Anatomical, histological and immunohistochemical study of the tongue in the rainbow trout (Oncorhynchus mykiss)
Abstract
The rainbow trout (Oncorhynchus mykiss Walbaum, 1792) is a fish commercially farmed all over the world. These fish are usually fed, in aquaculture, with pellets rich in proteins and fat. It is well known that there are close relationships among the adaptation of vertebrates to their environment, the capacity and the modality of feeding and the oral cavity morphology, especially the tongue one. No data are so far available about the morphology of the rainbow trout tongue, and therefore, the aim of this study was to investigate by light, scanning electron and confocal laser microscopy, the morphological characteristics of the tongue. An apex, a body and a root can be distinguished in the tongue, and the presence of teeth, taste buds and fungiform-like papillae was demonstrated. Light microscopy shows the presence of an adipose tissue pad in the deeper layer of the apex and in the most superficial layer of the root. In the deeper layer of the body, a triangular-shaped pad consisting of fusiform cells immersed in abundant extracellular matrix of the mesenchymal tissue was observed. The confocal laser microscopy shows the presence of cells with a fibroblast-like morphology positive for vimentin. In the deepest layer of the tongue root, a large area of osteo-cartilaginous tissue was observed. The results, besides the description of the morphological characteristics of the tongue, related to studies regarding the feeding, could be considered for the eventual applications of the use of mesenchymal cells, observed in adult fish, in cell therapies in different pathologies.
1 INTRODUCTION
The rainbow trout (Oncorhynchus mykiss Walbaum, 1792), belonging to the family Salmonidae, is a bony fish native to North America and later introduced and commercially farmed in several countries all over the world. The largest producers are Chile and Norway, but other significant trout-producing countries include the United States, the United Kingdom, Italy, France, Germany, Denmark and Spain. In Italy, the rainbow trout is the most used fish in aquaculture, because the adaptation to the rearing conditions and the capacity of growing are better than other species of trout. Rainbow trout are predators with a varied diet including aquatic insects, fish eggs, shrimps and other crustaceans. Feeds in aquaculture have been modified over the last years and are now based on high-energy food administered by compact nutritious pellets with high percentages of proteins and fat, all lifelong. The adaptation of vertebrates to their environment is in close relationship with the capacity and the modality of feeding, and a very significant role is carried out by the oral cavity, especially by the tongue. Several data are present in the literature on the fish oral cavity morphology (for review, see Kapoor & Khanna, 1994), and several evidences are present regarding the strict connection among the different manners of prehension, oral transport, mixing and swallowing, the feeding habits and the oral cavity morphology. An important role by different hormones in feeding, related to the morphological characteristics, has been recently demonstrated in the digestive system in teleosts (Audira et al., 2018; Carnovali, Luzi, Terruzzi, Banfi, & Mariotti, 2018; Mania et al., 2017; Montalbano et al., 2016, 2018). The most of the functional processes inside the oral cavity are carried out by the tongue, whose presence and description have been showed in different species of fish, demonstrating the variation in the presence of anatomical structures like taste buds, mucous cells and teeth in teleosts, also of commercial interest (Abbate et al., 2006,2017,2020a,2020b; Abbate, Guerrera, Montalbano, Ciriaco, et al., 2012; Abbate, Guerrera, Montalbano, De Carlos, et al., 2012; Alsafy, Bassuoni, & Hanafy, 2018; Amato et al., 2012; Dos Santos, Arantes, Santiago, & Dos Santos, 2015; El Bakary, 2014; Elgendy, Alsafy, & Tanekhy, 2016; Germanà et al., 2009; Guerrera et al., 2015; Ikpegbu, Ibe, & Nlebedum, 2019; Kasumyan, 2019; Kettratad, Senarat, Boonyoung, & Jiraungkoorsku, 2017; Levanti et al., 2017; Mahmoud, Essa, & Sayed, 2016; Sadeghinezhad, Rahmati-holasoo, Fayyaz, & Zargar, 2015; Sayed, Mahmoud, & Essa, 2019; Yu et al., 2019). Several significant papers, important as comparative data, are present in the literature in upper vertebrates like birds, reptiles and mammals (Abbate et al., 2008, 2009, 2010, 2020b; Abumandour & Kandyel, 2020; Barbosa et al., 2020; Bels et al., 2020; Cizek, Hamouzova, Goździewska-Harłajczuk, Klećkowska-Nawrot, & Kvapil, 2020; Cizek, Hamouzova, Kvapil, & Kyllar, 2019; Emura, 2016a,2016b,2016c,2017,2018a,2018b,2018c,2018d,2019; Erdoğan & Alan, 2012; Erdoğan & Iwasaki, 2014; Erdoğan Lima & Pérez, 2016; Erdoğan Villar Arias & Pérez, 2016; Erdoğan, Villar Arias, et al., 2016; Erdoğan et al., 2018a; Erdoğan and Sağsöz, 2018b; Freire et al., 2019; Gonçalves et al., 2020; Gunawan et al., 2020; Haddad et al., 2019; Herrel, Redding, Meyers, & Nishikawa, 2014; Iwasaki, Yoshimura, Shindo, & Kageyama, 2019; Massoud & Abumandour, 2020; Nabil & Tawfiek, 2020; Sadeghinezhad et al., 2015; Sadeghinezhad, Sheibani, Memarian, & Chiocchetti, 2017; Sadeghinezhad, Tootian, & Javadi, 2018; Saragih et al., 2020; Skieresz-Szewczyk Cornillie & Jackowiak, 2018; Skieresz-Szewczyk & Jackowiak, 2016,2017; Skieresz-Szewczyk Jackowiak, & Ratajczak, 2018,2019). Considering that no data are available in the literature about the morphology of the rainbow trout tongue and in order to give a contribution to the anatomical knowledge of this well-known farmed species of fish, the aim of this study was to investigate by light, scanning electron and confocal laser microscopy, the morphological characteristics of the tongue.
2 MATERIAL AND METHODS
The heads of n. 20 specimens of rainbow trouts of commercial size, weighing 600/700 g, aged 18 months, have been obtained by the Agroittica Macrostigma Company, located in Contrada Stafenna, Noto (SR—Sicily). No stabulation of fish has been carried out aimed to this investigation, because this company is a plant for the rainbow trout breeding and transformation into fillets for the final purpose of selling to the Italian and international market. Therefore, the heads represent a processing waste of this food industry. For a better exposure of the oral cavity, the temporo-mandibular joint was disarticulated. The tongues were withdrawn and washed in 5% neutral Extran (Merck), a cleansing solution commonly used to remove the mucus.
2.1 Scanning electron microscopy
The samples (n. 7) were fixed in 2.5% glutaraldehyde in Sörensen phosphate buffer 0.1 M. After several rinsing in the same buffer, they were dehydrated in a graded alcohol series, critical point dried in a Balzers CPD 030, sputter coated with 3-nm gold in a Balzers BAL-TEC SCD 050 and examined under a Zeiss EVO LS 10.
2.2 Light microscopy
The tongues (n. 7) were fixed in paraformaldehyde solution 4% in PBS for 48 hr to preserve tissues, dehydrated and routinely embedded in paraffin. Sagittal serial sections of 7 μm thick were obtained, mounted on microscope slides and processed for Masson Trichrome with aniline blue staining. Three different stains were used: Weigert's iron haematoxylin for nuclei, a mixture of acid dyes for cytoplasm and aniline blue for connective tissue. After rinsing in distilled water, the sections were submitted to the reagents, repeatedly washed in distilled water and rapidly dehydrated through ascending alcohols, clarified in xylene and mounted. Pictures were obtained under a Leica DMRB light microscope.
2.3 Laser confocal microscopy
The samples (n. 6) were fixed in paraformaldehyde solution 4% in PBS for 48 hr and processed for routine paraffin embedding. The blocks were cut in serial sagittal or horizontal sections 10 µm thick, mounted on gelatin-coated microscope slides and processed for immunofluorescence. The dehydrated and rehydrated sections were washed with Tris-HCL (0.05 M, pH 7.5) containing 0.1% bovine serum albumin and 0.2% Triton X-100. The endogenous peroxidase activity and non-specific binding were blocked (3% hydrogen peroxide and 50% fetal bovine serum), and the sections were incubated in a humid chamber overnight at 4°C with anti-vimentin (Serotec, 201100, dil. 1:100). Subsequently, the sections were rinsed in buffer and incubated for 90 min at room temperature with Alexa fluor 488 donkey anti-mouse IgG (H + L) (Invitrogen, A 21202 dil 1:300). To provide negative controls, representative sections were incubated with non-immune rabbit sera instead of the primary antibodies, or omitting the primary antibodies, following the same procedure described above. Under these conditions, no positive immunostaining was observed (data not shown).
The study was carried out following the EU Directive 2010/63/EU for animal experiments, following also the guidelines as recommended by the Science Council of Japan or the National Research Council's criteria (NIH No. 86–23).
3 RESULTS
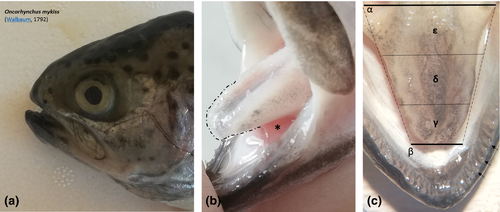
The tongue of Rainbow trout (Oncorhynchus mykiss, Walbaum, 1792 (Figure 1a)) has the appearance of a trunk of a dorso-ventrally compressed pyramid, with an aboral major and a rostral minor base. It is possible to distinguish an apex, a body and a root. The apex appears blunt and represents the only free portion of the tongue. The lower surface of the body and root is instead bound to the floor of the oral cavity, fitted, along the edges, with a row of sharp teeth (Figure 1b,c).
3.1 Scanning electron microscopy
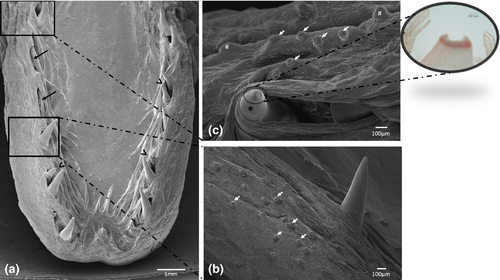
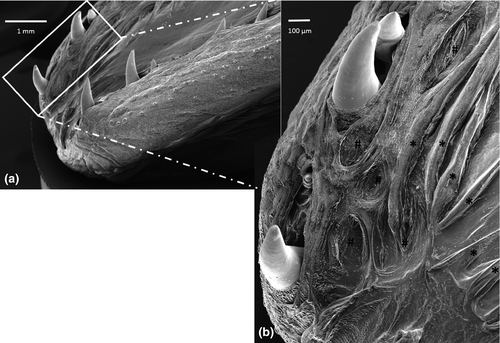
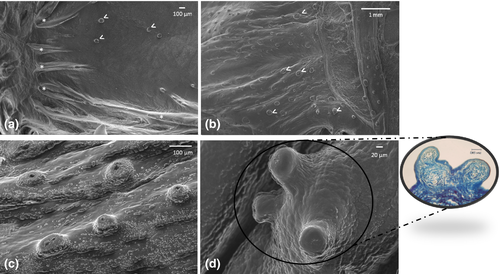
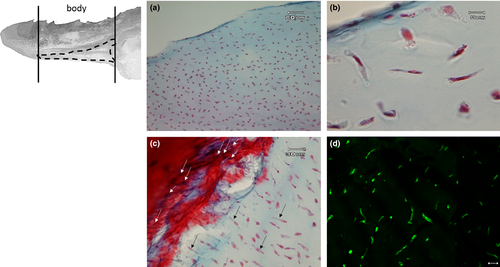
The margins of the tongue appear, along their entire extension, rather prominent, in comparison with the median and paramedian sagittal portion of the tongue and bear a single row of sharp caniniform teeth, with an oro-aboral orientation, erupting from several areas at the apex and body. Some alveolar-like niches appear empty as probable consequence of dental avulsions, others host up to two teeth at different stages of eruption (Figure 2a). The apex of some of them sometimes appears eroded (Figure 2b). Moreover, the lingual margins have, along the entire extension, numerous taste buds that make the surface irregular and scattered fungiform papillae are also evident (Figure 2b,c). Between the inner portion of the margins and the dorsal surface of the tongue, limited to the apex and to the sides of the body, folds of the lingual mucosa, similar to crests oriented towards the most depressed portion of the apex, can be observed (Figure 3a,b). It is also possible to highlight scarring outcomes to fill sites of avulsed teeth (Figure 3b). The dorsal surface of the body is mostly smooth with scattered taste buds (Figure 4a) that increase in number at root level (Figure 4b,c). In the lateral margins of the lingual root, clusters of fungiform-like papillae are observed (Figure 4d).
3.2 Light and confocal laser microscopy
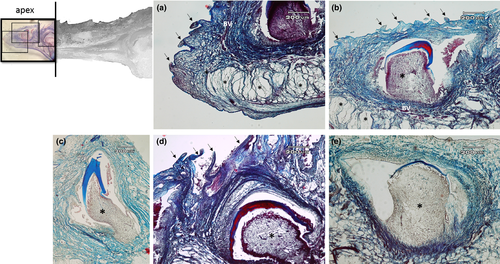
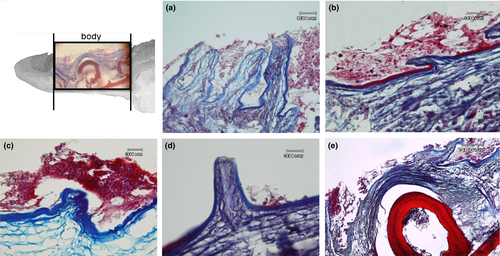
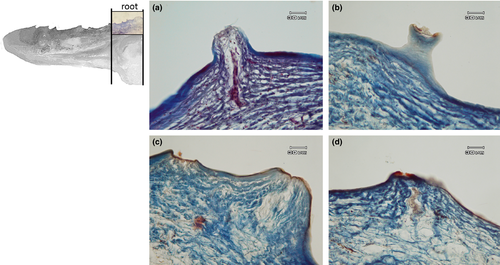
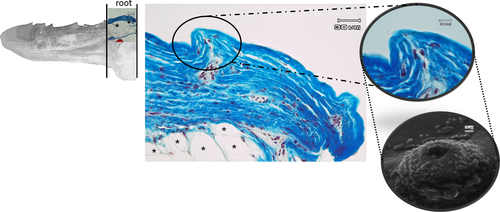
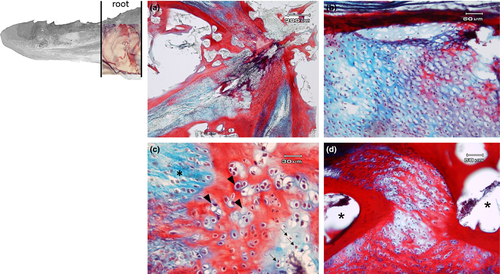
The apex is made up of abundant, dense, irregular, fibrillar connective tissue, richly vascularized, consisting mainly of collagen fibres arranged in bundles oriented in different directions, intensely stained by aniline blue (Figure 5a). The connective tissue rises to form fungiform-like papillae of various shapes (Figure 5a,b,d) and hosts, in its thickness, teeth at various stages of eruption and different degree of maturation (Figure 5a-e). A sketch of a tooth containing a mass of mesenchymal tissue or mesenchymal papilla is also visible in the deeper layer of the connective tissue, which will give rise to the dental pulp consisting of mature mucous tissue (Figure 5e). In the deeper layer of the apex, a white (unilocular) adipose tissue pad immersed in loose connective tissue is observed (Figure 5a,b). The body of the tongue, as well as the entire dorsal surface of the tongue, is covered with a non-keratinized, stratified pavement epithelium. Similarly to the apex, several papillae of various shapes with a mechanical function (Figure 6a-d) are showed and numerous teeth are also present (Figure 6e). In the deepest layer of the tongue body, there is a triangular-shaped pad with apex facing cranially, consisting of fusiform cells immersed in abundant extracellular matrix of the mesenchymal tissue (Figure 7a,b). This pad appears outlined by thin bony trabeculae probably originated by a process of intramembranous ossification. In fact, some fusiform and weakly coloured mesenchymal cells, in a position suggesting a migration towards ossified areas, and cells already differentiated into osteoprogenitor cells, are observed. In the trabeculae, osteocytes included in the bone matrix, which appears deep red stained are present (Figure 7c). Within the triangular structure described above, the presence of cells with a fibroblast-like morphology positive for vimentin is shown (Figure 7d). The root is characterized, in its lateral portion, by the presence of scattered fungiform-like papillae (Figure 8) and taste buds on the entire dorsal surface (Figure 9a). Below the dorsal surface, the thin layer of dense fibrillar connective tissue breaks abruptly and a pad of adipose tissue is present (Figure 9).
The muscular component is poorly represented by thin muscle fibres intercalated with collagen fibres, localized in the aboral parts of the root (data not shown).
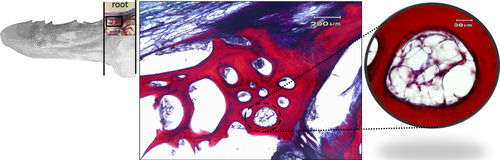
In the deepest layer of the tongue root, a large area of osteo-cartilaginous tissue can be observed (Figure 10a). The cells appear globular in shape and surrounded by abundant extracellular matrix of homogeneous appearance, forming hyaline cartilage that, at the periphery, shows areas during a process of ossification to replace the pre-existing cartilage (Figure 10b). The shade and intensity of the cartilaginous matrix staining appear heterogeneous. The cartilaginous matrix that is about to be replaced by bone tissue is characterized by red shades clearly in contrast to the soft blue staining typical of the GAG-rich cartilaginous matrix and fibrous cartilage in which bundles of collagen fibres and chondrocytes arranged parallel to the collagen fibres with homogeneous and poorly developed intercellular substance are evident (Figure 10c).
The bone matrix appears as small irregularly shaped spicules and trabeculae delimiting interconnected cavities (Figure 10d). At higher magnification circular spaces, resorption channels from which bone tissue has been removed to be replaced by bone marrow are observed (Figure 11a).
4 DISCUSSION
Until the last decade, the fish tongue was mainly described as a simple thickening of the oral cavity pavement mucosa (Buddington & Kuz'mina, 2000). Several studies demonstrated in different teleosts the presence of a true tongue (Abbate et al., 2006,2017,2020a,2020b; Abbate, Guerrera, Montalbano, Ciriaco, et al., 2012; Abbate, Guerrera, Montalbano, De Carlos, et al., 2012; Guerrera et al., 2015; Levanti et al., 2017; Yu et al., 2019). The morphological characteristics of the tongue in the rainbow trout match with that of other described teleost species; therefore, also in this fish of commercial interest an apex, a body and a root can be clearly showed, and these three distinguished zones could have different roles in the mechanics of food catching, prehension and retaining. Moreover, these functions are carried out by caniniform teeth observed in the apex and in the body, and the presence of alveolar-like niches, signs of dental avulsions, demonstrates the capacity of teeth replacement, typical of the most fish, for this reason called polyphyodonty. The oro-aboral orientation of the teeth is a clear sign of their role in prehension and retaining the nourishment. The fundamental role of the tongue in the mechanics of ingestion is confirmed by the presence of numerous fungiform-like papillae, while tasting is guaranteed by several taste buds showed on the tongue mucosa, especially on the root. The capacity of tasting is variable in teleosts, in fact while in sea bass, white sea bass and sea-bream, taste buds are widely localized in all the tongue dorsal surface (Abbate et al., 2012; Abbate, Guerrera, Montalbano, De Carlos, et al., 2012; Guerrera et al., 2015), and in swordfish and Atlantic salmon, there was no evidence of them (Abbate et al., 2017,2020a).
Therefore, a well-developed taste is the prove that the rainbow trout is significantly able to evaluate and so select the food palatability, sign of evolution of the species in the assessment of the food quality, with the consequent possible phenomenon of rejecting the nourishment. The simultaneous presence of well-developed teeth and taste buds is a further confirmation of the important role of the tongue in the early activities of the digestive system, strictly related to the processes of ingestion, swallowing and tasting.
Previous observations on the tongue of several teleosts have shown the presence of a well-developed muscular component (Abbate et al., 2006,2017,2020a,2020b; Abbate, Guerrera, Montalbano, Ciriaco, et al., 2012; Abbate, Guerrera, Montalbano, De Carlos, et al., 2012; Guerrera et al., 2015; Levanti et al., 2017), while our results demonstrate that the rainbow trout tongue shows several types of connective tissues, with different functional properties, from the embryonal tissue, the mesenchyme, to specialized connective tissues, as cartilaginous, bone and adipose tissues, as well as dense connective tissue raised at irregular intervals to form papillae with mechanical, sensory or mechano-sensory function. The functional properties are therefore correlated to different types of cells and fibres, present within the tissue and in the characteristics of the fundamental substance that constitutes the extracellular matrix. The highly significant presence of collagen fibres in the dense, irregular connective tissue gives significant strength at the apex of the tongue. In fact, the fibres are typically arranged in bundles oriented in different directions that allow this part of the tongue, which is particularly useful in prehension and therefore in the capture of the prey, to withstand mechanical stress. To strengthen our hypothesis, in the deepest layer of the apex, there is a pad made of adipose tissue that in our opinion plays an important role in supporting the function of the apex of the tongue in the mechanics of food intake. The presence of the lingual mucosal folds oriented towards the median sagittal part of the apex could be correlated to a potential increase in the size of the tongue, and the presence of scarring results of avulsed teeth is evidence of a continuous dental turnover in new sites, always maintaining the lateral positions. The fibrocartilaginous component, at root level, and adipose tissue pads found at the deepest layer of the apex give the tongue characteristics of flexibility and deformability, necessary to absorb strong mechanical stress, probably ancestrally correlated to the need to procure benthic macro-invertebrates in the stony seabed of rivers. On the other hand, the adipose pad presents at the level of the most superficial layer of the root, immediately below the thin layer of dense fibrillar connective tissue, that shows numerous taste buds, could represent the site of taste evaluation.
The muscular component, made up of thin bundles of fibres, in the tongue root, could play a role in the mechanisms related to food prehension. The presence of resorption channels in the deeper layers of the root indicates an active process of bone remodelling.
Moreover, another important aspect is the presence, in the deepest layer, of a triangular structure with spindle-shaped cells that for their morphological characteristics could be mesenchymal stromal cells. The laser confocal microscopy, in fact, showed a clear vimentin immunoreactivity. Vimentin is utilized as marker of mesenchymally derived cells as the major cytoskeletal component of mesenchymal cells (Musaelyan et al., 2018). The mesenchymal/stem stromal cells may be found in adult mesenchymal tissues other than bone marrow (Bernardo et al., 2009; Campagnoli et al., 2001). The mesenchyme, for its morphogenetic properties for the tissues derived from it, is considered a very important matrix. In Atlantic salmon it was demonstrated that adipose tissue originates from spindle mesenchymal cells (Abbate et al., 2020a), while In rainbow trout the undifferentiated cells, within the mesenchymal tissue, are precursors of cells of the bone tissue. These adult mesenchymal stem cells have a limited potential for differentiation, comparing with embryonic stem cells.
In conclusion, this study could be useful as a contribution to the knowledge of the morphological characteristics of the tongue in rainbow trout and the results could be related to further studies regarding the feeding in a very often farmed species in aquaculture. Moreover, the observation of tissues typical of the embryo-fetal phase in the adult specimens tongue also in this species of fish could be considered for the eventual applications in cell therapies in different pathologies.
ACKNOWLEDGEMENTS
The authors thank the “Azienda Agroittica Macrostigma by Civello Giuseppina” located in Contrada Stafenna, Noto (Siracusa), Sicily, Italy.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ETHICAL APPROVAL
All applicable international, national and/or institutional guidelines for the care and use of animals were followed (Directive 2010/63/EU).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




