Prevalence and geographical variation of dementia in New Zealand from 2012 to 2015: Brief report utilising routinely collected data within the Integrated Data Infrastructure
Abstract
Objectives
There are no national dementia epidemiological studies using New Zealand (NZ) data. NZ routinely collects health-care data within the Integrated Data Infrastructure (IDI). The study objectives were to 1) investigate late-onset dementia estimates using the IDI between 2012-2015 and compare these with 2) published estimates, and 3) variations between North and South Islands and ethnicity.
Methods
A population-based, retrospective cohort design was applied to routinely collected de-identified health/administrative IDI data. Dementia was defined by ICD-10-AM dementia codes or anti-dementia drugs.
Results
Approximately 2% of those aged ≥60 years had dementia, lower than published estimates. Dementia was higher in North Island; in 80- to 89-year-olds; among the Māori population when age-standardised, and 9% of all dementia cases had >1 dementia sub-type.
Conclusions
To our knowledge, this is the first study ascertaining dementia estimates using NZ’s whole-of-population IDI data. Estimates were lower than existing NZ estimates, for several reasons. Further work is required, including expanding IDI data sets, to develop future estimates that better reflect NZ’s diverse population.
Policy Impact
This research highlights discrepancies between estimates of New Zealand (NZ) dementia prevalence and those within the Integrated Data Infrastructure, emphasising the need for a more detailed epidemiological study within NZ to provide country-specific dementia data to help guide health care and policies.
1 INTRODUCTION
Dementia is an important public health concern, predicted to affect 75.62 million people worldwide in 2030.1 Understanding country-specific burden of disease facilitates effective health-care delivery. There have been no national New Zealand (NZ) epidemiological studies of dementia.2-4 One valuable study (LiLACS NZ) investigated dementia prevalence using NZ data but not at a national level.5, 6 This was using a small population within two districts of North Island and specifically in the older age groups (≥80-year-old Māori and ≥85-year-old Non-Māori).5, 6 However, estimates for national dementia prevalence historically have previously been produced by applying dementia prevalence rates for Australia to the estimated population of NZ3, 4 or by using incidence rates for Alzheimer dementia from an international systematic review modelled on NZ’s ageing population.2 The Alzheimer Disease International (ADI) 2015 report produced regional (Australasian) dementia prevalence estimates. Therefore, the recent NZ estimates apply the Australian prevalence rates to the United Nations projections for NZ’s population.4 Given the diverse ethnic compositions of NZ and Australia, we contend that whilst these ADI reports are useful, they are unlikely to accurately reflect the situation of NZ. Variations in dementia prevalence between and within countries are well recognised,7, 8 including dementia mortality differences between NZ’s North and South Islands.7
Greater information on dementia prevalence in NZ is required, and NZ’s linked health and administrative population data within the Integrated Data Infrastructure (IDI)9 provide some data to study dementia at a population level. The IDI, a large research database containing microdata about people and households,9 contains mortality, medication dispensing and hospital discharge data sets necessary to ascertain dementia, using methods similar to previous international research.8, 10-12
The aims of this study were to: (a) investigate NZ’s late-onset dementia prevalence using the IDI for 2012 to 2015 (inclusive); (b) compare this with published NZ estimates; and (c) compare variations between the North and South Islands and ethnicity.
2 METHODS
A population-based retrospective cohort study was used analysing routinely collected de-identified health and administrative data stored within NZ’s IDI system. Health data comprised Primary Health Organisation; publicly funded hospital discharges (National Minimum Dataset [NMDS]); subsidised dispensing (Ministry of Health [MOH] and Pharmaceutical Management Agency [PHARMAC]); and mortality, cause of death, data (MOH). Other administrative data comprised Inland Revenue (pensions) and Accident Compensation Corporation (ACC) data sets.
2.1 Data access, linkage and cohorts
Data were stored securely by Statistics NZ, with access granted following approvals.9
The IDI October 2016 version (Appendix S1) was used to create annual cohorts for 2012-2015. Our population at risk of dementia (denominator) was defined within the IDI as all individuals aged ≥60 years (alive or died during the year) and NZ residents who had interacted with any of the health data sets above, Inland Revenue (pensions) or ACC data sets. Our numerator therefore represented those individuals defined as having dementia in that year. We also obtained age, sex and multi-response ethnicity.13, 14
For each year, dementia cases (numerators) were counted using the case definition of dementia. Using SAS and SQL, the cohort was linked to health data sets (NMDS, dispensing and mortality data).
2.2 Dementia ascertainment
Dementia was defined by any mention of ICD-10-AM (Australian modification version) dementia codes in NMDS or mortality data sets; or anti-dementia drug prescriptions in the dispensing data set (Appendix S2). The mortality data set was only available for 2012 at the time of analysis due to temporal lag in public releases. Formulation codes identified anti-dementia drugs (Appendix S3). Only, two anticholinesterases (donepezil and rivastigmine) are funded, rivastigmine only since 2014. Dementia sub-types were classed by ICD-10-AM codes obtained from hospital or mortality data (Appendix S4). Appendix S5 refers to information on the SAS code.
2.3 Statistical analyses
Dementia cases were calculated by year, sub-type and geographical region. Released outputs were subject to statistical disclosure control in accordance with Statistics NZ including random rounding to base 3 (RR3), where numbers are rounded to the nearest multiple of 3, to protect confidentially.15, 16 For ethnic comparisons of dementia rates, we used direct age-sex standardisation to take account of differences in age and sex profile between different ethnic groups. Age-sex standardised rates were calculated using 5-year age-sex groups and weights derived from the total (all ethnicities) denominator population described above.
2.4 Ethics
Ethical approval was granted by The University of Auckland's Human Participant's Ethics Committee in April 2017 (reference 019125).
The study is reported according to RECORD (STROBE) guidelines.17
3 RESULTS
3.1 Denominator cohorts from 2012-2015
Table 1 details the study's population: 891 558; 916 065; 945 252; and 966 483 people for 2012-2015, respectively.
| Study population—population ≥60 years in NZ per year (denominator group, Population at risk) | 2012 | 2013 | 2014 | 2015 |
|---|---|---|---|---|
| Total people per year ≥60 years old in NZ | 891 558 | 916 065 | 945 252 | 966 483 |
| Sex (% of total study population)a | ||||
| Male | 409 173 (46%) | 422 199 (46%) | 436 506 (46%) | 446 574 (46%) |
| Female | 474 429 (53%) | 487 983 (53%) | 502 815 (53%) | 513 975 (53%) |
| Ethnicityb | ||||
| Māori | 59 145 | 62 208 | 65 508 | 68 670 |
| Pacific | 25 572 | 26 562 | 27 957 | 29 100 |
| European | 742 536 | 761 670 | 781 581 | 794 670 |
| Asian | 44 076 | 47 982 | 52 320 | 56 073 |
| Middle Eastern, Latin American and African (MELAA) | 2820 | 3042 | 3336 | 3627 |
| Other | 13 602 | 14 607 | 15 477 | 16 137 |
| Age rangea | ||||
| 60-64 | 234 312 | 237 288 | 242 226 | 242 553 |
| 65-69 | 201 471 | 214 593 | 224 991 | 231 792 |
| 70-74 | 159 402 | 162 735 | 167 277 | 171 084 |
| 75-79 | 114 567 | 118 638 | 123 531 | 131 064 |
| 80-84 | 88 539 | 88 965 | 90 126 | 90 180 |
| 85-89 | 55 209 | 56 601 | 58 026 | 59 397 |
| 90-94 | 23 409 | 24 549 | 26 139 | 26 802 |
| 95+ | 6696 | 6819 | 7002 | 7680 |
| Total people by North/South Islandc | ||||
| North Island | 637 245 | 660 852 | 683 220 | 699 813 |
| South Island | 230 001 | 237 825 | 244 248 | 248 934 |
| Dementia prevalence | ||||
| All dementia cases—numerator (% of total study population) | 13 677 (2%) | 13 155 (1%) | 13 800 (2%) | 14 391 (2%) |
| Dementia by sub-type: (% of total dementia cases per year) | ||||
| Alzheimer dementia | 2499 (18%) | 2094 (16%) | 2142 (16%) | 2166 (15%) |
| Vascular dementia | 1257 (9%) | 1284 (10%) | 1338 (10%) | 1407 (10%) |
| Parkinson's disease dementia | 447 (3%) | 432 (3%) | 441 (3%) | 447 (3%) |
| Other (including unspecified dementia sub-type) dementia | 10 647 (78%) | 10 563 (80%) | 11 166 (81%) | 11 736 (82%) |
| More than one type of dementia | 1110 (8%) | 1140 (9%) | 1194 (9%) | 1272 (9%) |
| Dementia by sex | ||||
| Male (% of total study population per year) | 5418 (1%) | 5601 (1%) | 5856 (1%) | 6240 (1%) |
| Female (% of total study population per year) | 8091 (1%) | 7542 (1%) | 7935 (1%) | 8136 (1%) |
| Dementia by ethnicity: (% of total study population ethnicity per year) | ||||
| Māori | 699 (1%) | 741 (1%) | 801 (1%) | 867 (1%) |
| Pacific | 279 (1%) | 330 (1%) | 351 (1%) | 348 (1%) |
| European | 12 057 (2%) | 11 730 (2%) | 12 225 (2%) | 12 750 (2%) |
| Asian | 321 (1%) | 357 (1%) | 411 (1%) | 444 (1%) |
| MELAA | 30 (1%) | 27 (1%) | 42 (1%) | 51 (1%) |
| Other | 36 (0.3%) | 57 (0.4%) | 75 (0.5%) | 78 (0.5%) |
| Dementia by ethnicity, age and sex standardised (% of total study population ethnicity per year) | ||||
| Maori | 2% | 2% | 2% | 2% |
| Pacific | 2% | 2% | 2% | 2% |
| Euro | 1% | 1% | 1% | 1% |
| Asian | 1% | 1% | 1% | 1% |
| MELAA | 2% | 1% | 2% | 2% |
| Other | 0.4% | 1% | 1% | 1% |
| Dementia by age range: (% of total dementia cases per year) | ||||
| 60-64 | 267 (2%) | 300 (2%) | 312 (2%) | 294 (2%) |
| 65-69 | 582 (4%) | 621 (5%) | 705 (5%) | 756 (5%) |
| 70-74 | 1257 (9%) | 1326 (10%) | 1404 (10%) | 1443 (10%) |
| 75-79 | 2175 (16%) | 2205 (17%) | 2340 (17%) | 2553 (18%) |
| 80-84 | 3264 (24%) | 3219 (24%) | 3255 (24%) | 3375 (23%) |
| 85-89 | 3486 (25%) | 3348 (25%) | 3447 (25%) | 3510 (24%) |
| 90-94 | 1860 (14%) | 1683 (13%) | 1869 (14%) | 1899 (13%) |
| 95+ | 618 (5%) | 438 (3%) | 465 (3%) | 546 (4%) |
| Dementia by geographical area: (% of total dementia case per year) | ||||
| North Island | 9696 (71%) | 9363 (71%) | 9870 (72%) | 10 197 (71%) |
| South Island | 3702 (27%) | 3492 (27%) | 3570 (26%) | 3810 (27%) |
Note
- In accordance with the journal style, percentages have been rounded up to the nearest whole number. Disparities in sum of counts: Mortality data were only available for 2012 at the time of analysis and so do not contribute to dementia counts for other years. Counts for dementia sub-type represent an ICD coding and not an individual (who may have more than one sub-type), so the totals differ from the total dementia cases (which also includes counts found from dementia drugs as stated in methods). Counts for ethnicity do not all add up as total response ethnicity coding is used in NZ where an individual can belong to more than one ethnic group.13 Released outputs were subject to statistical disclosure control in accordance with Statistics Protocols, including suppressing counts <6, and random rounding to the base of three (RR3) to avoid identification.14
- a Sex and age missing 7956 (2012), 5880 (2013), 5931 (2014), 5931 (2015).
- b Ethnicity was missing for 38 265 in the total study population.
- c Geographical information on Islands missing 24 315 (2012), 17 385 (2013), 17 781 (2014), 17 736 (2015).
3.1.1 Dementia prevalence
Dementia cases were 13 677 (2012), 13 155 (2013), 13 800 (2014) and 14 391 (2015)—0.3% of NZ’s total population in 2015 and 2% of our cohort ≥60 years. Most were ‘unspecified dementia’ sub-type; and around 9% had more than one dementia sub-type. Table 1 provides further details of dementia cases. The mean age for dementia ranged from 81.9 to 82.5 (SD 7.7-7.8). Most dementia was amongst the 80-89 age groups (47%-49% of all dementia cases). However, in 2012 the ≥95 age group had a slightly higher proportion (5%), potentially reflecting the additional data set (mortality) present for this year.
Europeans were the largest ethnic group contributing most to the overall dementia counts (88%-89% of dementia cases). Māori with dementia contributed 5%-6% of dementia cases, whilst the Asian ethnic group contributed 2%-3% overall. This roughly reflects the prevalence of each ethnic group within the study population. Interestingly, when dementia cases were separated by ethnicity and calculated as a proportion of the ethnic group overall, the numbers differed less substantially (Europeans 2% vs 1% of Māori, Table 1). However, following age and sex standardisation, the ethnic variation changed with dementia cases higher for Māori than Non-Māori (2% vs 1%, Table 1).
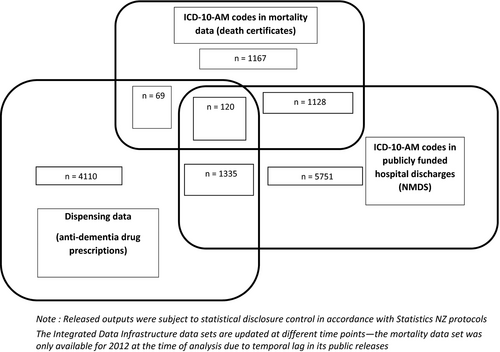
Figure 1 identifies dementia ascertainment overlap between IDI data sources in 2012. From individual data sets, most cases were ascertained from ICD-10-AM codes in NMDS (n = 5751), followed by dispensing data (n = 4110). Of 33 030 people who died in 2012, 1167 (4%) had dementia mentioned on death certificates (Figure 1). The remaining dementia cases were identifiable in more than one data set. However, only 120 dementia cases were found in all three data sets. In 2012, only 10% (n = 1335) were found in both NMDS and dispensing data sets and 8% (n = 1128) were found in NMDS and mortality data. This highlights that multiple data sets are beneficial for ascertaining dementia.
Donepezil prescriptions contributed 5634 (41%), 6474 (49%), 6843 (50%) and 7122 (49%) of dementia cases in 2012-2015, respectively. Rivastigmine prescriptions only contributed 57 (0.4%) and 264 (2%) dementia cases in 2014-2015, respectively (it was only publically available in NZ from 2014).
Given the population distribution, dementia was higher in North vs South Island. The overall percentage of total dementia remained relatively consistent across the years in both Islands (see Table 1).
4 DISCUSSION
Our study's purpose was to demonstrate the potential to estimate dementia prevalence using routinely collected data within NZ’s national repository, the IDI. To our knowledge, this is the first to do this. Our reported dementia estimates were as follows: lower than those previously published for NZ at 48 182 and 62 287 in 2011 and 2016, respectively3, 4; higher amongst Maori when results are age and sex standardised; higher in the North than the South Island; and 9% had more than one dementia sub-type.
However, our estimates should be interpreted cautiously and might be considered as ‘lower bound’ estimates. Several explanations for these variations should be considered for future work. Currently, NZ estimates use countries' data that may not be fully representative to NZ given it has previously been shown that significant intra-country variability in dementia rates exists.7, 8 Alternatively, secondary care and mortality data can underestimate dementia counts, a well-established issue with big data. Not everyone with dementia receives a diagnosis nor is hospitalised nor is prescribed anti-dementia drugs nor has dementia recorded on the death certificate. Many people with dementia are managed in primary care, and without these records being part of the IDI, or indeed a national requirement to collect this diagnostic information, the estimates may not include these people.
Unspecified dementia was the commonest sub-type. Around 9% of individuals had more than one dementia diagnosis/sub-type (Table 1). Without an agreed ICD coding for mixed dementia, this is not unexpected. Whilst Alzheimer's remains the commonest diagnosed dementia, recent work identified under-reporting of cerebrovascular pathology at postmortem.18 This could be important for NZ if cerebrovascular pathology is higher amongst some ethnic groups.5 However, inter-ethnic sub-type variations in NZ may be minor.19
Dementia counts varied between the North and South Islands. Possible explanations include age and ethnicity differences between North and South Islands; North Island substantially higher population (3/4 of NZ's population lives in North Island); or the accessibility to memory clinics to obtain a diagnosis (only seven of the 20 District Health Boards in NZ have at least one memory clinic with 5 of these in North Island).20 Additionally, Non-Maori living in urban locations are more likely to be prescribed anti-dementia drugs in urban locations.21 Further work is required to understand factors influencing geographical variation in dementia.
Recording of dementia may be lower in ethnic minorities.10 Despite representing 15% of NZ’s population, Māori had 6% of total dementia cases in 2015. However, following age and sex standardisation, Māori dementia was approximately 50% higher than Europeans. Recent NZ studies highlight possible ethnic differences in diagnosis-seeking and earlier onset of dementia,19, 22, 23 potentially also influenced by lower life expectancy.19, 22, 23 However, the LiLACS NZ study showed no significant difference in dementia rates between Māori and Non-Māori at older age (>80 years) within a smaller cohort (937 people).5 Nonetheless, the attitudes to dementia, including the stigma amongst indigenous people, are importantly highlighted in a recent ADI report,24 with a recent study discussing the complexities surrounding diagnosing mate wareware (dementia) in Māori communities.25 Further investigation, ideally within a national epidemiological study of dementia, is warranted.
Population-based studies using big data have several limitations. Of relevance is low diagnostic coverage for dementia26 or the ‘diagnosis gap’, which comprises three important and inter-related dimensions: patients who have dementia but do not have it recorded as a diagnosis; those who have dementia but do not interact with health/social services; and patients who have cognitive impairment that impacts on daily function but may not receive a dementia-related diagnosis. In the UK, specific population studies have been used to estimate the ‘diagnosis gap’ and then extrapolated to the whole population to better inform health-care delivery.27
Further limitations include the types and reliability of data sets for ascertaining dementia in NZ’s multi-ethnic population. Neither memory clinic nor primary care consultation data are available within the IDI. Primary care consultation data inferring cognitive impairment and dementia would be a valuable addition to the IDI, as would memory clinic outpatient data. Indeed, since our study, the IDI now incorporates NZ InterRAI database (an extensive resource for understanding cognition, dementia and care needs of NZ’s older people).28 This addition, along with including other data sets into the IDI, will allow validation of data sets for dementia coding and ascertainment specific to NZ and triangulation of dementia prevalence results to facilitate the delivery of NZ’s Framework for Dementia Care.29
Wilkinson's recent systematic review showed dementia coding and positive predictive values (PPVs) varied widely, with only <50% of dementia cases identified from routinely collected data and PPVs for dementia diagnoses ranging from 33% to 100%.30 This is comparable with other studies,10-12, 31-33 one highlighting only 53% of known dementia patients had dementia recorded on their hospital discharge.34 However, some studies achieved PPVs of >80%-90% within routinely collected data, meaning that those with a dementia diagnosis recorded were likely to have the diagnosis of dementia.30
Within secondary care data, dementia ascertainment had higher sensitivity if hospital admissions and mortality data were combined, and lower when only primary ICD codes were selected from death certificates.30 We mitigated for this by including ICD codes in any position on death certificates and combining hospital admissions and mortality data. Some recent studies have shown comparability of routinely collected hospital and primary care data for dementia ascertainment,11, 12 highlighting good sensitivity and specificity compared with memory clinic data.10 Whilst there is no ‘gold-standard’ data source, we attempted to overcome some of these limitations by including multiple data sets, as recommended by recent systematic reviews.30, 35
It is also important to recognise that routinely collected data will vary with time. For instance, rates may rise due to ageing populations, improved diagnosis, access to additional anti-dementia drugs (eg Rivastigmine in NZ in 2014) and awareness of dementia.
5 CONCLUSIONS
This study shows it is possible to ascertain dementia counts within the IDI and that these differ from current estimates. Traditional ‘gold-standard’ country-wide epidemiological studies are costly and labour-intensive, but the IDI could provide a future tool to enhance understanding of dementia prevalence in NZ at a national level. Our research highlights the need for further work including a national epidemiological study of dementia using the InterRAI within the IDI, and adding further relevant data sets (memory clinics and primary care). This will also allow validation for dementia recording accuracy in NZ’s routinely collected data sets;, improve dementia ascertainment; and understand the ‘diagnosis gap’ specific to NZ, and ultimately better inform health-care delivery.
ACKNOWLEDGEMENTS
KEW is supported by clinical research fellowship from Alzheimer Scotland and the University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross-council Lifelong Health and Wellbeing initiative (MR/L501530/1). Funding from the Biotechnology and Biological Sciences Research Council (BBSRC) and Medical Research Council (MRC) is gratefully acknowledged. KEW, JMS and TCR are members of the Alzheimer Scotland Dementia Research Centre funded by Alzheimer Scotland. KEW additionally received a PhD studentship travel grant from the MRC in 2016 for a preliminary visit to New Zealand for planning the research and an additional grant from Alzheimer Scotland to undertake the research in 2017. The funders played no part in the design or conduct of this manuscript. Thanks to the IDI team at Statistics New Zealand for their valuable input, assistance with the study and use of data, in particular thanks to Simon McBeth and Stephen Challands. Additionally, we would like to thank Dr Jinfeng Zhao for the original VARIANZ population SAS code (co-written) with Dr Sheree Gibb that this study's SAS code was based on. Lastly, we would like to thank Professor John Starr (co-author) for his huge lifetime contribution to dementia research worldwide. We are deeply saddened by his sudden unexpected death on 9 December 2018 and miss our colleague greatly.
CONFLICTS OF INTEREST
No conflicts of interest declared.
DISCLAIMER STATEMENT
Access to the data presented was managed by Statistics New Zealand under strict microdata access protocols and in accordance with the security and confidentiality provisions of the Statistic Act 1975. Our findings are not Official Statistics. The opinions, findings, recommendations and conclusions expressed are those of the researchers, not Statistics NZ, or the Universities of Edinburgh or Auckland.




