Morphological Aspects of Oral Denticles in the Sharpnose Shark Rhizoprionodon lalandii (Müller and Henle, 1839) (Elasmobranchii, Carcharhinidae)
Summary
The oral denticles of some elasmobranchs are found on the surface of the oral cavity and are homologous to those on the body surface, being well developed, independent and non-growing, with varying morphology and distribution depending on the species. The structural and three-dimensional characteristics of oral denticles from the rostro-ventral surface of the sharpnose shark Rhizoprionodon lalandii were described following imaging by both light and scanning electron microscopy. The light microscopy results showed that the triangular shape of the denticles consisted of a base and an apex. Picrosirius staining showed the arrangement of collagen fibres and oral denticles, and a predominance of type-I collagen was found in both structures under polarized light. There was a broad homogeneous distribution of denticles on the ventral surface, forming a leaf-like shape with the cusp facing the caudal region. Interlocking, hexagonal, geometric structures on its rostral side and ridges on the rostral side of the oral denticles were observed under increased magnification. We concluded that the denticle morphology found in R. lalandii differ of others analysed species, and the descriptions of these structures therefore provide important information for the classification of the species. In this species, the main functions can be assigned to help reduce hydrodynamic drag, particularly by this being a species that uses ram ventilation, and to protect the epithelium of the oropharynx of abrasion and parasites.
Introduction
Sharks are cartilaginous fish, which appeared approximately 400 million years ago, and along with the rays form the subclass Elasmobranchii, approximately 1100 species are known throughout the world (Castro, 1987; Compagno, 2001). Ecologically, the sharks and rays play a vital role in marine ecosystems, as they are positioned on the apical portion of the trophic chain, due mainly to large size animals and feed on a wide range of prey, playing the prime role of regulators of this ecosystem, especially tropical and subtropical (Garrick, 1982; Camhi et al., 1998; Ferretti et al., 2010).
In elasmobranchs, placoid scales or dermal denticles, gifts across the surface of the skin, are also in the oral mucosa in a similar way, with modifications of the dermal denticles (Nelson, 1970; Kemp, 1999), remaining little differentiated along the evolutionary process (Sire and Huysseune, 2003). The function of dermal denticles has been modified along several functional lines, including protection from predators and ectoparasites, reduction of mechanical abrasion, accommodation of bioluminescent and sensory organs, and reduction of frictional drag (Reif, 1978; Raschi and Tabit, 1992).
They exhibit inter-specific variation in oral denticles and can be numerous, small or large, sparse or closely grouped so simple or highly differentiated (Imms, 1905; Daniel, 1934; Nelson, 1970; Atkinson and Collin, 2012), appear to help reduce hydrodynamic drag, particularly in species that use ram ventilation and to protect the oropharyngeal epithelium from abrasion and parasites (Atkinson and Collin, 2012).
The denticles and teeth are composed of calcified dentin, covered by a layer of enamel. By having a composition and hardness more resistant to decay than the cartilaginous skeleton, denticles can form an important and historical record in identifying fossil species (Gravendeel et al., 2002).
The sharpnose shark Rhizoprionodon lalandii (Muller; Henle, 1839), from the order Carcharhiniformes, family Carcharhinidae, is a small-sized shark species that reaches up to 80 cm. Sharpnose sharks are associated with the continental shelf, inhabiting shallow waters along the Atlantic coast from Panama to the State of Santa Catarina in Brazil for their entire life cycle (Figueiredo, 1977; Compagno, 1984; Compagno et al., 2005). This species represents approximately 60% of the total number of sharks caught in artisanal fisheries in the Itanhaem region, in the south of São Paulo State (Namora, 2003; Motta et al., 2005). Population and catch data are unreliable due to a scarcity of records, and R. lalandii is classified as ‘Data Deficient’ (DD) on the IUCN red list of threatened species. However, increased mortality at all ages associated with coastal fisheries most likely threatens populations of this species (IUCN, 2012). Caught with bottom lines, longlines and gillnets, R. lalandii is of significant commercial importance in the markets of small-sized coastal towns (Figueiredo, 1977).
The diet of sharpnose sharks in southern Brazil comprises mainly on teleosts, followed by cephalopods and crustaceans (Lima et al., 2000; Bornatowski et al., 2012, 2014). Given its abundance and diet, R. lalandii may be an important predator of demersal and pelagic prey in coastal waters of Brazil (Bornatowski et al., 2012).
The aim of this study was to describe the structure of the oral denticles of the ventro-rostral surface of sharpnose shark employing light microscopic and scanning electron microscopy methods.
Material and Methods
Animals
A total of eight male sharpnose sharks, Rhizoprionodon lalandii (Muller; Henle, 1839), identified according to Figueiredo (1977), were used. The terminology used in this paper referring to the oral cavity follows Atkinson and Collin (2012) and Collin (2012). All specimens were donated by fishermen trawling (Austria captain) in southern Brazil (25°00–25°34′S and 46°30–46°55′W) to the Instituto de Pesca, from Santos-SP and transferred to the Department of Anatomy (FMVZ/USP). This study was approved by the Commission for Ethics on Animal Use (CEUA) no 9971141113 of the School of Veterinary Medicine and Animal Science of the University of São Paulo (USP).
Light microscopy
Samples of the ventro-rostral surface (n = 4) were dissected and fixed in 4% paraformaldehyde solution. After complete fixation, the samples were dehydrated in a series of increasing ethanol concentrations (70–100%), then cleared in xylene, and subsequently embedded in histological paraffin. Sagittal sections of 5 μm thickness were cut using a Leica microtome (Leica Biosystems, Bensheim, Germany) and stained with haematoxylin–eosin (HE) and picrosirius (PS). Images were obtained using a Nikon Eclipse E-800 light microscope (Nikon Corp., Tokyo, Japan).
Scanning electron microscopy
Samples of the ventro-rostral surface (n = 4) were immersed in a modified Karnovsky fixative solution, with 2.5% glutaraldehyde and 2% paraformaldehyde in a sodium phosphate buffer solution (0.1 m, pH 7.4), for 48 h at 4°C (Watanabe et al., 1989; Duro et al., 2012; Bolina et al., 2013). The samples were then post-fixed in a 1% aqueous osmium tetroxide solution for 2 h at 4°C, washed in distilled water, dehydrated in an increasing alcohol series and dried in a Balzers CPD-020 critical point dryer (Bal-Tec, Balzers, Liechtenstein) using liquid CO2 (Ciena et al., 2013; Reginato et al., 2014). The samples were then positioned and mounted on aluminium stubs and sputter coated with gold ions using an Emitech-K550 device and examined under a LEO 435 VP scanning electron microscope (LEO Electron Microscopy Ltd., Cambridge, UK) at the Department of Surgery, School of Veterinary Medicine and Animal Science (FMVZ-USP).
Results
Macroscopy
Macroscopic observations of the ventro-rostral region of the oral cavity (4 cm length and 2.5 cm wide) showed a rounded apex with extremity concave following the jaw line. The surface epithelium revealed a rough appearance with major density of denticles in the caudal region adjacent to the gills (Fig. 1).

Light microscopy
The oral denticles of the ventro-rostral surface are constituted by calcified dentin. This were arranged vertically, with a triangular shape and were composed of a base and apex both (Fig. 2a). The base featured two rods with epithelial attachment; the caudal rod was disposed vertically, and a wide rostral rod was disposed obliquely. The rods converged to form the caudally inclined apex. The epithelial tissue revealed thin mucous membrane in the surface with varied disposition of the epithelial fibres and communicating projections between the rods (Fig. 2a,b). In the bottom with regular disposition presented the submucous tissue (Fig. 2a,c,e).
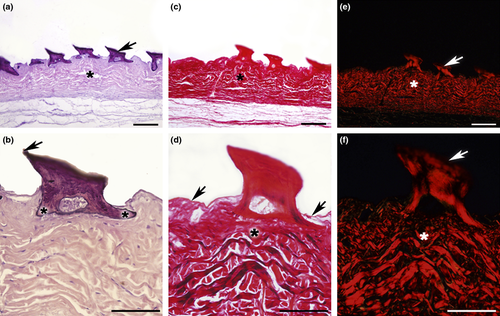
Figure 2c,d shows the arrangement of both the collagen fibres of the epithelium and oral denticles. A predominance of type-I collagen could be observed in both structures under polarized light (Fig. 2e,f).
Scanning electron microscopy
The uniform three-dimensional aspects of the denticles revealed a broad homogeneous distribution along the ventral surface (Fig. 3a,b), with a leaf-like shape. Broad lateral margins converged to form the protruding apex, with a cusp faced towards the caudal region (Fig. 3c,d). Interwoven collagen fibres were observed between the bases of the oral denticles (Fig. 3c,d). The surface presented smooth in the caudal side of the oral denticles, while one to three ridges were observed on the rostral side extending from the base to the distal third of the apex (Fig. 3e). At greater magnifications, the hexagonal geometric structures interlocking were observed on the rostral side of the oral denticle (Fig. 3f).

Discussion
Similar to dermal denticles in body surface of the sharks, the denticles of the oral cavity are well developed, independent and non-growing. They vary in morphology, distribution and function depending on the species (Daniel, 1934; Nelson, 1970; Atkinson and Collin, 2012). Consist of a crown, neck and base, an enamelled layer overlies dentin which extends from the crown down into the neck. A pulp cavity lies within the denticles (Sire et al., 2009; Motta et al., 2012). According to Fraser et al. (2010), the dermal denticles and teeth have independent origins from different tissue layers. In this present study, the oral denticles and dermal denticles present similar morphology, in hypothesis by your ectodermal origin.
Few studies have been made describing the morphology of oral denticles in sharks. In a thorough study of more than 15 species, Nelson (1970) observed that the pharyngeal denticles participate in the formation of cohesive units termed pharyngeal pads. The macroscopic characteristics, such as the shape of the ventral oral cavity, and the distribution and abundance of oral denticles, of the shark Rhizoprionodon terraenovae (Richardson, 1836), described by Nelson (1970), were similar to those found in R. lalandii. Reif (1980) describes the development of dermal denticles and also from the cavity of the mouth of the shark Scyliorhinus canicula, where the formation of the denticles in the cavity of the mouth starts much later than on the surface of the body.
In R. lalandii, the oral denticles were found to be numerous, with a homogeneous distribution, and were often close to each other; the same distribution was observed in the sharks Carcharhinus melanopterus and Negaprion acutidens (Atkinson and Collin, 2012), both from the family Carcharhinidae. The three-dimensional aspects observed in R. lalandii were also similar to the two species cited above.
Histologically, the denticles on the ventro-rostral surface exhibit the same characteristics as those found in the tongue of the megamouth shark Megachasma pelagios described by Yano et al. (1997), which has connective tissue containing fibres, denticles with a cusp that is curved posteriorly, and a basal root composed of bone tissue.
The presence of denticles on the ventral surface of the oral cavity in R. lalandii can provide a hard surface to protect the epithelium from abrasion during the consumption of hard-bodied prey. During feeding, the processing of prey is as important as their capture, especially in the case of prey that has both digestible portions and indigestible portions such as bones, spines and shells. In many tetrapods, this processing task is performed by manipulation with ‘hands’ and tongue (Dean et al., 2005).
Megacarnivorous sharks are particularly effective in handling prey, with the capture and processing involving the coordinated use of the upper and lower jaws, mechanically and where external to these sharks, the flexibility of the upper jaw in the handling of the prey is larger (Dean et al., 2005). According to Liem et al. (2001), the fish tongues play no role in food transport or handling. Elasmobranchs have basihyal and ventral hyoid and brachial cartilage on the floor of the oropharyngeal cavity, which function as a reduced tongue (Daniel, 1934; Dean et al., 2005; Wilga et al., 2007).
The high number of denticles on the ventral surface of the oral cavity in R. lalandii suggests that this species has good prey handling efficiency. Because the diet of R. lalandii is based on small fish, cephalopods and crustaceans, such traits are most likely evolutionary adaptations to predation.
The most frequently discussed function for dermal denticles involves the swimming performance through drag reduction. Mounting evidence indicates that drag reduction most likely occurs by reducing turbulent cross-flow near the scale surface, thereby reducing shear stress, and by control of flow separation around the body, which would reduce pressure drag (Raschi and Tabit, 1992; Bechert et al., 2000; Lang et al., 2008). It is therefore proposed that the sharks which use ram ventilation (Wegner and Graham, 2010) have these keeled denticles to reduce drag on the epithelium of the oropharyngeal cavity when they swim with their mouths open (Atkinson and Collin, 2012).
Conclusion
We concluded that oral denticles on the ventral surface of the oral cavity in Rhizoprionodon lalandii have distinct morphological characteristics that are different from other species and possibly relevance to the taxonomic classification. In this species, the main functions can be assigned to help reduce hydrodynamic drag, particularly by this being a species that uses ram ventilation, and to protect the epithelium of the oropharynx of abrasion and parasites.
Conflict of Interest
The authors declare that there is no conflict of interest in this manuscript.




