical carbon sink by delaying or preventing recovery. Tree growth can be stimulated by liana cutting; however, its applicability for conservation management remains uncertain, particularly in Africa (the least-studied continent for ecological restoration) and against pervasive barriers such as wildfires. We conducted a small-scale trial to investigate tree sapling regeneration following liana cutting in a lowland African forest prone to low intensity wildfires. We employed a BACI design comprising eighteen 25 m2 plots of sapling trees in liana-infested areas. After 5 years of liana cutting, we saw greater recruitment, stem growth and net biomass. Wildfires caused 51% mortality and probably masked liana cutting influences on species and survival, but may have encouraged stem recruitment through interaction with liana cutting. Incorporating our data into a first quantitative review of previous studies, we found that tree growth, recruitment and net growth rates were all consistently higher where lianas were either absent or removed (respectively: 80%, 215%, 633%; n = 14, 3, 4). Tree growth impacts were approximately equivalent across size-classes and continents. We give recommendations for improved plot and sample sizes, but conclude that liana cutting is a promising restoration method for lowland tropical forests, including Africa.
Résumé
frLa croissance de lianes après les perturbations des forêts menace le puits de carbone tropical en retardant, voire en empêchant la restauration. Il est possible de stimuler la croissance des arbres en coupant les lianes mais la faisabilité de cette mesure de conservation reste incertaine, spécialement en Afrique (le continent où la restauration écologique est la moins étudiée), vu aussi des obstacles aussi généralisés que les feux de brousse. Nous avons réalisé un essai à petite échelle pour étudier la régénération de jeunes arbres après la coupe des lianes dans une forêt de basse altitude sujette à des feux de faible intensité. Nous avons utilisé une approche expérimentale-témoin avant-après (BACI) sur 18 parcelles de 25 m² contenant de jeunes arbres dans une zone infestée de lianes. Après cinq ans de coupes de lianes, nous avons vu un recrutement, une croissance des troncs et une biomasse nette plus importants. Les feux ont causé une mortalité de 51% et ont probablement masqué l'influence de la coupe des lianes sur les espèces et leur survie, mais ils pourraient avoir favorisé le recrutement des jeunes arbres par leur interaction avec la coupe des lianes. En intégrant nos données dans une première revue quantitative d’études antérieures, nous avons découvert que la croissance des arbres, leur recrutement et les taux de croissance nette étaient tous chaque fois plus élevés là où les lianes étaient soit absentes, soit enlevées (respectivement : 80%, 215%, 633%; n = 14, 3, 4). Les impacts sur la croissance des arbres étaient à peu près équivalents dans toutes les classes de taille et sur tous les continents. Nous donnons des recommandations pour améliorer la taille des parcelles et des échantillons, mais nous concluons que la coupe des lianes est une méthode de restauration prometteuse pour les forêts tropicales de basse altitude, y compris en Afrique.
Introduction
Restoration of degraded tropical forests is vital for averting substantial impacts on biodiversity, carbon sinks and global economy. An estimated 33–60% of tropical forests have been degraded by logging (Asner et al., 2009; FAO, 2010) with 1.4 Bha identified for potential restoration (Minnemeyer et al., 2011). Despite this potential, attempts at ecological restoration have addressed only a meagre proportion of tropical forests. Tropical forest management has even failed to prevent biodiversity loss within and around protected areas (Laurance et al., 2012). Attempts at improved management through restoration must use site-specific biological and social conditions to develop tailored approaches (Stanturf, Palik & Dumroese, 2014). Passive restoration is possible where soils, seeds and plant dispersal are conducive (Barnes & Chapman, 2014), but succession from heavily degraded tropical forest is significantly slower without active management (Chazdon, 2003).
Landscape-scale restoration methods for tropical forests are needed that are practical and affordable for developing nations. Tree-planting has considerable demands on time, expertise and cost (Chazdon, 2008). Planted trees may even grow slower than naturally regenerating trees (Omeja, Chapman & Obua, 2009) and will affect genetic diversity unless sourced from a range of parent trees at the immediate locality. One promising alternative for conservation management widely employed by commercial forestry (Putz, 1991) is to temporarily exclude lianas (woody climbers) by cutting.
Lianas are a disturbance-favouring guild (Ledo & Schnitzer, 2014), growing rapidly in canopy gaps or edges of tropical forest (Schnitzer & Bongers, 2011). While various nonclimbing plants compete with forest trees, those that climb are particularly rigorous (Paul & Yavitt, 2011). Lianas physically restrict trees and compete for light and nutrients, causing reduced growth, sap flow, fecundity, leaf production and survival (Hegarty, 1991; Álvarez-Cansino et al., 2015; Toledo-Aceves, 2015) and ultimately biomass accumulation and carbon sequestration (Schnitzer et al., 2014). Consequently, forest succession may be slowed or arrested (Schnitzer, Dalling & Carson, 2000; Tymen et al., 2015). Accordingly, the global distribution of lianas is negatively related to carbon stocks (Durán & Gianoli, 2013). Increasing tropical forest degradation has stimulated 1.7–4.6% annual increase in liana abundance, threatening the tropical carbon sink (Phillips et al., 2002; Schnitzer & Bongers, 2011; van der Heijden et al., 2013).
In selectively logged neotropical forests, liana cutting has improved growth, survival and recruitment of tree saplings (Gerwing, 2001; Campanello et al., 2012). Focal timber species have also shown increased growth and survival of saplings following liana cutting (Grauel & Putz, 2004) and increased growth of mature trees by 9–64% (Pérez-Salicrup & Barker, 2000; Peña-Claros et al., 2008). The only studies outside of timber concessions or plantation have focussed on Panama, where liana cutting has improved tree growth, recruitment, net carbon uptake and community composition (Schnitzer et al., 2014; Álvarez-Cansino et al., 2015; van der Heijden, Powers & Schnitzer, 2015; Wright et al., 2015), whereas pioneer species may benefit from liana presence (Schnitzer & Carson, 2010). Liana cutting also has positive effects on leaf, fruit and seed production, damage caused by treefall, and reduced subsequent liana colonization of canopy gaps (Parren & Bongers, 2005; Nabe-Neilsen, Kollmann & Peña-Claros, 2009).
Studies of tree regeneration following liana cutting in the palaeotropics have been limited to focal species in combination with other forestry techniques. Africa is the least-studied continent for ecological restoration (Wortley, Hero & Howes, 2013) despite having the most degraded forest (Minnemeyer et al., 2011) and huge restoration opportunity cost for rural livelihoods (Chamshama & Vyamana, 2010). The value of liana cutting for restoration has been questioned for Africa because of insufficient logging degradation (Bongers, Schnitzer & Traore, 2002). Accordingly, liana removal did not affect tree size or density in Cameroon (Schnitzer, Parren & Bongers, 2004), nor tree species or growth in Uganda (Duncan & Chapman, 2003). Conversely, tree growth elsewhere in Uganda was slow in liana-dominated areas (Babaasa et al., 2004). Tree seedling growth also declined 19% when planted with lianas in Ghana (Toledo-Aceves & Swaine, 2007) and increased fivefold after liana cutting in Côte d'Ivoire (Schnitzer, Kuzee & Bongers, 2005). Liana cutting combined with liberation thinning also increased timber tree growth in Côte d'Ivoire (Parren & Doumbia, 2005), Malaysia (Putz, Lee & Goh, 1984) and Nigeria (Lowe & Walker, 1977).
While liana cutting may encourage tree growth, it may harm ecosystem functioning. Lianas contribute up to 44% of biodiversity and ~25% of stem density in tropical forests (Schnitzer & Bongers, 2002) and are important for structure (Gentry, 1991) and animals (Emmons & Gentry, 1983; Arroyo-Rodríguez et al., 2015). Cutting large lianas also damages regenerating trees (Campanello et al., 2007). Importantly for ecosystem functioning, liana cutting does not negatively affect subsequent liana regrowth, species composition or tree:liana ratio (Parren & Bongers, 2005; Parren & Doumbia, 2005; Campanello et al., 2012). However, few liana cutting studies have presented data for small trees (Gerwing, 2001; Pérez-Salicrup, 2001; Duncan & Chapman, 2003; Schnitzer, Parren & Bongers, 2004; Wright et al., 2015), thus overlooking recruitment (and hence succession) and compromising ecosystem function through removal of large lianas.
Limited evidence suggests that tree growth following liana cutting may be robust to periodic wildfires (Peña-Claros et al., 2008). This is an important consideration because fire risk increases following heavy tropical forest degradation (Chazdon, 2003; Blate, 2005) and may trigger conversion to scrub or savannah (Cochrane et al., 1999). Forest fires are 94% unplanned (FAO, 2010), and human-induced increases have reduced global tree basal area (Lehmann et al., 2014), releasing carbon equivalent to 41% of global fossil fuels (Cochrane, 2003). Wildfires burned >85% of sub-Saharan Africa between 1996 and 2009 (Krawchuk & Moritz, 2014) including >37 Mha of forest (FAO, 2010).
While prescribed fire can maintain forest by removing deadwood and thicket, evidence from Bolivia suggests that fire is inappropriate for managing lianas because it stimulates rapid regrowth (Gerwing, 2001), especially for small lianas (Pinard, Putz & Licona, 1999). Furthermore, the biggest impact of wildfires is on sapling trees, causing >50% annual mortality, increasing with fire intensity and frequency (Balch et al., 2011). Therefore, prevention of wildfires is often deemed essential for tropical forest restoration (Holl, 2002). However, wildfires have affected most terrestrial ecosystems throughout their history (Marlon et al., 2008) including many tropical forests (Chazdon, 2003). For example, the East African Coastal Forests have survived conversion to woodland for >150,000 years of low frequency fires (Clarke & Karoma, 2000). Hence, restoration strategies are required that do not assume complete fire exclusion. However, fire-vegetation dynamics remain poorly known, prompting calls for more explicit inclusion in conservation research and planning (Krawchuk et al., 2009).
This study employs liana cutting in a before-after-control-impact design to determine its effectiveness for regenerating sapling trees in an East African forest. The study is small scale and designed as a precursor for later work, but provides a rare palaeotropical trial that is unique in that we test (i) the combined influence of liana cutting on growth, recruitment, mortality, biomass and species for sapling trees, and (ii) the robustness of the method by incorporating covariance with pervasive low frequency wildfires. The results are combined with previous studies to make a first quantitative review of liana influence on tropical forest tree growth and to discuss practicalities for liana cutting as a conservation tool.
Material and methods
Study site
The study was conducted in Magombera Forest, southern Tanzania (Fig. 1; 11 km2; elevation 270 m; annual rainfall 1514 mm; wet season March–May; sandy-loam pH 4.7; C:N 8.6). The habitat was semi-deciduous Zanzibar-Inhambane forest with an understorey comprising small herbs, vines, sparse evergreen shrubs and lianas predominant in disturbed areas. Magombera Forest has high biodiversity value, including a remarkable proportion of threatened trees and a unique plant community comprising both lowland and montane species typical of the Eastern Arc Mountains and Coastal Forests of East Africa (Rodgers, Homewood & Hall, 1980; Marshall, 2008). The area supports several regionally endemic species including the Udzungwa red colobus monkey (Procolobus gordonorum) and Magombera chameleon (Kinyongia magomberae). Magombera Forest had no legal protection at the time of study, but with interim management by a conservation collaboration, which ensured limited disturbance to research sites.
Magombera Forest was chosen for its restoration potential following timber-felling in the 1970s/1980s, and widespread pole-sized tree removal for local domestic use and sale. The area comprised ~20% ‘secondary forest’ (≥90% canopy loss with mostly stunted/damaged trees and isolated tall trees) and ~80% ‘degraded primary forest’ (<90% canopy loss with a mostly heavily degraded understorey; Fig. 1). Most primary and secondary forest comprised a high density of understorey lianas, particularly Uncaria africana G.Don, forming thickets fastened by hooked spines (Fig. 2). Across the region, many of these thickets had persisted for ≥30 years, similar to liana forest elsewhere in East Africa (Chapman & Chapman, 1997). Tanzanian forest habitats were mostly not actively managed at the time of study. However, in Magombera Forest, managers and villagers maintained fire-breaks and extinguished some wildfires, reducing the extent of burning by ~50%. Wildfires were typical of lowland forests in the region, comprising creeping low intensity ground fires spreading from neighbouring agriculture and woodland management.

Plots
In July 2007 (year 1), we established eighteen 5 m × 5 m plots in areas with lianas touching or obstructing all ‘sapling’ tree stems 1–4.9 cm diameter at breast height (130 cm; dbh). Plots were placed at 100 m intervals along a transect to ease relocation, randomly offsetting 100 m north or south to avoid disturbance. We did not randomize across the forest as dense thickets would have precluded relocation of plots and required extensive cutting for access. Instead, the transect location was selected for its representation of the Magombera Forest tree species community (Marshall, 2008). One randomized plot location had few lianas and hence was repositioned. The plot size and number were chosen to allow rapid survey while giving sufficient sample size to assess growth following a previous East African study (Duncan & Chapman, 2003). Among the 18 plots, we managed ten alternate plots by cutting lianas. We used secateurs to cut liana stems or branches (mostly <1 cm dbh) that either touched or obstructed ‘seedling’ (<1 cm dbh) or sapling trees, leaving cut vegetation in situ. Cuts were made at ground level and around 1.5 m. Annually in February and July, any new liana growth was cut again. The sample size was uneven (ten managed by liana cutting; eight unmanaged) because two intended plot locations had few lianas anywhere nearby.
In year 1, we measured, marked and identified all sapling tree stems, and painted the point of measurement (re-labelling annually if deteriorated). To ease relocation in subsequent years, we dug L-shaped ditches at plot corners (15 cm deep × 30 cm long) and marked nearby trees with paint and tags. In July 2012 (year 5), we re-measured all surviving year 1 stems, plus any new ‘recruited’ stems. Height was measured using a tape measure (stems 1.3–2.5 m), wooden pole (2.5–7 m), or Bushnell Yardage Pro rangefinder (≥7 m). We measured dbh using a girthing tape, adjusting the point of measurement for deformed trees (Kuebler, 2003). Specimens were collected for identification at the Royal Botanic Gardens, Kew. To determine potential for future restoration of the mature tree community, we also made basic quantitative comparison (% species overlap) of the sapling species composition to ‘mature’ stems ≥10 cm dbh along the same transect (0.875 ha; 5 m × 1750 m), and to a species inventory of mature stems ≥10 cm dbh for the whole forest (Marshall, 2008 and A.R. Marshall, unpublished data).
Analysis
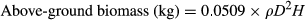 (1)
(1)Biomass calculation for moist forest trees using WSG (ρ; g cm−3), dbh (D; cm) and height (H; m) (Chave et al., 2005).
We performed statistical analyses using R 3.0.2 (http://cran.r-project.org). To determine the impact of liana cutting, we used one-way analysis of variance (ANOVA) models to compare five regeneration indicators between managed and unmanaged plots: (i) new sapling stem biomass (recruitment), (ii) proportional biomass increase of year 1 stems (growth), (iii) proportion of year 1 biomass lost through deaths (mortality), (iv) proportional change in total biomass (net biomass change; recruitment plus growth minus mortality) and (v) proportional species richness change (community change). We did not manage fire, but included wildfire frequency per plot in ANOVA models, including its statistical interaction with liana cutting (ANCOVA). To ensure parsimony, wildfire frequency (and in one instance liana cutting) was dropped from models where it did not improve the effect size according to likelihood ratio tests (Crawley, 2005). For all modelling, we employed transformations to remove skew, including twice natural log for recruitment and growth, and square root for proportional net biomass change (respectively, adding one or two to address negative values). Residual diagnostics were used to verify normality.
For regeneration indicators dependent on year 1 biomass (all except recruitment), we preferred proportional over absolute measures of change to reduce bias from variation in year 1 stem size and density. For these models using proportions, we excluded either one or two plots with zero values in year 1 (i.e. infinite proportional change). To address this removal of plots, we also calculated means and 95% bootstrapped confidence intervals (10,000 iterations; 95CI) for absolute measurements, in comparison with proportional changes.
To avoid Type I errors we used false discovery rate correction of alpha values (αFDR; Benjamini & Hochberg, 1995). To determine sampling adequacy for significant indicators, we used power analysis to calculate the proportion of discernible change, under our sampling level and increased sampling to fifteen or twenty plots per treatment (80% power; Crawley, 2005).
Results
Biomass
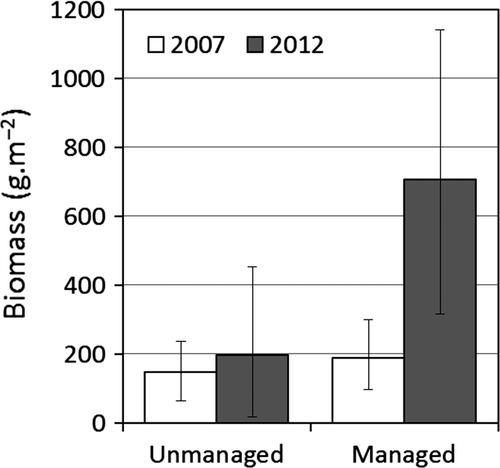
Net biomass increase among stems 1–4.9 cm dbh was higher in plots managed by liana cutting than unmanaged plots (total 129.9 versus 9.6 kg, respectively; Fig. 3; Table 1). The increase in biomass comprising recruitment of new stems was higher in managed plots (total 31.6 kg; 0.42 stems m−2, 95CI 0.16–0.75) than unmanaged plots (total 5.7 kg; 0.10 stems m−2, 0.03–0.18) (Table 1). In year 1, we measured 107 stems, with no significant difference between managed and unmanaged plots prior to management (W = 59; P = 0.098; managed: 0.4–5.6 stems 10 m−2; unmanaged: 0.0–3.2 stems 10 m−2). Stem numbers increased with recruitment to 171 in year 5, with significantly more stems in managed plots (W = 65.5; P = 0.026; managed: 0.8–17.2 stems 10 m−2; unmanaged 0.0–4.0 stems 10 m−2).
| Regeneration measure | Unmanaged | Managed (lianas cut) | Models [and covariates] |
|---|---|---|---|
| Recruitment: Biomass recruited (g m−2)a1 |
28(6–59) |
126(53–211) |
b(i) F = 6.2, r 2 =0.28, P = 0.024 (ii) F = 4.3, r2=0.37, P = 0.024 [liana cutting(+): δr2 = 0.37, P = 0.72; wildfires(+): δr2 = 0.09, P = 0.061; interaction(+): δr2=0.18, P = 0.038] |
| Growth: Proportional (and g m−2) biomass growth of liberated year 1 saplingsa1 |
0.46(−0.05 to 1.33) 142(−5 to 404) |
1.98(0.86–3.15) 467(168–793) |
F = 6.3, r 2 =0.28, P = 0.023 |
| Mortality: Proportion (and g m−2) of year 1 biomass lost through deaths |
0.68 (0.43 to 0.91) 116(49 to 187) |
0.57(0.36–0.77)74(33–119) |
F = 0.39, r2=0.03, P = 0.541 |
| Net biomass increase: Proportion (and g m−2) change in total biomass (recruitment + growth – death)a1 |
−0.10(−0.79 to 0.83) 48(−118 to 269) |
3.43(1.92–4.95) 520(205–859) |
F = 13.1, r 2 =0.46, P = 0.0026 |
| Community change: Proportional (and absolute) change in species richness per plot |
−0.33 (−0.67 to 0.17) 0.00(−0.75 to 0.88) |
−0.06 (−0.32 to 0.24) −0.70 (−1.70 to 0.20) |
cF = 3.1, r2=0.22, P = 0.080 [liana cutting(−): δr2=−0.08, P = 0.502; wildfires(−): δr2=0.17, P = 0.038] |
- aTransformed for modelling: 1twice ln; 2square root.
- bModels (i) and (ii) explained equivalent variance (F = 2.69; P = 0.103) and hence (i) preferred for parsimony. However, model (ii) also presented to highlight interaction.
- cModel using wildfire frequency alone (Fig. 4b) preferred for parsimony (equivalent variance explained: F = 0.48; P = 0.503).
The net biomass increase also comprised growth of year 1 stems, again higher in managed plots (total 116.7 kg; 3.4 kg stem−1, 95CI 1.8–5.5; 17.7 mm dbh stem−1, 95CI 12.3–23.4) than unmanaged plots (total 21.3 kg; 1.6 kg stem−1, 0.1–4.4; 7.9 mm dbh stem−1, 0.5–18.4) (Table 1). Biomass loss from mortality was no different between managed plots (total 18.5 kg; 0.17 stems m−2, 95CI 0.10–0.24) and unmanaged plots (total 17.4 kg; 0.12 stems m−2, 0.07–0.17) (Table 1).
At 80% power, our sample size was sufficient for detecting ≥152% change in recruitment, ≥267% proportion growth and ≥35% proportion net biomass. Increasing the sample size 15–20 plots per treatment would have increased the expected detection to ≥102–87%, ≥180–152% and ≥24–20%, respectively.
Species
Four species were recruited to plots that were absent in year 1, but seven species were lost, resulting in a net loss (2007: 29 species; 2012: 26 species). The proportional loss of species was not significantly different between managed and unmanaged plots (Table 1). Community composition was similar between treatments, with 76% of stems from species found in both managed and unmanaged plots. However, managed and unmanaged plots shared only five of their top ten species in year 1 (four in year 5; Table S1). This difference did not account for the proportional net biomass increase in managed plots, which persisted using only stems from species found in both treatments (F = 6.79; r2=0.31; P = 0.020). Precision for assessing relative species abundance within and between plots was limited, as seen from frequent zero values and complete overlap in 95CIs (Table S1).
Of 86 tree species known to reach 10 cm dbh across the forest, we identified 38 sapling species in restoration plots, compared to 29 mature tree species along the transect. Only 48% of mature tree species were found among saplings. The top ten sapling species included only one of the top ten mature trees and only three of the top ten from the whole forest (Table S1; two and three year 5 species, respectively). Nearly all stems (>99%) were species typical of the Eastern Arc Mountains and Coastal Forests (EAMCF) of eastern Africa, with drier woodland species comprising <1% of stems. Mature trees were dominated by canopy species, whereas restoration plots were dominated by understorey/midstrata species (Table S1).
Wildfire
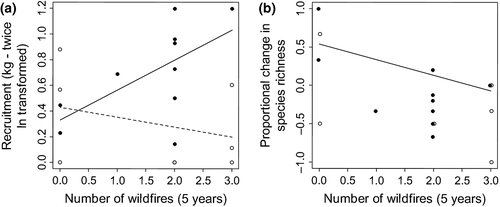
Pervasive wildfire frequency was 1.61 plot−1 (range 1.0–3.0 plot−1; 0.32 yr−1, 0.20–0.60) and equivalent between managed (1.60 plot−1, 95CI 1.00–2.10) and unmanaged plots (1.63 plot−1, 0.63–2.50). Wildfires killed ~55 year 1 stems (91.7% of deaths), but wildfire frequency did not directly influence growth, recruitment, mortality or net biomass change (r2 ≤ 0.08; P ≥ 0.27) and hence was not confidently retained in models (Table 1). Interaction was seen between wildfire frequency and liana cutting management for recruitment (greater cutting influence at high wildfire frequency; Fig. 4a) but with no significant improvement on liana cutting alone (Table 1). Increasing wildfire frequency explained 29% of variation in proportional species loss, enhancing a univariate liana cutting model but not significant at the 95% level (Table 1; Fig. 4b). Plots with zero to one wildfire showed increased proportional community change (0.23, 95CI −0.27 to 0.73), while those with two to three wildfires decreased (−0.3, 95CI −0.52 to −0.13).
Discussion
The results suggest that liana cutting is a promising restoration strategy, expanding its potential beyond the geographic bias of previous studies. We here compare our tree regeneration rates to previous liana cutting studies and studies comparing liana presence/absence through natural growth or planting.
Biomass
Our observed growth rate increase for liana cutting compared to unmanaged plots (dbh: 119%; biomass: 109%) is greater than most previous studies comparing tree dbh, circumference or height with/without lianas (mean 66%, 95CI 39–99, n = 12 [including this study: 69%, 43–100, n = 20]), but more typical of studies comparing biomass or basal area (80%, 35–150, n = 13 [83%, 39–147, n = 14]) (Table 2). Previous studies have been biased towards low elevation (median 200 m; mean 215 m), with the only high elevation study showing no significant influence of liana and shrub removal on tree growth (Duncan & Chapman, 2003). Our 350% increase in recruitment with liana cutting is higher than the only two comparable studies of stems ≤5 cm dbh, both in Panama, which increased by 46% (stems ≥1.3 m tall; Schnitzer & Carson, 2010) and 250% (stems <1 cm dbh; Grauel & Putz, 2004), giving a mean of 215% across studies. Two further studies showed (a) comparable stem size and density between liana cut and control plots in Cameroon (Schnitzer, Parren & Bongers, 2004) and (b) increased sapling density with liana cutting and liberation thinning after 19 years (but not 42 years) in Malaysia (Addo-Fordjour, Rahmad & Asyraf, 2013), but did not present pretreatment measurements for calculation of % increases.
| Study | Country (elevation) | Lianas | No lianas | % | Size | Years |
|---|---|---|---|---|---|---|
| This study | Tanzania (270 m) | 1.6 dbh (0.33 bio) | 3.5 dbh (0.69 bio) |
119 (109) |
1–4.9 | 5 |
| Lowe & Walker (1977)b,d,e | Nigeria (143 m) | 19.1 ba | 22.9 ba | 20 | 10.5–36.2 | 6 |
| Putz (1984)a1,d,e | Panama (85mk) | 635 ba | 893 ba | 41 | 30–50 | 10 |
| Putz, Lee & Goh (1984) site 1b,e | Malaysia (124mk) | 15.4 ba | 26.6 ba | 73 | ≥10 | 5 |
| Putz, Lee & Goh (1984) site 2b,e | Malaysia (47mk) | 23.9 ba* | 26.0 ba* | 9 | ≥10 | 4 |
| Putz, Lee & Goh (1984) site 3b,e | Malaysia (200mk) | 28.2 ba | 40.1 ba | 42 | ≥10 | 4 |
| Stanley (1997)a1,f | Brazil (30mk) | 2.1 dbh* (0.17 h) |
2.4 dbh* (0.23 h) |
14 (35) |
≥2.0 h | 9 |
| Pérez-Salicrup & Barker (2000)e | Bolivia (200 m) | 0.2 circ | 0.4 circ | 100 | 10–20 | 1 |
| Gerwing (2001) | Brazil (50 mk) | 1.3 dbh | 3.0 dbh | 131 | >5 | 2 |
| Gerwing (2001) | Brazil (50mk) | 0.8 dbh | 2.5 dbh | 213 | 2–5 | 2 |
| Pérez-Salicrup (2001)e | Bolivia (200m) | 0.18 RGRh | 0.28 RGRh | 54 | Seedlings | 1.5 |
| Duncan & Chapman (2003)c | Uganda (1500) | 0.2 h* | 0.1 h* | −50 | <1.6 h | 6 |
| Grauel & Putz (2004)e | Panama (50 mk) | 1.1 dbh* | 1.9 dbh* | 73 | 4–15 | 5 |
| Grauel & Putz (2004)e | Panama (50 m) | 2.8 dbh | 6.0 dbh | 114 | ≥15 | 5 |
| Dauber, Fredericksen & Peña (2005) site 1a1 | Bolivia (500 mk) | 2.2 dbh | 2.6 dbh | 18 | ≥10 | 3–8 |
| Dauber, Fredericksen & Peña (2005) site 2a1 | Bolivia (250 mk) | 4.1 dbh | 7.0 dbh | 71 | ≥10 | 2–3 |
| Dauber, Fredericksen & Peña (2005) site 3a1 | Bolivia (300 mk) | 2.6 dbh | 4.1 dbh | 58 | ≥10 | 7 |
| Dauber, Fredericksen & Peña (2005) site 4a1 | Bolivia (250mk) | 3.2 dbh | 3.6 dbh | 13 | ≥10 | 1–4 |
| Schnitzer, Kuzee & Bongers (2005)a,c,e | Cote d'Ivoire (30mj) | 0.22 bio | 1.18 bio | 436 | Seedlings | 2 |
| Parren & Doumbia (2005)e,g | Cote d'Ivoire (125 mk) | 6.0 dbh | 9.0 dbh | 50 | 5–50 | 3 |
| Campanello et al. (h) | Argentina (200mk) | 3.6 RGRc | 6.4 RGRc | 78 | ≥10 | 1.75 |
| Peña-Claros et al. (2008) | Bolivia (300mk) | 0.24 dbh | 0.52 dbh | 116 | ≥10 | 4 |
| Toledo-Aceves & Swaine (2007)a2,c,e | Ghana (210m) | 0.062 RGRd (0.064 RGRh) (0.119 RGRb*) |
0.069 RGRd (0.076 RGRh) (0.144 RGRb*) |
11 (19) (21) |
Seedlings | 1 |
| Toledo-Aceves & Swaine (2008)a2,e,h | Ghana (210m) |
0.037 RGRd* (0.040 RGRh) (0.014 bio*) |
0.041 RGRd* (0.044 RGRh) (0.017 bio*) |
10 (9) (22) |
Seedlings | 1–1.25 |
| van der Heijden & Phillips (2009)a,d,i | Peru (260m) |
2.8 dbh (2.92 bioMg ha−1) |
4.6 dbh (3.30 bioMg ha−1) |
63 (13) |
≥10 | 3 |
| Villegas et al. (2009)e | Bolivia (450m) | 2.0 dbh | 3.0 dbh | 50 | 10–50 | 4 |
| Ingwell et al. (2010)a1 | Panama (145m) | Sun 0.004 dbhgrShade–0.02 dbhgr | 0.022 dbhgr–0.04 dbhgr 1 |
450 100 |
4–193 | 10 |
| Schnitzer & Carson (2010)j | Panama (30mk) | 0.09 dbh (1.12 bio m−2) | 0.14 dbh (2.23 bio m−2) |
56 (99) |
>1.3 h | 8 |
| Álvarez-Cansino et al. (2015)e | Panama (30 mk) | 1.98 ba | 5.18 ba | 162 | 10–20 | 1 |
| van der Heijden, Powers & Schnitzer (2015) | Panama (30mk) | 3.79 bio Mg ha−1 | 5.50 bio Mg ha−1 | 45 | ≥10 | 3 |
| Wright et al. (2015) | Panama (30mk) | 2.08 RGRb | 2.69 RGRb | 29 | ≥0.5 | 3 |
| Wright et al. (2015)e | Panama (30mk) | −0.115 RGRb | 0.006 RGRb | 105 | Seedlings | 3 |
- aLianas not removed, instead compared (1) trees naturally with/without lianas, or (2) trees planted with/without planted lianas.
- bLiana-cutting combined with other silvicultural treatments.
- cPlantation.
- dIncrements estimated from empirical models.
- eMean of focal species, mostly commercial except for Álvarez-Cansino et al. (2015) and Wright et al. (2015).
- fForest trees regenerating in fire-managed pasture.
- gData cited from an unpublished conference presentation (H. Fickinger); size class inferred.
- hTree growth benefitted from liana absence in large gaps (mean RGR: +14%; bio: +28%) but not small gaps/understorey (mean RGR: −1, +5; bio: −12, −12); Only RGRh was significant, for just one tree species in large gaps.
- iMean of low, medium and high light levels (18, 36 and 89% growth increase without lianas).
- jBiomass data from Schnitzer et al. (2014).
- kElevation estimated from online map.
- 1Contrary to other studies, however the highest liana density had a negative impact.
- *Not significant.
The impact of liana absence on tree stem growth in Africa (dbh circ−1 h−1: +28%, −18 to 7, n = 5; ba bio−1: +122%, 21–288, n = 5), Asia (dbh circ−1 h−1: no data; ba bio−1: +41%, 9–73, n = 3) and the palaeotropics combined (dbh circ−1 h−1: +28%, −18 to 77, n = 5; ba bio−1: +92%, 26–197, n = 8) did not differ from the more extensive neotropical studies (dbh circ−1 h−1: +83%, 54–119, n = 15; ba bio−1: +71%, 37–112, n = 6). This provides a first quantitative indication that lianas (and presumably liana removal) have approximately equivalent pantropical impact on biomass.
Our lack of liana cutting influence on mortality is inconsistent with neotropical studies showing greater mortality with lianas present (Ingwell et al., 2010; Schnitzer & Carson, 2010; van der Heijden, Powers & Schnitzer, 2015). However, wildfires were not present in these studies and may have masked a positive impact of liana cutting on survival. Accordingly, our 56–64% stem mortality (57–68% biomass loss) is within the range of other tropical forests impacted by wildfires (Woods, 1989: >80%, Malaysia; Pinard, Putz & Licona, 1999: 74%, Bolivia; Kinnaird & O'Brien, 1998: 25–70%, Sumatra). However, without controlling for fire the expected positive influence of liana cutting on survival remains uncertain. Previous African studies of tree mortality with/without lianas have found only ambiguous (Schnitzer, Kuzee & Bongers, 2005; Toledo-Aceves & Swaine, 2007) or zero relationships (Toledo-Aceves & Swaine, 2008). Liana cutting also did not influence seedling survival for two tree species in Bolivia (Pérez-Salicrup, 2001).
Bringing together growth, recruitment and mortality, our observed 765% increase in relative net biomass gain from 0.32 kg kg−1 in managed plots to 2.77 kg kg−1 in unmanaged plots is of similar magnitude to two previous estimates from Panama of 370% (stems >1.3 m height; unmanaged 0.23 kg kg−1; managed 1.08 kg kg−1; Schnitzer et al., 2014) and 646% (stems ≥10 cm dbh; unmanaged 0.017 kg kg−1; managed 0.11 kg kg−1; van der Heijden, Powers & Schnitzer, 2015). A similar magnitude of increase (750%) was seen for basal area in Brazil (stems >5 cm dbh; unmanaged 64 cm2 m−2; managed 544 cm2 m−2; Gerwing, 2001). The mean 633% increase across these studies suggests increasing pantropical liana growth will have increasing impact on biomass loss beyond existing estimates of 9–76% (Schnitzer et al., 2014; van der Heijden, Powers & Schnitzer, 2015). Liana influence can vary with initial tree biomass (Schnitzer et al., 2014), and hence, we have made these comparisons using biomass (or basal area) increase per pretreatment stem size, that is kg kg−1 and cm2 m−2, to control for initial differences in and plot and stem size between studies.
The reason for our generally high regeneration is uncertain. Variation within previous studies has been ascribed to shade and liana density (Ingwell et al., 2010; Schnitzer & Carson, 2010). Variation across studies may also have arisen from inconsistencies in tree size, measurement units and time span. Across studies, the growth increase without lianas was unrelated to tree size and most variable for small stems (<5 cm dbh: 135% [95CI: 47–250], n = 7; >5 cm dbh: 75% [51–104], n = 20; unspecified size: 75% [36–124], n = 5). Regarding units, dbh narrowly predominates over biomass in the literature, presumably because of its relevance to forestry and uncertainty regarding biomass calculation. Indeed, region-specific biomass estimation methods are lacking across the tropics, particularly for saplings. Regarding time span, the impact of liana cutting in isolation is unknown beyond 10 years and 71% of all restoration studies have spanned ≤15 years (Wortley, Hero & Howes, 2013).
Importantly, wildfires in Magombera Forest have not obscured the positive influence of liana cutting on biomass. Furthermore, the observed positive interaction between liana cutting and wildfire frequency provides weak evidence that liana cutting is more effective for encouraging recruitment in the presence of fire. However, our observed lack of biomass gain in the absence of liana cutting does not suggest a positive ‘prescribed burn’ influence.
Species
Our difference in species between sapling and mature stems suggests typical negative density dependent growth (Wright, 2002). However, our high sapling species richness shows good restoration potential. Our data are inconclusive regarding liana cutting impacts on community restoration, perhaps a result of limited scale. In Panama, tree species richness increased 65% following liana cutting (Schnitzer & Carson, 2010) and tree-liana competition was similar between species (Álvarez-Cansino et al., 2015). Conversely, liana cutting in Brazil caused both increases and decreases across species (Gerwing, 2001). Community change following liana cutting remains unverified for Africa; however, pioneer trees recruited faster than mid-successional species following liana/shrub removal in Uganda (Duncan & Chapman, 2003). Moreover, tropical forest recovery often requires establishment of single species (Chazdon, 2003) or several ‘framework species’ (Goosem & Tucker, 1995) before soil fertility, seed dispersal and shade are sufficient to stimulate succession. Hence, longer-term monitoring is required.
Similar to mortality, our lack of liana cutting influence on species composition may have been confounded by wildfires, which can favour pioneers (Cochrane & Schulze, 1999) or drought-resistant species with thick bark (Uhl & Kauffman, 1990). However, our observed prevalence of forest species gives no evidence for wildfire-driven conversion to woodland. Yet while our regression was weak, wildfires caused ≥51% stem mortality and net species loss where they exceeded one per 5 years. As saplings grow, their resilience to fire improves, with increasing evidence for a bark resistance threshold (Hoffmann et al., 2012).
Practicalities
An important practical consideration is cost. Using a cutting rate of sixteen person-h ha−1 (Grauel & Putz, 2004), it would take four people 18 months to manage the 11 km2 Magombera Forest. However, given our flat topography, this could be achievable within 12 months (one person-day ha−1). This would cost ~US$6000 yr−1 for labour and equipment (US$5.45 ha−1), compared to US$1-4 ha−1 for West Africa (Bongers, Schnitzer & Traore, 2002) and mean $11 ha−1 for Bolivia and Brazil (range $1–16 ha−1; Vidal et al., 1997; Pérez-Salicrup et al., 2001; Dauber, Fredericksen & Peña, 2005). The equivalent cost for alternative tree-planting in neighbouring Uganda was more than 44 times more expensive (US$1200 ha−1.5 yr−1; Omeja, Chapman & Obua, 2009).
Future monitoring of saplings would benefit from increased sampling. Plots used previously for stems <5 cm dbh have varied in size and number per treatment (Duncan & Chapman, 2003: 25 m2, n = 19; Schnitzer, Kuzee & Bongers, 2005: 108 m2, n = 10; Gerwing, 2001: 400 m2, n = 6). Our own sampling enabled statistically verifiable conclusions for biomass. However, larger plots would reduce zero values and improve precision. Increased samples would further improve precision, with our power analysis suggesting twenty plots per treatment would have nearly doubled our ability to detect biomass change. While our plots were too small to estimate an optimal size for measuring biodiversity, previous work suggests 300–400 stems per plot (Gimaret-Carpentier et al., 1998). At our observed sapling density, this would need plots of ~790–1053 m2, similar to stems 1–9.9 cm dbh in Amazonia (0.1 ha; Magnusson et al., 2005).
Thirdly, lianas (and cutting) may not influence regeneration where light or liana abundance is low, for example shaded forest or small canopy gaps (Toledo-Aceves & Swaine, 2008; van der Heijden & Phillips, 2009; Ingwell et al., 2010). Liana removal may even be damaging to healthy forests, for example maintaining small canopy gaps can preserve biodiversity by supporting pioneers and lianas (Schnitzer & Carson, 2000). Hence, liana cutting is best employed on a tree-by-tree basis (Schnitzer & Bongers, 2002) with focus on abundant species (Sfair et al., 2011) in large canopy gaps/thickets (Schnitzer & Carson, 2010) and heavily degraded understorey (this study). Cutting must also be temporary to allow liana recovery (Campanello et al., 2012). Trees were bent by lianas in Magombera up to ~2 cm dbh, and we estimate that six to 7 years of cutting would allow 50% of recruited stems to reach this size.
Accurate assessment of liana cutting is also hindered because, for geographic completeness our review of previous work includes liana presence/absence through natural growth or planting. These studies may suffer from species bias in host tree preferences (Putz, Lee & Goh, 1984; Campanello et al., 2007) or choice of planted seedlings. Indeed, the increase in growth without lianas was higher among cutting studies (mean 90%; n = 19) than the rest (57%; n = 10), but not significant (95CI 53–139 and 23–108, respectively). A previous comparison of growth between liberated and naturally liana-free trees found ambiguous results for timber species in Bolivia (Villegas et al., 2009). Therefore, more liana cutting studies are needed across stem sizes in natural forest, especially regarding recruitment and biodiversity.
Finally, restoration must form part of a holistic strategy. Effective restoration benefits from legal and stakeholder support, economic value, improvement in soil, hydrology and water quality, and understanding the interactions between ecosystems and socio-political systems (ITTO, 2002; SER, 2004). Arguably no tropical forest restoration study has addressed situations where all of these factors have been true. This includes our study, where there was limited legal protection, little direct income from the forest, and no soil or hydrology data. Moreover, the persistence of wildfires emphasizes that burning practices in adjacent areas were not employed with landscape management in mind. Nevertheless, liana cutting has here proved effective for regenerating biomass even without full wildfire control.
Acknowledgements
Thanks to Philip Platts, Dave Raffaelli and journal editors/reviewers for comments; TAWIRI and COSTECH for permits; Nicolas Deere, Freddie Sutton and Aloysi Mwakisoma for mapping; Martijn Snoep for restoration costings. Donors: Flamingo Land; CEPF; NERC, NER/S/A/2002/11177.




