Testis Morphometry and Stages of the Seminiferous Epithelium Cycle in an Epididymal Sperm-storing Neotropical Vespertilionid, Myotis levis (Chiroptera)
Summary
Yellowish myotis, Myotis levis, is a seasonal, epididymal sperm-storing Neotropical vespertilionid. In the dry season, males show simultaneous testis regression and sperm storage in cauda epididymis, enabling them to mate during this season. In this study, we investigated seasonal variations in body mass, diameter and height of seminiferous tubules and nuclei of Leydig cells in a population of southeastern Brazil. We also determined the frequencies of the stages of the seminiferous epithelium cycle (SEC) of mature individuals of this population. Body mass and diameter of Leydig cell nuclei showed no significant differences between dry and rainy seasons and stages of annual reproductive cycle; however, all other morphometric parameters varied significantly. The relative cumulative frequency of pre-meiotic stages of the SEC (1–3) was 51%, of meiotic stage (4) was 2% and of post-meiotic stages (5–8) was 47%. We confirmed that the yellowish myotis presents seasonal sperm production as revealed by testis regression and epididymal sperm storage during the dry season.
Introduction
Vespertilionid bats living in temperate zones tend to be seasonally monoestrous and possess reproductive mechanisms that reflect the need for hibernation or extended torpor during the winter which disrupts the reproductive cycle. Among these mechanisms, the storage of spermatozoa by males and females, delayed ovulation, delayed fertilization in the female genital tract and embryonic diapause are particularly significant (Weir and Rowlands, 1973; Oxberry, 1979; Racey et al., 1987; Rydell, 1992; Heideman, 2000). In tropical areas where there is little seasonal variation to restrict insect availability and impose restrictions on reproduction, insectivorous vespertilionid bats may have a polyestrous pattern with more than one reproductive peak during the year (Wilson and LaVal, 1974; Myers, 1977; Wilson, 2007). However, there are tropical species that have seasonal reproduction in which males show regressive testes and may store sperm in the cauda epididymis in response to temporal asynchrony that exists between spermatogenesis and mating, and between spermatogenesis and accessory genital gland activity (Gustafson, 1979; Krutzsch, 1979, 2000; Racey, 1979; Crichton, 2000; Araújo et al., 2013).
The family Vespertilionidae is the most diverse within Chiroptera, including species distributed in tropical and temperate regions of the planet. In this family, the genus Myotis has the widest geographical distribution among bats (Nowak, 1994) and currently is represented by 103 species (Simmons, 2005). These two characteristics make the species of this genus promising models for the study of reproduction as they occur in a variety of different environmental conditions.
The yellowish myotis, Myotis levis (I. Geoffroy Saint-Hilaire, 1824), is insectivorous and generally forages in open areas near water by capturing insects in flight. It is a small bat (about 6 g) that ranges in colour from tones of grey to brown and lacks any facial ornamentation (Nowak, 1994; Eisenberg and Redford, 1999; Araújo et al., 2013). The yellowish myotis has been recorded from Uruguay, southern Brazil, southern Bolivia, Paraguay and northern Argentina (Wilson, 2007). However, because of the taxonomic difficulty with southern South American Myotis, the systematic status and distribution of this species remain unresolved (Stevens et al., 2010).
A recent study (Araújo et al., 2013) showed that a population of yellowish myotis in a highland area in southeastern Brazil has seasonal monoestry, asynchrony in maturation of male and female gonads and sperm storage in cauda epididymis, similar to those of epididymal sperm-storing temperate vespertilionid species. In the present study, we use morphological and morphometric techniques to characterize seasonal variations of the following biometric testis parameters: diameter of the seminiferous tubules, the height of the seminiferous epithelium and the diameter of the nucleus of Leydig cells. Using the tubular morphology method, we also define the stages of the seminiferous epithelium cycle (SEC) and document their frequencies. Thus, we provide, for the first time for a Neotropical insectivorous species, information on testicular morphology and sperm occurrence in cauda epididymis over the annual reproductive cycle that can be used in future comparative analyses.
Materials and Methods
Study area
The study was conducted in the Santuário do Caraça, a preserved area in the Serra do Caraça, southeastern Brazil (20°04′30″S, 43°24′28″W). The area encompasses 10 187 ha with elevations of 750–2072 m. It is located in the transition between the Atlantic Forest and Cerrado domains, thus supporting various ecosystems such as seasonal semideciduous forest, riparian forest and rocky fields (Giulietti et al., 1997). Climate in the region is seasonal with a rainy summer – rainy season – from October to March and a dry winter – dry season – from April to September. Annual rainfall in the Serra do Caraça region averages 1373 mm, of which 81.5% occurs during the rainy season (Sá et al., 2012). The temperature inside the yellowish myotis colony roost shows that August (within the dry season) was the coolest period, with temperatures between 9 and 18°C. The warmest period corresponds to February and March (23–25°C; within the rainy season) (Araújo et al., 2013).
Animal processing and data analysis
The individuals were captured each month from March 2010 to May 2011 by placing two mist-nets, measuring 2.5 × 7 m each, on the attic exit pass from 18:00 h to 21:00 h. We captured a total of 48 males, which were placed individually in cloth bags and euthanized with an overdose injection of sodium thiopental (0.9 mg/g body weight). We recorded the date of capture and body mass of each bat. Only bats with ossified finger epiphyseal cartilages and thus categorized as adults (Anthony, 1988) were used.
For histological analyses, small fragments of the right testis and epididymis were fixed by immersion in Bouin liquid for up to 12 h at room temperature. The fragments were then dehydrated in an ethanol series for subsequent paraffin embedding. The tissues were cut at 5 μm of thickness and stained with haematoxylin-eosin. Corrections for tissue shrinkage during histological processing were not made. Analyses of accessory genital glands were not performed.
A total of 436 near circular cross-sections of seminiferous tubules, corresponding to circa 9 seminiferous tubules per bat, were digitally photographed in an optical microscope, and the diameter of the seminiferous tubules and height of their epithelium were measured using the program image j (Rasband, 2012). The photographed cross-sections were used to identify the stages of the SEC and their frequencies using the method of tubular morphology (Clermont, 1972; Berndtson, 1977; Hess and França, 2007) which is based on the following characteristics: shape of the spermatid nuclei, occurrence of meiotic divisions and position of spermatids in the seminiferous epithelium.
The annual reproductive cycle of the yellowish myotis male is characterized by four stages: rest, maturing, mature and mating (Araújo et al., 2013). Mating refers to the stage in which the testis is in a regressed condition when the seminiferous epithelium shows only Sertoli cells and rare spermatogonia, but the cauda epididymis is enlarged and packed with spermatozoa (Araújo et al., 2013). In this study, we adopted the terminology of testicular regression instead of mating (Araújo et al., 2013) as our focus was to evaluate mainly the morphological changes of the seminiferous epithelium over the annual reproductive cycle. Images of cauda epididymis associated to the corresponding stage of the reproductive cycle are provided in order to illustrate the reproductive seasonality of both organs.
All individuals were classified according to one of these stages of the reproductive cycle, and the frequencies of individuals in each stage and in each season were determined. The stages of the SEC and the frequency of each stage were obtained in the testis of individuals in mature stage. Furthermore, we measured the diameter of the nucleus of 480 Leydig cells (10 cells of each individual in the rainy and dry seasons) that were found surrounding the seminiferous tubules.
Comparisons of the means of the morphological and morphometric parameters for rainy and dry seasons were made by the Student's t-test and Mann–Whitney test (Zar, 1999). One-way analysis of variance (anova) was used to evaluate variations of these parameters between reproductive stages. The Tukey test for posteriori comparisons was performed when necessary (Zar, 1999). Statistical analyses were performed using biostat 5.3 (Ayres et al., 2007).
All specimens are deposited in the collection of the Pontifícia Universidade Católica de Minas Gerais. Captures were performed under licences (# 22231-1 and 28120-1) granted by the Brazilian Chico Mendes Institute for Biodiversity Conservation. The procedures used in this study were previously approved by the University Ethics Committee on Animal Use.
Results
Testis parameters
We registered statistically significant variation in most testis parameters between rainy and dry seasons (Table 1), and among the four stages of the annual reproductive cycle (Table 2); the exceptions were diameter of Leydig cell nuclei and body mass which did not show significant variation (P > 0.05) in any of the analyses (Tables 1 and 2).
| Season | DST | HSE | DLC | CM |
|---|---|---|---|---|
| Rainy (n = 27) | 87.4 ± 43.9 | 29.2 ± 14.3 | 4.8 ± 0.3 | 5.6 ± 0.6 |
| Dry (n = 21) | 59.3 ± 15.3 | 17.9 ± 3 | 5.0 ± 0.3 | 5.3 ± 0.5 |
| Mann–Whitney (U) | 147.5 | 117 | 2.448* | 174.5 |
| Significance level | s | s | ns | ns |
- s = P < 0.05, ns = P > 0.05; *t-test of student.
| Reproductive stage | DST | HSE | DLC | CM |
|---|---|---|---|---|
| Rest (n = 23) | 53.5 ± 6.6A | 17.0 ± 3.7A | 5.1 ± 0.5 | 5.5 ± 0.5 |
| Maturing (n = 15) | 67.8 ± 15.8B | 24.9 ± 7.8B | 4.8 ± 1.4 | 6.0 ± 0.5 |
| Mature (n = 7) | 154.2 ± 23.4C | 47.6 ± 11.7C | 3.9 ± 2.2 | 5.5 ± 0.6 |
| Regressed (n = 3) | 92.6 ± 7.7D | 22.5 ± 1.4D | 5.3 ± 0.4 | 5.7 ± 0.3 |
| anova (F) | 105.1 | 38.4 | 0.45 | 2.7 |
| Significance level | s | s | ns | ns |
- Different column superscripts show statistically significant differences.
- P < 0.05.
Stages of the annual reproductive cycle of males and their frequencies
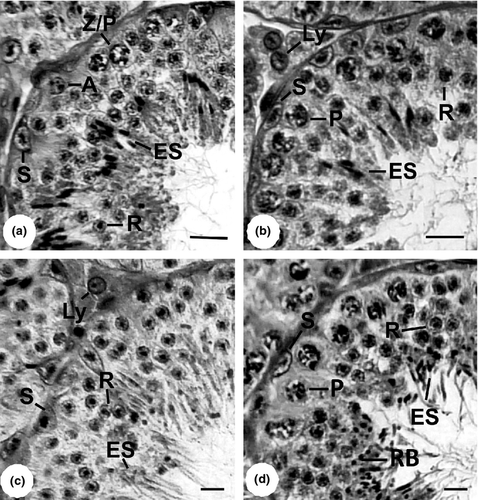
The stages of the annual reproductive cycle as rest, maturing, mature and regressed (Fig. 1, including inserts of cauda epididymis at the corresponding testis stage) were registered in both seasons. During the rainy season, most bats were maturing (48.1%), whereas 29.6% had reached the mature stage, and 22.2% were still resting; no regressed bats were captured during the rainy season. In the dry season, most bats were resting (81.0%), 14.3% were in regression, and only 4.8% were maturing; no mature bats were registered during this season. A brief description of the stages follows.

In the rest stage, the seminiferous tubules had reduced diameter, and the height of the epithelium was decreased (Table 2). The seminiferous epithelium was composed of numerous Sertoli cells and some type A spermatogonia supported by the basal membrane. In addition, there was a higher proportion of Leydig cells among the seminiferous tubules; no spermatozoa were present in cauda epididymis (Fig. 1a).
The maturing stage was characterized by a still reduced seminiferous tubules diameter and epithelium (Table 2), reduced lumen and by the presence of primary spermatocytes at pre-leptotene, spermatogonia and Sertoli cells; few spermatozoa were seen in cauda epididymis (Fig. 1b).
The mature stage, which occurred only in the rainy season, was characterized by the presence of all cells of the spermatogenic lineage. The lumen of the tubule was well defined; cauda epididymis showed larger amount of spermatozoa than in previous stage (Fig. 1c), and the height of the seminiferous epithelium and diameter of the tubules showed the highest values (Table 2).
The regressed stage occurred only in the dry season and was characterized by cellular disorganization of the testis, wide seminiferous tubule lumen, reduced number of cells in the seminiferous epithelium (Fig. 1d) and reduced morphometric parameters when compared to the mature stage (Table 2). Noticeably, the cauda epididymis was packed with spermatozoa.
Frequency of the stages of the seminiferous epithelium cycle
The frequencies of the eight stages of the SEC are shown in Fig. 2. The cumulative relative frequency of pre-meiotic stages (stages 1 to 3) was 51%, meiotic stage (stage 4) 2%, and post-meiotic stages (stages 5 to 8) 47%. Descriptions of cellular associations of each stage (Figs 3 and 4) are provided below:
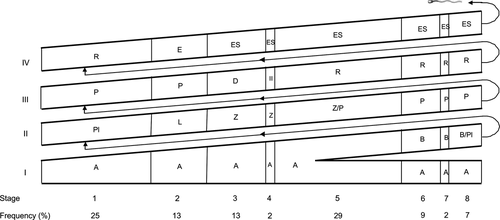

- Stage 1: The cell types recorded were A spermatogonia (not shown in Fig. 3a), primary spermatocytes in pre-leptotene, a generation of primary spermatocytes in pachytene, a generation of round spermatids and Sertoli cells whose cytoplasmic extensions supported the spermatogenic cells (Fig. 3a).
- Stage 2: Characterized by having several layers of elongated spermatids with their respective nuclei facing the Sertoli cells, type A spermatogonia (not shown in Fig. 3b), primary spermatocytes in leptotene and one or two layers of primary spermatocytes in pachytene (Fig. 3b).
- Stage 3: The cell association in this stage contained early formation of bundles of elongated spermatids in the intermediate portion of the seminiferous epithelium, type A spermatogonia (not shown in Fig. 3c), a generation of primary spermatocytes in zygotene, and a most advanced generation of primary spermatocytes in diplotene (Fig. 3c).
- Stage 4: Characterized by the presence of metaphase plates resulting from two meiotic divisions (one from primary spermatocytes in diplotene to secondary spermatocytes and another from secondary spermatocytes to spermatids), primary spermatocytes in zygotene, primary spermatocytes in diplotene (not shown in Fig. 3d), secondary spermatocytes (not shown in Fig. 3d), elongated spermatids in bundles located in the intermediate region of the seminiferous epithelium, and type A spermatogonia and Sertoli cells (Fig. 3d).
- Stage 5: Presents a layer of elongated spermatids, organized in bundles located in the deep region of the seminiferous epithelium, besides round spermatids, type A spermatogonia and primary spermatocytes in zygotene and pachytene (Fig. 4a).
- Stage 6: Shows type B spermatogonia, type A spermatogonia (not shown in Fig. 4b), a layer of primary spermatocytes in pachytene, layers of rounded spermatids and elongated spermatids arranged in bundles directing to the lumen of the seminiferous tubule (Fig. 4b).
- Stage 7: Shows type A and B spermatogonia (not shown in Fig. 4c), a layer of primary spermatocytes in pachytene (not shown in Fig. 4c), several layers of round spermatids and a large number of elongated spermatids located close to the lumen and not organized in bundles (Fig. 4c).
- Stage 8: Shows elongated spermatids attached to a large extent to the edge of the seminiferous epithelium, residual bodies, several layers of round spermatids, a layer of primary spermatocytes in pachytene (not shown in Fig. 4d), type A spermatogonia (not shown in Fig. 4d) and type B spermatogonia (Fig. 4d).
Discussion
In this study, we found statistically significant differences in testis morphology of the yellowish myotis between rainy and dry seasons, thereby confirming that reproductive activity of yellowish myotis is seasonal (Miranda et al., 2010; Araújo et al., 2013). The former authors reported the capture of a pregnant or lactating female during the rainy season in Paraguay.
The yellowish myotis, despite having regressed testes during the dry season, exhibits sperm storage in the cauda epididymis, being able to reproduce at the end of the dry season and during the rainy season (Araújo et al., 2013), as seen in some other Neotropical vespertilionids species (Myers, 1977; Beguelini et al., 2013a), and similar to tropical (Medway, 1972; Bernard et al., 1997) and temperate vespertilionid species (Racey, 1979; Crichton, 2000; Encarnação et al., 2004; Krutzsch, 2009), except that there are no hibernation events.
Positive correlation between temperature and rain and reproductive cycle of bats in the Neotropics has been widely demonstrated (McWilliam, 1988; Heideman and Bronson, 1994; Sosa and Soriano, 1996; Arlettaz et al., 2001; Estrada and Coates-Estrada, 2001). In this study, we found that, in general, testicular rest and regression occur during the dry season, and maturing and mature during the rainy season. The occurrence of maturing and mature during the rainy season seems to be an appropriate strategy as this period experiences an increased availability of insects needed as energy source (Wolda, 1978; Racey and Entwistle, 2000; Silva et al., 2011). Males may allocate more energy to gonadal maturation and production and storage of sperm, as gonadal maturation is energetically expensive (Kenagy and Trombulak, 1986; Heideman, 2000). However, studies performed under controlled conditions are needed to provide a good understanding of how bats respond reproductively to environmental conditions (Heideman, 2000).
Despite the apparent fit of reproductive activity of males allocating greater energy to gonads (Kenagy and Trombulak, 1986) during favourable times, the reproductive cycle of the yellowish myotis is conditioned by seasonal receptivity of the female, which occurs in the middle of the dry season. Such a reproductive strategy allows births to coincide with the rainy season (Araújo et al., 2013), when conditions are more suitable for the survival of offspring (Bronson, 1985; Speakman and Thomas, 2003). Thus, the male reproductive cycle of the yellowish myotis may be timed by the female reproductive cycle, as it is for other species of vespertilionids (Pfeiffer and Mayer, 2013).
Significant differences in diameter of seminiferous tubules and height of the seminiferous epithelium are related to greater sperm activity (França and Russell, 1998) as registered in the present work during the mature stage in the rainy season. The diameter of Leydig cell nuclei showed no significant variation between seasons (present study) as the morphology of these cells does not vary according to the season (Gustafson, 1979).
From histomorphological point of view, there are not yet clearly established patterns of annual reproductive cycle for Neotropical bats. For the vespertilionids Lasiurus blossevillii, Histiotus velatus, Myotis nigricans, Eptesicus furinalis and M. albescens, the reproductive stages were classified into maturation, activity and quiescence based on the presence of spermatozoa in the testis or cauda epididymis (Beguelini et al., 2013a). For the phyllostomid Artibeus lituratus, two reproductive periods, activity and regression were described (Oliveira et al., 2009). However, continually active pattern of spermatogenesis throughout the year was found in the later species (Duarte and Talamoni, 2010) and in A. planirostris (Beguelini et al., 2013b).
The presence of eight stages of the SEC, based on tubular morphology (Clermont, 1972), described for yellowish myotis (present study) has been similarly described for the Neotropical phyllostomid bats Artibeus lituratus, Artibeus planirostris, Carollia perspicillata, Platyrrhinus lineatus and Sturnira lilium (Beguelini et al., 2009; Oliveira et al., 2009; Morais et al., 2013), but not for the vespertilionid M. nigricans in which different cell associations were registered in seminiferous tubule cross-sections (Beguelini et al., 2009).
There have been few works investigating the SEC in Neotropical microchiropteran species. The frequencies of pre-meiotic, meiotic and post-meiotic stages have been computed in few bats (Beguelini et al., 2009; Morais et al., 2013). In these bats, including ours, pre-meiotic and post-meiotic frequencies prevail, and meiotic stage frequency is usually low. The reduced number of bats which had the seminiferous cycle studied up until now hinders an evaluation of a phylogenetic relationship among them. The frequency of disappearance of a given cellular association and its reappearance, characterizing a sequence of the SEC (Clermont, 1972), is a species-specific constant and fundamental to knowing the duration of the spermatogenic process (Hess and França, 2007).
In this study, we confirmed that the yellowish myotis presents seasonal sperm production as revealed by testis regression and epididymal sperm storage during the dry season. These findings are the first reported for a Neotropical insectivorous species and may be useful in future comparative analyses.
Acknowledgements
We thank the FIP-PUC Minas for funding the project and providing scholarships (TOF, AAN), and Raissa A. Araújo for help in data collection. We also thank the staff of the Reserva Particular do Patrimônio Natural Santuário do Caraça for allowing us to collect the bats.




